Introduction
Bone marrow involvement by malignant lymphoma is
generally considered as a systemic dissemination of the disease
arising elsewhere, such as the lymph nodes and extranodal organs.
However, albeit extremely rare, malignant lymphomas exclusively
involving the bone marrow have been previously reported (1). Recently, the diagnostic criteria for
primary bone marrow lymphoma (PBML) have been proposed and the
clinicopathological features of this extremely rare tumor have also
been documented (1).
Cold agglutinin disease (CAD) accounts for 13–15% of
patients with autoimmune hemolytic anemia and is characterized by
the presence of cold agglutinins, which are antibodies that
agglutinate erythrocytes at an optimum temperature of 0–4°C
(2,3). Traditionally, CAD has been classified
into a primary type, not associated with malignant lymphoma or
other diseases and a secondary type, accompanying infection (such
as Epstein-Barr virus and Mycoplasma pneumoniae) and
malignant disease, most often malignant lymphoma. However, it has
been previously recognized that even the primary form is frequently
associated with an underlying lymphoproliferative disease in the
bone marrow (3). Although 76% of
CAD patients have been found to exhibit underlying malignant B-cell
lymphoma in the bone marrow (2,3), the
incidence of CAD in patients with non-Hodgkin lymphoma is low
(4). Previously, Varoczy et
al reported that of 421 non-Hodgkin lymphoma patients, 7.6%
exhibited an autoimmune disease and only one patient exhibited
autoimmune hemolytic anemia (5). To
the best of our knowledge, only one case of primary bone marrow
diffuse large B cell lymphoma (DLBCL) presenting with CAD has been
previously reported (6). The
present report describes the second case of primary bone marrow
DLBCL accompanying CAD. Written informed consent was obtained from
the patient.
Case report
Case presentation
A 76-year-old male with a past history of traumatic
epilepsy presented to Shiga University of Medical Science Hospital
(Otsu, Japan) with fever and fatigue. Physical examination revealed
jaundice in the patient’s bulbar conjunctiva and no superficial
lymph nodes were palpable. The patient did not describe symptoms of
acrocyanosis. Laboratory tests demonstrated marked anemia and
elevation of total bilirubin and lactate dehydrogenase (hemoglobin,
7.4 g/dl; mean cell volume, 90 fl; white blood cell count,
5.3×109/l; lymphocytes, 2.3×109/l; lactate
dehydrogenase, 558 IU/l; bilirubin, 131.7 μmol/l; and soluble
interleukin-2 receptor, 1,220 U/ml). Systemic surveillance by
imaging studies failed to detect any tumorous lesions,
lymphadenopathy or hepatosplenomegaly.
The direct antiglobulin test was positive, with
anti-C3d specificity. Anti-IgG was negative and indirect
antiglobulin test was also positive. Cold agglutinins were present
with a titer of 8,192 at 4°C and <1 at 37°C. Bone marrow
aspiration and biopsy were performed.
Subsequently, R-THP-COP (rituximab, 375
mg/m2; pirarubicin, 40 mg/m2; vincristine,
0.8 mg/m2; cyclophosphamide, 650 mg/m2; and
prednisolone, 40 mg/m2) therapy was performed. Following
six cycles of R-THP-COP therapy, the cold agglutinin titer was
markedly decreased (by <4), and bilirubin (15.6 μmol/l) and
lactate dehydrogenase (186 IU/l) levels were also reduced. Bone
marrow aspiration revealed no neoplastic lymphocytes. Following 19
months of the initial chemotherapy, malignant lymphoma relapsed.
Cold agglutinins were present again with a titer of 4,096 at 4°C.
The patient ultimately succumbed to the disease.
Immunohistochemistry
The formalin-fixed, paraffin-embedded tissue blocks
were sectioned (3-μm-thick), deparaffinized and rehydrated. Each
section was stained with hematoxylin and eosin and used for
immunostaining. Immunohistochemical analyses were performed using
an autostainer (Benchmark XT system; Ventana Medical Systems, Inc.,
Tucson, AZ, USA) according to the manufacturer’s instructions. The
following primary antibodies were used: Mouse monoclonal antibodies
against bcl-2 (bcl-2/100/D5), bcl-6 (P1F6), CD3 (PS1), CD5 (4C7),
CD10 (56C6), CD20 (L26) (all Novocastra Laboratories, Ltd.,
Newcastle upon Tyne, UK), CD138 (B-A38; Cell Marque Corp., Rocklin,
CA, USA) and MUM-1 (MUM1p; DakoCytomation, Glostrup, Denmark), as
well as rabbit monoclonal antibody against cyclin D1 (SP4; Nichirei
Biosciences Inc., Tokyo, Japan).
Histopathological findings
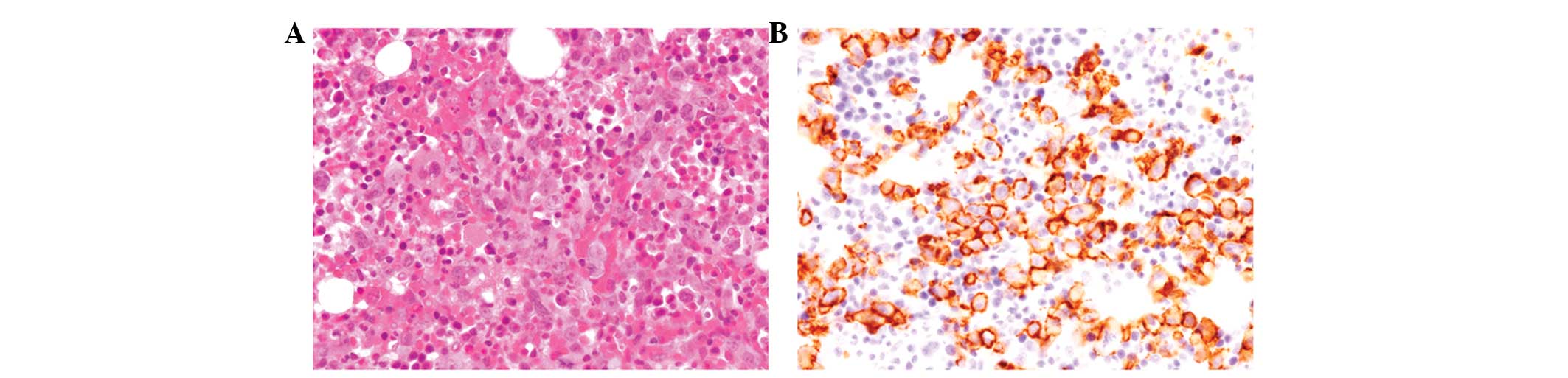
Bone marrow aspiration and biopsy revealed
hypercellularity with proliferation of large-sized lymphoid cells
containing irregular-shaped large nuclei with conspicuous nucleoli
(Fig. 1A). No bone trabeculae
destruction was noted and no lymphoma cells were detected in the
peripheral blood.
Immunohistochemical findings
Lymphoid cells in the bone marrow were positive for
CD20, bcl-2, bcl-6 and MUM1 (Fig.
1B), but negative for CD3, CD5, CD10, CD138 and cyclin D1.
According to these results, an ultimate diagnosis of primary bone
marrow DLBCL accompanying CAD was concluded.
Discussion
The present report describes the second documented
case of primary bone marrow DLBCL accompanying CAD. Recently, the
diagnostic criteria of PBML have been proposed, as follows: i)
isolated bone marrow infiltration of lymphoma cells regardless of
peripheral blood involvement; ii) no evidence of lymph node,
spleen, liver, or other extra bone marrow involvement on physical
examination or imaging studies; iii) absence of localized bone
tumors; iv) no evidence of bone trabeculae destruction in the bone
marrow biopsy; and v) exclusion of leukemia/lymphoma cases
(1). The present case corresponded
to the abovementioned criteria of PBML. Previously, Martinez et
al analyzed the clinicopathological features of 21 cases of
PBML (1). In total, 15 cases were
DLBCL and four were follicular lymphoma. The remaining two cases
were peripheral T-cell lymphoma, not otherwise specified. Notably,
peripheral blood involvement of lymphoma cells was observed in only
one DLBCL case, whereas three of the four follicular lymphoma cases
presented peripheral blood involvement (1). No cases of PBML accompanying CAD were
identified in the study (1).
Níáinle et al reported the first documented case of primary
bone marrow DLBCL with secondary CAD in a 69-year-old male
(6). The patient presented with
severe lethargy, and laboratory tests revealed anemia and elevated
lactate dehydrogenase and bilirubin. Cold agglutinins were present
with a titer of 128 at 4°C and bone marrow biopsy demonstrated
DLBCL. The patient was administered R-CHOP therapy, resulting in
remission of hemolytic anemia and malignant lymphoma (6). The clinical course of the present case
was similar to that of the patient previously reported by Níáinle
et al(6). Cold agglutinin
titer was decreased following chemotherapy in the two cases and
increased again at relapse in the present case. These results
indicate that lymphoma cells of primary bone marrow DLBCL produce
cold agglutinins.
Cold agglutinins in CAD are usually specific for the
I antigen, an erythrocyte surface carbohydrate macromolecule.
Cooling allows high-thermal amplitude cold agglutinins to bind to
the antigen in the peripheral circulation, resulting in the
agglutination of erythrocytes and impaired microcirculation. The
antigen-antibody complex activates the classical complement
pathway, leading to extravascular hemolysis, occurring mainly in
the liver (3). The autoantibody in
the majority of cases of CAD is IgM-κ (3). Moreover, it has now been recognized
that even primary CAD is frequently associated with an underlying
lymphoproliferative disease in the bone marrow (3). Previously, Berentsen reported that 76%
of primary CAD cases exhibited non-Hodgkin lymphoma in the bone
marrow and that the most common type of lymphoma is
lymphoplasmacytic lymphoma, followed by marginal zone lymphoma
(3). DLBCL accompanying CAD has
been extremely rarely reported (4,7).
Although, cases of primary pulmonary DLBCL associated with
autoimmune hemolytic anemia, primary bone marrow DLBCL complicated
with autoimmune hemolytic anemia and erythroid hypoplasia and DLBCL
involving the adrenal gland and kidney associated with CAD have
also been previously documented (7–9).
Primary CAD has now been recognized as a spectrum of clonal
lymphoproliferative bone marrow disorder, including DLBCL, as
observed in the present case. Therefore, the bone marrow of
patients with CAD must be examined for the detection of underlying
lymphoproliferative disorders.
Moreover, PBML is an extremely rare and aggressive
subtype of malignant lymphoma and <40 cases have been previously
reported in the literature (1,6,9,10).
In total, three cases of primary bone marrow DLBCL complicated with
hemolytic anemia, including the present case, have been documented
(6,9). The incidence of hemolytic anemia in
this extremely rare type of malignant lymphoma may be high as that
of conventional nodal and extranodal DLBCL. Therefore, additional
clinicopathological analyses are required to clarify the biological
and clinical features of this extremely rare type of tumor.
References
|
1
|
Martinez A, Ponzoni M, Agostinelli C, et
al: Primary bone marrow lymphoma: an uncommon extranodal
presentation of aggressive non-Hodgkin lymphomas. Am J Surg Pathol.
36:296–304. 2012. View Article : Google Scholar
|
|
2
|
Berentsen S: How I manage cold agglutinin
disease. Br J Haematol. 153:309–317. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ulvestad E, Berentsen S, Bo K and Shammas
FV: Clinical immunology of chronic cold agglutinin disease. Eur J
Haematol. 63:259–266. 1999. View Article : Google Scholar
|
|
4
|
Hauswirth A, Skrabs C, Schützinger C,
Gaiger A, Lechner K and Jäger U: Autoimmune hemolytic anemias,
Evans’ syndromes, and pure red cell aplasia in non-Hodgkin
lymphomas. Leuk Lymph. 48:1139–1149. 2007.
|
|
5
|
Váróczy L, Gergely L, Zeher M, Szegedi G
and Illés A: Malignant lymphoma-associated autoimmune diseases-a
descriptive epidemiological study. Rheumatol Int. 22:233–237.
2002.
|
|
6
|
Níáinle F, Hamnvik OP, Gulmann C, et al:
Diffuse large B-cell lymphoma with isolated bone marrow involvement
presenting with secondary cold agglutinin disease. Int J Lab
Hematol. 30:444–445. 2008.PubMed/NCBI
|
|
7
|
Airaghi L, Greco I, Carrabba M, et al:
Unusual presentation of large B cell lymphoma: a case report and
review of the literature. Clin Lab Haem. 28:338–342. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kubota Y: A case of primary pulmonary
malignant lymphoma associated with autoimmune hemolytic anemia.
Nihon Kokyuki Gakkai Zasshi. 44:453–457. 2006.(In Japanese).
|
|
9
|
Sumi M, Ichikawa N, Shimizu I, Yotsumoto
M, Ueno M and Kobayashi H: Primary diffuse large B-cell lymphoma of
the bone marrow complicated with autoimmune hemolytic anemia and
erythroid hypoplasia. Rinsho Ketsueki. 48:571–575. 2007.(In
Japanese).
|
|
10
|
Alvares CL, Matutes E, Scully MA, et al:
Isolated bone marrow involvement in diffuse large B cell lymphoma:
a report of three cases with review of morphological,
immunophenotypic and cytogenetic findings. Leuk Lymphoma.
45:769–775. 2004. View Article : Google Scholar
|