Introduction
The efficacy of epidermal growth factor
receptor-tyrosine kinase inhibitor (EGFR-TKI) for treatment of
non-small cell lung carcinoma (NSCLC) patients with EGFR
activating mutations is well established. Several prospective
studies have observed that first-line EGFR-TKI treatment leads to
longer progression-free survival in NSCLC patients with such
EGFR mutations compared with platinum-based doublet
chemotherapy (1–4). Hence the presence of
EGFR-activating mutations can be used to determine whether
to administer EGFR-TKI to NSCLC patients. In addition, EGFR
mutations associated with primary resistance or acquired resistance
to EGFR-TKI have been identified (5). Therefore, the ability to detect both
types of EGFR mutations is important in making treatment
decisions for NSCLC patients. To date, there have been numerous
reports of EGFR mutations, including alterations associated
with drug sensitivity or drug resistance. EGFR-activating
mutations, specifically a deletion in exon 19 and the missense
mutation L858R in exon 21, have been reported most frequently,
accounting for >90% of mutations in EGFR(6,7). By
contrast, insertions in exon 20 and missense mutation T790M in exon
21 are described as primary resistance mutations for EGFR-TKI. Four
EGFR mutations have been associated with acquired resistance
to EGFR-TKI, L747S, D761Y, T790M and T854A (8,9), but
the mechanism for acquiring these mutations remains unclear. In the
present study, L747S was identified in three patients who had never
received EGFR-TKI therapy, indicating that the initial tumor
contained resistant clones carrying L747S in varying
proportions.
Case report
Method
Gene alterations in 77 lung carcinoma patients were
analyzed by collecting and studying curette lavage fluid at the
time of diagnosis. DNA was extracted from cells attached to the
curette and PCR was performed to amplify mutation hotspot regions
in the EGFR genes, as described previously (10). The PCR products were
direct-sequenced and the mutations were then confirmed by
resequencing using different primers. Approval for the study was
obtained in advance from the Ethics Committee for Genomic Research
at Showa University (Tokyo, Japan; approval number 113). The
patients provided written informed consent.
Results
Overall, 27% (21 of 77) were found with EGFR
mutations, including L747S detected in three patients.
Case 1
Table I shows the
patient characteristics and their mutation statuses. A 78-year-old
Japanese male with a current smoking history of 45 pack-years was
referred to Showa University Fujiguoka Hospital (Yokohama, Japan)
due to a screen-detected abnormal chest X-ray. As a result, the
patient was diagnosed with stage IB lung adenocarcinoma. A
computed-tomography (CT) scan of the chest at diagnosis revealed a
primary tumor, 15-mm in size, in the right upper lobe. The levels
of the tumor markers, carcinoembryonic antigen (CEA), CYFRA21-1 and
pro-gastrin-releasing peptide (Pro-GRP), were within normal limits.
The lobe was completely resected with a lymph node dissection. No
recurrence was observed during two years after surgery. The tumor
was found to have two EGFR mutations, G719S and L747S.
Neither mutation was detected in the genomic DNA extracted from the
normal tissue of the lung.
 | Table IClinical characteristics and mutation
status. |
Table I
Clinical characteristics and mutation
status.
| Characteristic | Case 1 | Case 2 | Case 3 |
|---|
| Age, years | 78 | 73 | 82 |
| Gender | M | M | M |
| Stage | IB | IV | IIIB |
| Cytological
diagnosis | Ad | Sq | SCLC |
| Tobacco,
pack-years | 45 | 80 | 30 |
| EGFR
mutation | G719S, L747S | L747S | L747S |
| Tumor marker |
| CEA, ng/ml | 1.7 | 1.9 | 10.5 |
| CYFRA21-1,
ng/ml | 2.0 | 2.4 | 1.4 |
| Pro-GRP, pg/ml | 25.3 | 27.2 | 468.1 |
Case 2
A 73-year-old Japanese male with a smoking history
of 80 pack-years was diagnosed with stage IV squamous cell lung
carcinoma and bone metastasis, found due to a chest X-ray
abnormality that was detected during a routine checkup. A CT scan
of the chest showed that the majority of the right upper lobe
consisted of a neoplasm, and that the tumor had infiltrated the
mediastinum. The levels of the tumor markers, CEA, CYFRA21-1 and
Pro-GRP, were within normal limits. The patient possessed the
EGFR mutation, L747S. Single-agent chemotherapy consisting
of 1000 mg/m2 gemcitabine was administered at on days 1,
8 and 15. The therapy was repeated every four weeks for two cycles;
however, the patient failed this therapy and succumbed within four
months of diagnosis. Neither normal tissue nor whole blood was
available for further analysis.
Case 3
An 82-year-old Japanese male with a smoking history
of 30 pack-years was hospitalized (Showa University Fujiguoka
Hospital) due to extreme fatigue. The patient’s serum sodium level
was 113 mEq/ml upon admission. The patient was diagnosed with
hyponatremia, due to the inappropriate secretion of antidiuretic
hormone, and with stage IIIB small cell lung carcinoma (SCLC). As
shown in Table I, the tumor
markers, CEA and Pro-GRP, were elevated with values of 10.5 and
468.1 pg/ml, respectively. The patient possessed the EGFR
mutation, L747S. The patient received carboplatin at a dose of AUC
5.0 every four weeks on day 1, and etoposide at a dose of 80
mg/m2 every four weeks on days 1, 2 and 3. A total of 16
cycles were administered in the last two years. L747S was not
detected in the genomic DNA from a whole blood sample.
Sequencing
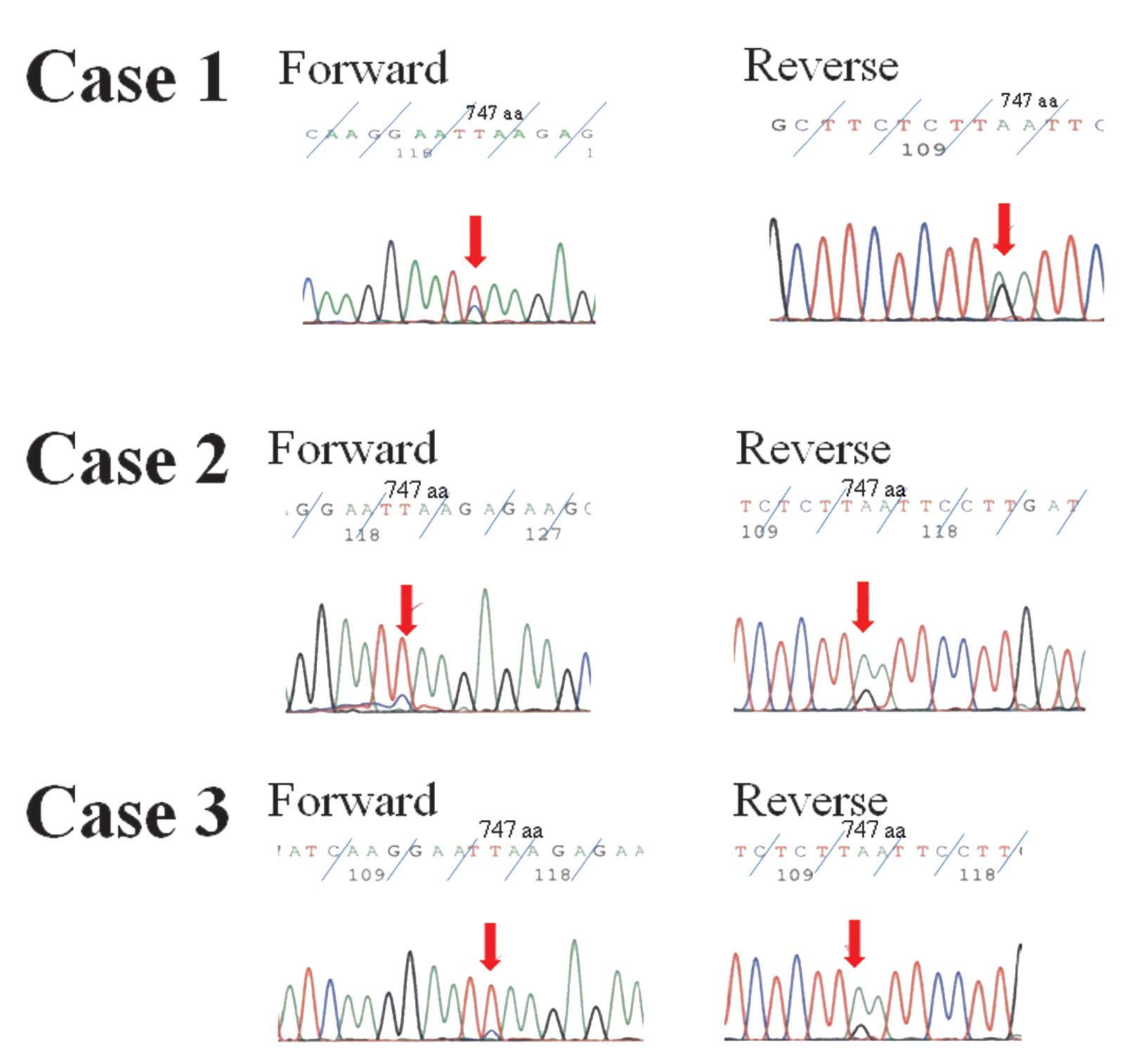
As shown in Fig. 1,
samples from each subject were direct-sequenced and the mutations
were then confirmed by sequencing using different forward and
reverse primers. In summary, the present study revealed that the
EGFR mutation, L747S, was detected in male smokers with
>30 pack-years each, and who were diagnosed with adenocarcinoma,
squamous cell carcinoma and SCLC. Among these cases, two of the
L747S mutations were confirmed to be somatic.
Discussion
The purpose of this study was to report cases of
EGFR-TKI-naïve patients carrying the EGFR mutation, L747S,
which is associated with acquired resistance to the drug. Previous
studies have revealed that acquired resistance mutations, including
L747S, are rare (8,9). There have also been few reports of
L747S detection in lung carcinoma patients who have not yet
received treatment (11,12). In the present study, 3.9% (3/77)
EGFR-TKI-naïve patients were identified with L747S, as reported
previously (10). Tumor cells
carrying L747S may have an advantage in carcinoma progression.
L747S was identified in two NSCLC patients and in one SCLC patient,
all of whom were male smokers. L747S was not associated with
staging. As summarized in Table I,
no patients showed overlap with a deletion in exon 19 or L858R in
exon 21, whereas one adenocarcinoma patient carried G719S in exon
18. No pathogenesis of an acquired resistance mutation was evident.
Two possible mechanisms of acquiring EGFR-TKI resistance have been
proposed. One hypothesis is that the initial tumor includes cells
carrying resistance mutations that exist prior to EGFR-TKI therapy.
The second hypothesis is that tumor cells acquire novel resistance
mutations during therapy. These two hypotheses assume that
resistant clones are selected through EGFR-TKI therapy. The present
study revealed that three smokers who had never received EGFR-TKI
therapy harbored the EGFR mutation, L747S. The initial tumor
in each case likely contained resistant clones carrying L747S in
varying proportions, consistent with the former hypothesis. This
mutation may also be associated with the primary resistance to
EGFR-TKI therapy. The presence of L747S in EGFR should
therefore be noted, particularly for patients with
EGFR-activating mutations, since dose escalation of EGFR-TKI
may overcome resistance due to the mutation (13,14).
Notably, L747S may be detected only by direct-sequencing, and not
by the Scorpion-amplification refractory mutation system or peptide
nucleic acid-locked nucleic acid PCR clamp methods commonly used in
clinical practice (15).
There have been several reports of EGFR
mutations in SCLC (16–18), thus the mutation status of
EGFR should be analyzed in NSCLC and SCLC. EGFR-TKI has been
shown to induce a partial response in SCLC patients carrying
EGFR-activating mutations, which are described as SCLC
combined adenocarcinoma components (19–21).
In case 3, the Pro-GRP and CEA levels were elevated simultaneously,
indicating that adenocarcinoma cells were included in the tumor.
Studies have revealed a histological transformation from NSCLC into
SCLC in combination with alterations of tumor markers in
EGFR mutant patients who acquired EGFR-TKI resistance
(22,23). The present study is the first report
showing an EGFR mutation associated with resistance to
EGFR-TKI therapy in an SCLC patient. This indicates that the
mutation status in SCLC requires further investigation, and that it
will be useful to detect EGFR-activating mutations or
resistance mutations of EGFR-TKI in SCLC and NSCLC patients.
The EGFR mutation, L747S, was detected in two
NSCLC patients and one SCLC patient, none of whom had ever received
EGFR-TKI therapy. In addition to clonal selection of resistant
cells in the initial tumor, cells carrying L747S may predominate
following EGFR-TKI therapy. L747S may be associated with primary
EGFR-TKI resistance. The presence of L747S in EGFR should
therefore be considered, particularly for patients with
EGFR-activating mutations. The early detection of EGFR-TKI
resistance mutations may be beneficial in making treatment
decisions for lung carcinoma patients. In the future, analyses of
EGFR mutations in lung carcinoma, including SCLC, should be
continued.
Acknowledgements
This study was supported by a grant from Eli Lilly
Japan K.K. The authors would like to thank NAI, Inc., (http://www.nai.co.jp/) for proofreading this
manuscript.
References
|
1
|
Maemondo M, Inoue A, Kobayashi K, Sugawara
S, Oizumi S, et al: Gefitinib or chemotherapy for non-small-cell
lung cancer with mutated EGFR. N Engl J Med. 362:2380–2388. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Mitsudomi T, Morita S, Yatabe Y, Negoro S,
Okamoto I, et al: Gefitinib versus cisplatin plus docetaxel in
patients with non-small-cell lung cancer harbouring mutations of
the epidermal growth factor receptor (WJTOG3405): an open label,
randomised phase 3 trial. Lancet Oncol. 11:121–128. 2010.
View Article : Google Scholar
|
|
3
|
Zhou C, Wu YL, Chen G, Feng J, Liu XQ, et
al: Erlotinib versus chemotherapy as first-line treatment for
patients with advanced EGFR mutation-positive non-small-cell lung
cancer (OPTIMAL, CTONG-0802): a multicentre, open-label,
randomised, phase 3 study. Lancet Oncol. 12:735–742. 2011.
View Article : Google Scholar
|
|
4
|
Rosell R, Carcereny E, Gervais R,
Vergnenegre A, Massuti B, et al: Erlotinib versus standard
chemotherapy as first-line treatment for European patients with
advanced EGFR mutation-positive non-small-cell lung cancer
(EURTAC): a multicentre, open-label, randomised phase 3 trial.
Lancet Oncol. 13:239–246. 2012. View Article : Google Scholar
|
|
5
|
Pao W and Girard N: New driver mutations
in non-small-cell lung cancer. Lancet Oncol. 12:175–180. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mitsudomi T and Yatabe Y: Mutations of the
epidermal growth factor receptor gene and related genes as
determinants of epidermal growth factor receptor tyrosine kinase
inhibitors sensitivity in lung cancer. Cancer Sci. 98:1817–1824.
2007. View Article : Google Scholar
|
|
7
|
Tanaka T, Matsuoka M, Sutani A, Gemma A,
Maemondo M, et al: Frequency of and variables associated with the
EGFR mutation and its subtypes. Int J Cancer. 126:651–655. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lin L and Bivona TG: Mechanisms of
resistance to epidermal growth factor receptor inhibitors and novel
therapeutic strategies to overcome resistance in NSCLC patients.
Chemother Res Pract. 2012:8172972012.
|
|
9
|
Suda K, Mizuuchi H, Maehara Y and
Mitsudomi T: Acquired resistance mechanisms to tyrosine kinase
inhibitors in lung cancer with activating epidermal growth factor
receptor mutation-diversity, ductility, and destiny. Cancer
Metastasis Rev. 31:807–814. 2012. View Article : Google Scholar
|
|
10
|
Yamaguchi F, Kugawa S, Tateno H, Kokubu F
and Fukuchi K: Analysis of EGFR, KRAS and P53 mutations in lung
cancer using cells in the curette lavage fluid obtained by
bronchoscopy. Lung Cancer. 78:201–206. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pallis AG, Voutsina A, Kalikaki A,
Souqlakos J, Briasoulis E, et al: ‘Classical’ but not ‘other’
mutations of EGFR kinase domain are associated with clinical
outcome in gefitinib-treated patients with non-small cell lung
cancer. Br J Cancer. 97:1560–1566. 2007.
|
|
12
|
Jia XL and Chen G: EGFR and KRAS mutations
in Chinese patients with adenosquamous carcinoma of the lung. Lung
Cancer. 74:396–400. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Costa DB, Schumer ST, Tenen DG and
Kobayashi S: Differential responses to erlotinib in epidermal
growth factor receptor (EGFR)-mutated lung cancers with acquired
resistance to gefitinib carrying the L747S or T790M secondary
mutations. J Clin Oncol. 26:1182–1184. 2008. View Article : Google Scholar
|
|
14
|
Costa DB, Nguyen KS, Cho BC, Sequist LV,
Jackman DM, et al: Effects of erlotinib in EGFR mutated non-small
cell lung cancers with resistance to gefitinib. Clin Cancer Res.
14:7060–7067. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Goto K, Satouchi M, Ishii G, Nishio K,
Hagiwara K, et al: An evaluation study of EGFR mutation tests
utilized for non-small-cell lung cancer in the diagnostic setting.
Ann Oncol. 23:2914–2919. 2012. View Article : Google Scholar
|
|
16
|
Fukui T, Tsuta K, Furuta K, Watanabe S,
Asamura H, et al: Epidermal growth factor receptor mutation status
and clinicopathological features of combined small cell carcinoma
with adenocarcinoma of the lung. Cancer Sci. 98:1714–1719. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morinaga R, Okamoto I, Furuta K, Kawano Y,
Sekijima M, et al: Sequential occurrence of non-small cell and
small cell lung cancer with the same EGFR mutation. Lung Cancer.
58:411–413. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Tatematsu A, Shimizu J, Murakami Y, Horio
Y, Nakamura S, et al: Epidermal growth factor receptor mutations in
small cell lung cancer. Clin Cancer Res. 14:6092–6096. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zakowski MF, Ladanyi M and Kris MG;
Memorial Sloan-Kettering Cancer Center Lung Cancer OncoGenome
Group. EGFR mutations in small-cell lung cancers in patients who
have never smoked. N Engl J Med. 355:213–215. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Okamoto I, Araki J, Suto R, Shimada M,
Nakagawa K and Fukuoka M: EGFR mutation in gefitinib-responsive
small-cell lung cancer. Ann Oncol. 17:1028–1029. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lu HY, Mao WM, Cheng QY, Chen B, Cai JF,
et al: Mutation status of epidermal growth factor receptor and
clinical features of patients with combined small cell lung cancer
who received surgical treatment. Oncol Lett. 3:1288–1292. 2012.
|
|
22
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, et al: Genotypic and histological evolution
of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl
Med. 3:75ra262011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Alam N, Gustafson KS, Ladanyi M, Zakowski
MF, Kapoor A, et al: Small-cell carcinoma with an epidermal growth
factor receptor mutation in a never-smoker with
gefitinib-responsive adenocarcinoma of the lung. Clin lung Cancer.
11:E1–E4. 2010. View Article : Google Scholar : PubMed/NCBI
|