Introduction
Pancreatic cancer is the fourth leading cause of
cancer-related mortality (1).
Furthermore, pancreatic cancer has a poor prognosis, with a 5-year
survival rate of 6% (1), in part
due to its ability to metastasize at an early stage (2). Non-cohesive pancreatic cancer is
caused by the loss of expression of certain proteins, including
E-cadherin, which results in cancer cell dissemination and
subsequently, a poor prognosis (3,4).
Consequently, only 10% of patients with pancreatic cancer are
treated with curative therapy, whilst 90% are treated
conservatively (5). Therefore,
there is a requirement for the development of novel treatments for
pancreatic cancer.
Small-molecule receptor tyrosine kinase inhibitors
are currently under investigation as anticancer agents (6). Insulin-like growth factor-I receptor
inhibitors are particularly promising, as they inhibit the
dissemination of pancreatic cancer cells (7,8). One
of the disadvantages of these inhibitors, however, is the observed
co-inhibition of the insulin receptor (9).
The Wnt signaling pathway is pivotal for cell growth
and differentiation. There are 3 signaling branches of the Wnt
pathway, the canonical, planar-cell polarity and
Wnt-Ca2+ pathways (10),
with the former being the best characterized. During the canonical
Wnt pathway, β-catenin is first phosphorylated and degraded in
normal cells, following which, it enters the nucleus where it
increases the expression of target genes, including cyclin D1, a
protein involved in cell proliferation (11). Frizzled (Fz) is a receptor of Wnt
ligand. Upon binding of Wnt ligand to Fz, the Wnt pathway is
activated. In total, 10 members of the Fz family of genes have been
identified based on structural and functional homology studies
(12). Fz genes have been
previously implicated in carcinogenesis and embryogenesis and their
expression is upregulated in gastric cancer and hepatoma cells
(13,14). We investigated the possibility of
Fz2 as a potential target of molecular therapy for pancreatic
cancer.
Materials and methods
Cell culture
Pancreatic cancer cell lines, PANC-1, MIA-Paca2,
NOR-P1, PK-45H, PK-1, PK-59 and KP4, were purchased from Cell Bank,
RIKEN BioResource Center (Tsukuba, Japan). MIA-Paca2 was cultured
in Dulbecco’s modified Eagle’s medium (DMEM), KP4 was cultured in
DMEM:F12 and the remaining lines were cultured in Roswell Park
Memorial Institute medium-1640. Media were purchased from
Sigma-Aldrich (St. Louis, MO, USA) and supplemented with 10% fetal
bovine serum (FBS; Life Technologies, Carlsbad, CA, USA). The cell
lines were cultured with 5% carbon dioxide at 37°C in a humidified
chamber.
Reverse transcription (RT) and
quantitative polymerase chain reaction (PCR)
The cells were spread in 6-well plates (Asahi Glass
Co., Ltd., Tokyo, Japan) and cultured. When the cells had reached
80% confluency, they were further cultured for 48 h after
transfection. Total RNA (5 μg), isolated with Isogen (Nippon Gene
Co., Ltd., Tokyo, Japan), was used to generate cDNA with Super
Script III and oligo dT primers, according to the manufacturer’s
instructions (Life Technologies). Human whole pancreas RNA was
purchased from Takara and used as a positive control (Takara Bio,
Inc., Shiga, Japan). The PCR primers, annealing temperatures,
reaction cycle numbers and product sizes were as follows: Fz1
(GenBank accession no. NM_003505) forward (F), 5′-AATGACAAGTTCGCC
GAGGAC-3′ and reverse (R), 5′-GCCAGGTGAAAATACTGT GAGTTGG-3′ (59°C
for 30 cycles; 206 bp); Fz2 (NM_001466) F,
5′-CAAGGTGCCATCCTATCTCAGC-3′ and R, 5′-GTA GCAGCCCGACAGAAAAATG-3′
(59°C for 30 cycles; 247 bp); Fz3 (NM_017412) F,
5′-AGAGAAGAACTGTCATTT GCTCGC-3′ and R, 5′-TCCTTGTGTCACTGTGGAAGCC-3′
(53°C for 30 cycles; 255 bp); Fz4 (NM_012193) F, 5′-CAAGTG
ATTCTCCTGCCACAGC-3′ and R, 5′-CAACTCTCTCCA GTGTCCTCCATC-3′ (57°C
for 30 cycles; 270 bp); Fz5 (NM_003468) F, 5′-CCCTCATCCCCTAAGAGAGAC
AAAG-3′ and R, 5′-GCTGGCTGTGAAGAAGTTGCTG-3′ (55°C for 30 cycles;
230 bp); Fz6 (NM_003506) F, 5′-AGCAGC ATCCATCTCCAGACTCTC-3′ and R,
5′-CTGAATGACAAC CACCTCCCTG-3′, (57°C for 30 cycles, 251 bp); Fz7
(NM_003507) F, 5′-AGACTTAGCCACAGCAGCAAGG-3′ and R,
5′-CGCCGTTATCATCATCTTCCTG-3′ (58°C for 30 cycles; 287 bp); Fz8
(NM_031866) F, 5′-ATCCAAAGCAGA TGCCATTGTC-3′ and R,
5′-AACACTGTGAAGGGGTGG GAAC-3′ (59°C for 30 cycles; 137 bp); Fz9
(BC_026333) F, 5′-TCTTTGGAGAACCCCACACACC-3′ and R, 5′-TGC
TCACTTGCCTGACCTTGAC-3′ (60°C for 30 cycles; 148 bp); Fz10
(NM_007197) F, 5′-AAACGCTGGACTGCCTGATG-3′ and R,
5′-GCTTTTTTGTAAATCCCACCGC-3′ (58°C for 30 cycles; 217 bp); and
GAPDH (NM_002046) F, 5′-ACCTGA CCTGCCGTCTAGAA-3′ and R,
5′-TCCACCACCCTGTTG CTGTA-3′ (63°C for 30 cycles; 246 bp). PCR was
performed using Taq DNA polymerase (Life Technologies) and products
were subjected to analysis by gel electrophoresis in 2% agarose in
1X TAE (40 mM Tris-acetate/1 mM EDTA). Quantitative PCR was
performed using Fast SYBR Green Master Mix (Life Technologies) and
analyzed with the MiniOpticon Detection System (Bio-Rad, Hercules,
CA, USA). The primer pairs for quantitative PCR and the resultant
product sizes were as follows: Fz2 (NM_001466) F,
5′-TCCTCAAGGTGCCAT CCTATCTC-3′ and R, 5′-TGGTGACAGTGAAGAAGGTGG
AAG-3′ (183 bp); cyclin D1 (NM_053056) F, 5′-AGAGGCGGA
GGAGAACAAACAG-3′ and R, 5′-AGGCGGTAGTAGGAC AGGAAGTTG-3′ (180 bp);
and ribosomal protein L19 (RPL19; BC095445) F,
5′-CGAATGCCAGAGAAGGTCAC-3′ and R, 5′-CCATGAGAATCCGCTTGTTT-3′ (157
bp). Quantitative PCR was performed for 40 cycles of 5 sec for
denaturation and 5 sec for annealing/extension. GAPDH and RPL19
were used as internal controls.
Immunostaining and microscopy
Serial sections of human pancreatic cancer tissue
(62-year-old male with adenocarcinoma; BioChain Institute, Inc.,
Newark, CA, USA) were deparaffinized, autoclaved and incubated
first with hydrogen peroxide, followed by a 30-min incubation with
2% normal goat serum in phosphate-buffered saline (PBS; washing
buffer). Following overnight incubation with a rabbit polyclonal
anti-Fz2 antibody (1:5,000; Sigma-Aldrich), the specimens were
rinsed with PBS and subsequently incubated with horseradish
peroxidase-labeled anti-rabbit antibody (1:500; GE Healthcare,
Amersham, UK) for 2 h. Next, diaminobenzidine (Dako, Carpinteria,
CA, USA) was applied to the tissue sections as a chromogen, and the
nuclei were stained with hematoxylin (Muto Pure Chemicals Co.,
Ltd., Tokyo, Japan) for 15 sec. The specimens were observed and
images were captured under an AX80 microscope (Olympus Corp.,
Tokyo, Japan).
Cell proliferation analysis
The MIA-Paca2 cells were trypsinized, harvested and
spread onto 96-well flat-bottom plates (Asahi Techno Glass) at a
density of 1,000 cells per well. Next, the cells were incubated for
24 h in DMEM supplemented with 10% FBS. Following culture, the
cells were transfected with the short interference RNA (siRNA) of
Fz2 (siRNA-Fz2) or the short hairpin RNA (shRNA) of Fz2 (shRNA-Fz2)
for 72 h. Cell cultures were then used in
3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium
inner salt (MTS) assays, according to the manufacturer’s
instructions (Promega Corporation, Madison, MI, USA). MTS is
bio-reduced by cells into a colored formazan product that reduces
absorbance at 490 nm. Absorbance was analyzed at a wavelength of
490 nm with an iMark Microplate Absorbance Reader (Bio-Rad).
siRNA and shRNA transfection
Transfection of the cells with siRNA-Fz2 (Life
Technologies) was carried out using Lipofectamine 2000 and Opti-MEM
(both Life Technologies), according to the manufacturer’s
instructions. Briefly, siRNA and Lipofectamine 2000 were separately
diluted in Opti-MEM at room temperature for 5 min. The diluted
siRNA and Lipofectamine 2000 were then incubated together for an
additional 20 min at room temperature to facilitate complex
formation. Next, culture medium was aspirated from dishes or wells
containing cells and the complexes were added to cultured cells.
shRNA-Fz2 (OriGene Technologies Inc., Rockville, MD, USA) was
transfected into cells using Lipofectamine LTX (Life Technologies),
according to the manufacturer’s instructions. Briefly, shRNA was
incubated with PLUS reagent for 5 min, following which, LTX reagent
was added. A 30-min incubation at room temperature ensued and the
complex was subsequently applied to the cell culture medium.
Statistical analysis
Cell proliferation and quantitative PCR data were
analyzed by a one-way analysis of variance. The statistical
analysis was performed using JMP 5.0 software (SAS Institute Inc.,
Cary, NC, USA) and P<0.05 was considered to indicate a
statistically significant difference.
Results
Fz gene expression levels in pancreatic
tissue
The expression levels of the Fz genes were analyzed
by RT-PCR in the normal pancreatic tissue and the pancreatic cancer
cell lines (PANC-1, MIA-Paca2, NOR-P1, PK-45H, PK-1, PK-59 and KP4;
Fig. 1A). Fz1, Fz3, Fz7 and Fz10
were expressed not only in the pancreatic cancer cells, but also in
the normal pancreatic tissues, while Fz6 was not expressed in
either of the two cell types. Fz4 was only weakly expressed in the
pancreatic cancer cell lines. In addition, Fz5 and Fz9 were not
expressed in the normal pancreatic tissues, but were expressed in 3
and 1 of the pancreatic cancer cell lines, respectively. Fz8 was
expressed only in the normal pancreatic tissues, while Fz2 was
weakly expressed in the normal pancreatic tissues and expressed in
all other pancreatic cancer cell lines examined, with the exception
of NOR-P1. Relative to its expression in MIA-Paca2, the expression
levels of Fz2 in the normal pancreatic tissue was 4.1±1.8%
(Fig. 1B). The expression of Fz2
was 1.3±0.7% of NOR-P1. These expression results were consistent
with the RT-PCR results, and Fz2 was consequently used for further
analysis.
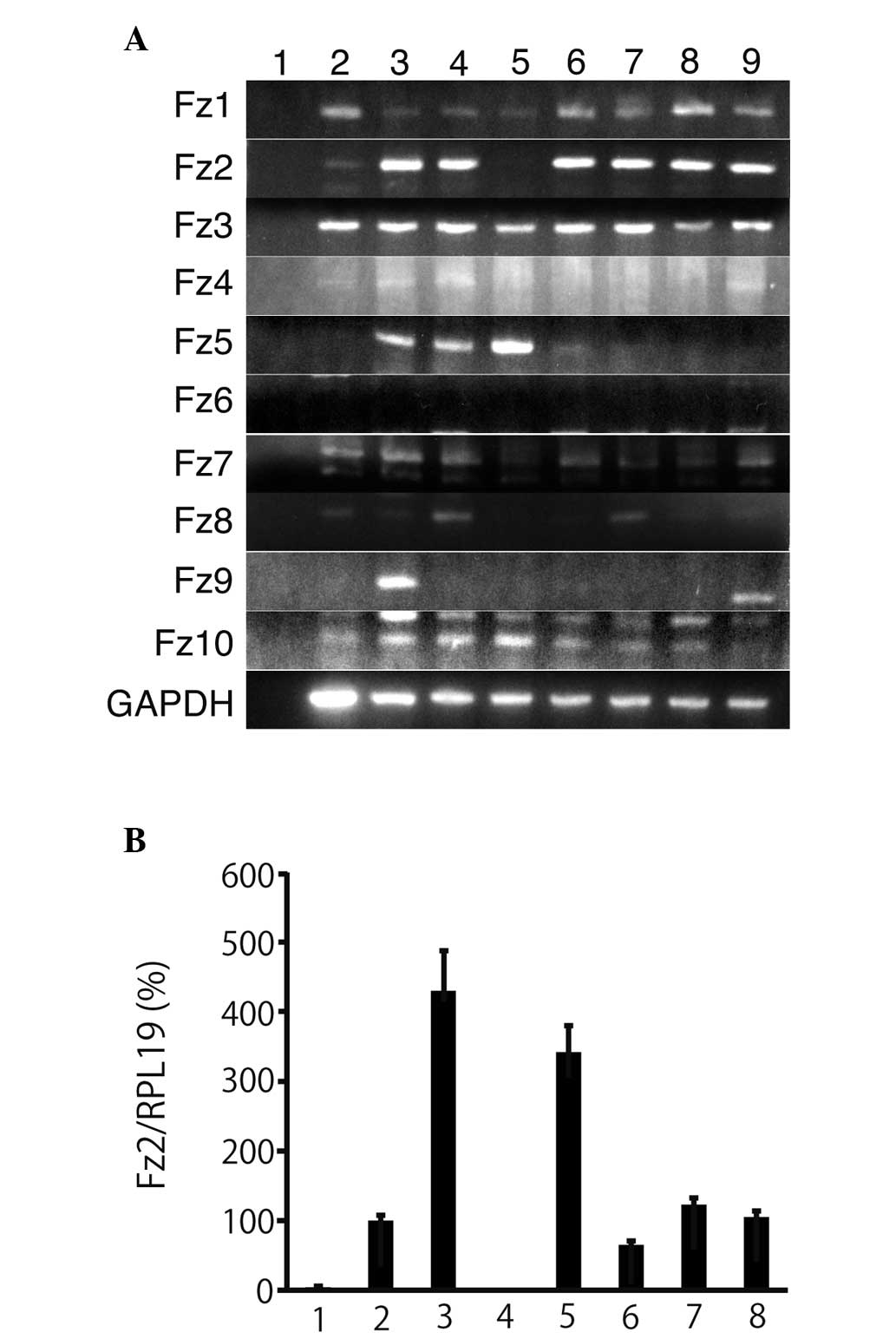 | Figure 1Expression of Fz genes in pancreatic
cancer cell lines. (A) Expression of Fz genes was analyzed in
pancreatic cancer cell lines by RT-PCR. Lane 1, H2O; 2,
normal pancreatic tissue; 3, PANC-1; 4, MIA-Paca2; 5, NOR-P1; 6,
PK-45H; 7, PK-59; 8, PK-1; and 9, KP4. Fz2/RLP19 was calculated as
the expression level of Fz2. All experiments were performed in
triplicate. (B) Expression levels of Fz2 were analyzed in
pancreatic cancer cell lines by quantitative PCR. Lane 1, normal
pancreas; 2, MIA-Paca2; 3, PANC-1; 4, NOR-P1; 5, PK-45H; 6, PK-59;
7, PK-1; and 8, KP4. Fz, frizzled; RT-PCR, reverse transcription
polymerase chain reation; RPL19, ribosomal protein L19. |
Immunostaining
Immunostaining was performed to determine the
expression of Fz2 at the protein level in the normal pancreatic and
pancreatic cancer tissues (Fig. 2).
Fz2 was not expressed in the glands or normal pancreatic duct
tissues (Fig. 2A). By contrast, Fz2
was expressed in the pancreatic cancer cells (Fig. 2B).
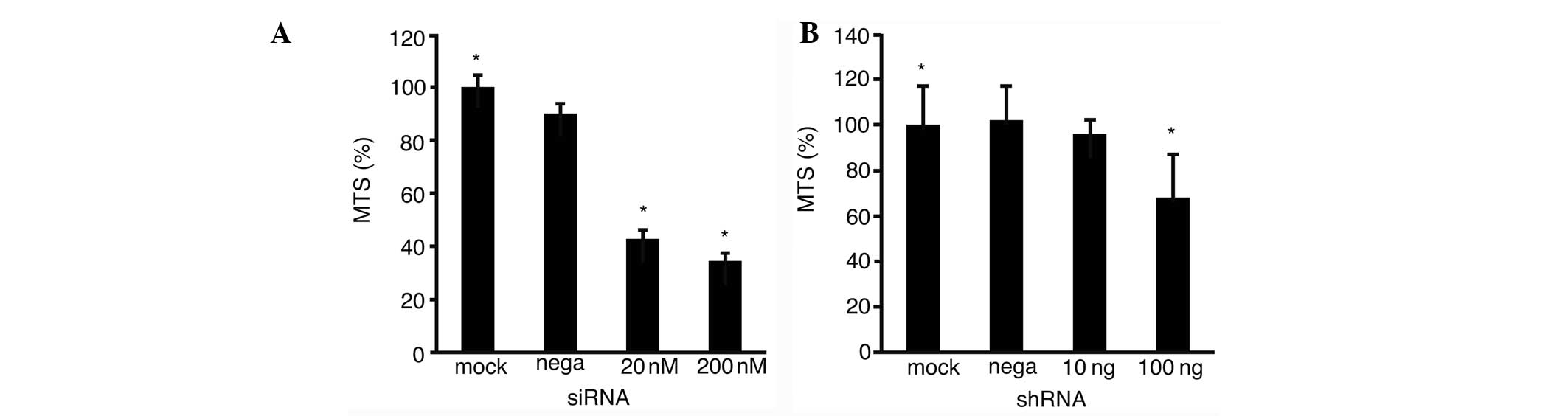
Inhibition of Fz2 expression
These results prompted the study of the suppression
of pancreatic cancer cell proliferation following the inhibition of
Fz2 expression. MIA-Paca2 cells were cultured with siRNA- or
shRNA-Fz2 and subjected to MTS assays. Relative to the mock
transfection, the proliferation of the MIA-Paca2 cells was
suppressed to 34.5±2.8% (P<0.05; Fig. 3A) following transfection with 200 nM
siRNA-Fz2, and to 68.0±19.0% (P<0.05) following transfection
with 100 ng/well shRNA-Fz2 (Fig.
3B). The expression levels of Fz2 were analyzed by quantitative
PCR to confirm suppression with siRNA- or shRNA-Fz2 (Fig. 4). Fz2 expression was suppressed to
12.9±0.9% (P<0.05) following transfection with 200 nM siRNA-Fz2
(Fig. 4A), and to 42.0±4.1%
(P<0.05) following transfection with 2.5 μg/well shRNA-Fz2
(Fig. 4C).
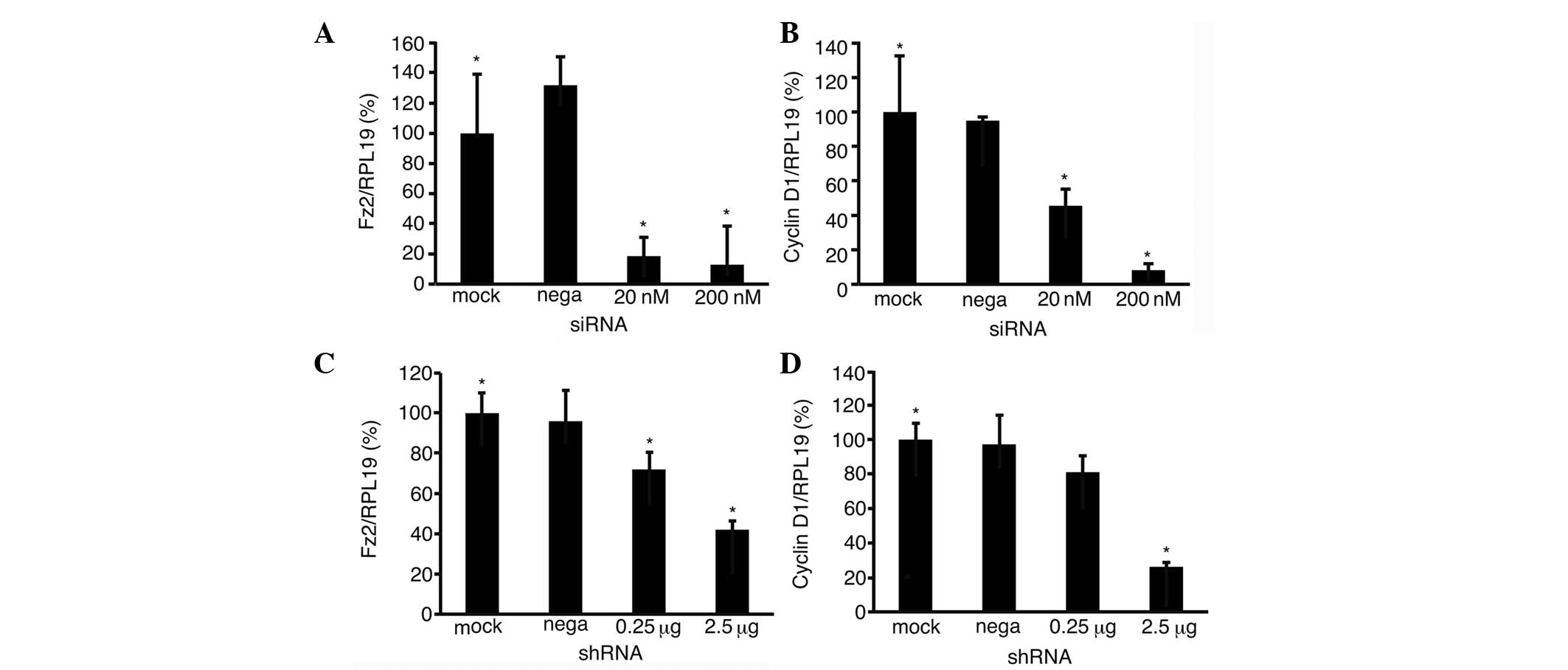 | Figure 4Quantitative PCR. Expression levels of
Fz2 and cyclin D1 were analyzed in MIA-Paca2 cells transfected with
(A and B) the siRNA or (C and D) shRNA of Fz2. The groups were as
follows: Mock, mock transfected; nega, transfected with negative
control; 20 nM, transfected with 20 nM siRNA; 200 nM, transfected
with 200 nM siRNA; 0.25 μg, transfected with 0.25 μg shRNA; and 2.5
μg, transfected with 25 μg shRNA. *P<0.05 (one-way
analysis of variance). Experiments were performed in triplicate.
PCR, polymerase chain reaction; Fz2, frizzled-2; siRNA, small
interfering RNA; shRNA, short hairpin RNA; RPL19, ribosomal protein
L19. |
Cyclin D1 expression
Finally, the expression of cyclin D1 was analyzed to
investigate the mechanism of the observed suppression of cell
proliferation in the MIA-Paca2 cells. Fz2 expression levels were
suppressed to 8.2±3.7% (P<0.05) following transfection with 200
nM siRNA-Fz2 (Fig. 4B), and to
26.2±2.6% (P<0.05) following transfection with 2.5 μg/well
shRNA-Fz2 (Fig. 4D).
Discussion
Sagara et al(1998) reported that Fz2 was not
expressed in the normal pancreas (15). Although the RT-PCR results of the
current study revealed a weak band of Fz2 expression in the normal
pancreatic tissue, the expression was significantly low. The
discrepancy between the RT-PCR and quantitative PCR results may be
attributed to the saturation of PCR products in the former method.
The results of the quantitative PCR were consistent with that of
previous studies. In pancreatic cancer patients, Fz2 is upregulated
in 8 cases out of 15 (16). In the
present study, relative to normal pancreatic tissues, Fz2 was
overexpressed in 6 out of the 7 pancreatic cancer cell lines. The
results of the quantitative PCR indicate, therefore, that Fz2 is
upregulated in pancreatic cancer cell lines more often than in
pancreatic cancer tissues. We hypothesize that the upregulation of
Fz2 is necessary for cells to proliferate in vitro. Notably,
β-catenin protein levels have been shown to be upregulated in
patients whose cancerous pancreatic tissue showed increased
expression levels of Fz2 (16),
even though the gene encoding β-catenin was only mutated in 13.3%
(2/15) of the patients (16). Using
a global genomic analysis, a previous study found that 12 pathways
were altered in 24 pancreatic cancer patients, to varying degrees
(17). Alteration of the Wnt
pathway is not specific to pancreatic cancer, but also occurs in
colorectal cancer and hepatocellular carcinoma (18). Fz2 was has been found to be
upregulated in 61.5% (8/13) of pancreatic cancer patients in the
absence of the β-catenin mutation (16). APC is not mutated and β-catenin is
not upregulated in MIA-Paca2 cells, a representative cell line of
pancreatic cancer (19). In the
present study, cellular transfection with siRNA- and shRNA-Fz2
suppressed the proliferation of the MIA-Paca2 cells, a result that
we now expect to obtain in patient pancreatic cancer tissues
containing normal APC and β-catenin.
Cyclin D1 is upregulated in pancreatic cancer
tissues compared with surrounding normal tissues (20). Moreover, antisense oligonucleotides
specific to cyclin D1 inhibit the growth of pancreatic cancer in
vivo(21). Together, these
results indicate that pancreatic cancer cell proliferation is
suppressed with the inhibition of cyclin D1 expression. In cells of
hepatocellular carcinoma, proliferation is suppressed with
siRNA-Fz9 and is associated with the decreased expression of cyclin
D1 (14). Similarly, in the present
study, pancreatic cancer cell proliferation and the expression of
cyclin D1 were suppressed following siRNA- and shRNA-Fz2 treatment,
the latter as revealed by quantitative PCR analysis. The results
are, therefore, consistent with previous observations. The main
disadvantages of using antisense RNA in cancer treatment are that
its half-life is short and its delivery is difficult (22). In contrast to cyclin D1, a
cytoplasmic protein, Fz2, is a receptor. A novel molecular therapy
for pancreatic cancer may, therefore, be developed using monoclonal
antibodies and small molecule receptor inhibitors specific to Fz2.
Future studies are likely to analyze the signaling pathway between
Fz2 and cyclin D1 in pancreatic cells and tissues.
References
|
1
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
2
|
Mao C, Domenico DR, Kim K, Hanson DJ and
Howard JM: Observations on the developmental patterns and the
consequences of pancreatic exocrine adenocarcinoma. Findings of 154
autopsies. Arch Surg. 130:125–134. 1995. View Article : Google Scholar
|
|
3
|
Vogel I, Krüger U, Marxsen J, et al:
Disseminated tumor cells in pancreatic cancer patients detected by
immunocytology: a new prognostic factor. Clin Cancer Res.
5:593–599. 1999.
|
|
4
|
Winter JM, Ting AH, Vilardell F, et al:
Absence of E-cadherin expression distinguishes noncohesive from
cohesive pancreatic cancer. Clin Cancer Res. 14:412–418. 2008.
View Article : Google Scholar
|
|
5
|
Iovanna J, Mallmann MC, Gonçalves A,
Turrini O and Dagorn JC: Current knowledge on pancreatic cancer.
Front Oncol. 2:62012. View Article : Google Scholar
|
|
6
|
Asuthkar S, Rao JS and Gondi CS: Drugs in
preclinical and early-stage clinical development for pancreatic
cancer. Expert Opin Investig Drugs. 21:143–152. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Insulin-like growth factor-I
receptor in proliferation and motility of pancreatic cancer. World
J Gastroenterol. 16:1854–1858. 2010. View Article : Google Scholar
|
|
8
|
Tomizawa M, Shinozaki F, Sugiyama T,
Yamamoto S, Sueishi M and Yoshida T: Insulin-like growth factor I
receptor involvement in proliferation of NOR-P1 cells in serum-free
media. J Cell Biochem. 113:2714–2720. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rajpathak SN, Gunter MJ, Wylie-Rosett J,
et al: The role of insulin-like growth factor-I and its binding
proteins in glucose homeostasis and type 2 diabetes. Diabetes Metab
Res Rev. 25:3–12. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Katoh M and Katoh M: WNT signaling pathway
and stem cell signaling network. Clin Cancer Res. 13:4042–4045.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen HJ, Hsu LS, Shia YT, Lin MW and Lin
CM: The β-catenin/TCF complex as a novel target of resveratrol in
the Wnt/β-catenin signaling pathway. Biochem Pharmacol.
84:1143–1153. 2012.
|
|
12
|
Wang HY, Liu T and Malbon CC:
Structure-function analysis of Frizzleds. Cell Signal. 18:934–941.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kirikoshi H, Sekihara H and Katoh M:
Expression profiles of 10 members of Frizzled gene family in human
gastric cancer. Int J Oncol. 19:767–771. 2001.PubMed/NCBI
|
|
14
|
Fujimoto T, Tomizawa M and Yokosuka O:
SiRNA of frizzled-9 suppresses proliferation and motility of
hepatoma cells. Int J Oncol. 35:861–866. 2009.PubMed/NCBI
|
|
15
|
Sagara N, Toda G, Hirai M, Terada M and
Katoh M: Molecular cloning, differential expression, and
chromosomal localization of human frizzled-1, frizzled-2, and
frizzled-7. Biochem Biophys Res Commun. 252:117–122. 1998.
View Article : Google Scholar
|
|
16
|
Zeng G, Germinaro M, Micsenyi A, et al:
Aberrant Wnt/beta-catenin signaling in pancreatic adenocarcinoma.
Neoplasia. 8:279–289. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Jones S, Zhang X, Parsons DW, et al: Core
signaling pathways in human pancreatic cancers revealed by global
genomic analyses. Science. 321:1801–1806. 2008. View Article : Google Scholar
|
|
18
|
White BD, Chien AJ and Dawson DW:
Dysregulation of Wnt/β-catenin signaling in gastrointestinal
cancers. Gastroenterology. 142:219–232. 2012.
|
|
19
|
Pujal J, Capellá G and Real FX: The Wnt
pathway is active in a small subset of pancreas cancer cell lines.
Biochim Biophys Acta. 1762:73–79. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Luo Y, Qiu Z, Tian L, et al:
Identification of novel predictive markers for the prognosis of
pancreatic ductal adenocarcinoma. Hum Pathol. 44:69–76. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wang JC, Thiere M, Henne-Bruns D,
Knippschild U and Kornmann M: Inhibition of pancreatic cancer cell
growth in vivo using a tetracycline-inducible cyclin D1 antisense
expression system. Pancreas. 42:141–148. 2012. View Article : Google Scholar
|
|
22
|
Heidegger I, Pircher A, Klocker H and
Massoner P: Targeting the insulin-like growth factor network in
cancer therapy. Cancer Biol Ther. 11:701–707. 2011. View Article : Google Scholar : PubMed/NCBI
|