Introduction
Capecitabine is a novel oral fluoropyrimidine
carbamate rationally designed to allow selective 5-fluorouracil
(5-FU) activation in tumor tissues (1). Capecitabine is metabolized to 5-FU
within tumors and produces certain side-effects characteristic to
5-FU. Although capecitabine is generally well tolerated, hand-foot
syndrome (HFS) is the most common clinical adverse reaction. HFS,
also called palmar-plantar erythrodysesthesia, is a distinctive and
relatively frequent dermatological toxic reaction associated with
certain chemotherapeutic agents, specifically capecitabine,
infusional fluorouracil and liposomal doxorubicin (2). The manifestations of HFS have been
classified into 3 grades according to their severity (3). HFS (of all grades) occurred in ~50% of
patients in the early phase II studies of capecitabine as a
single-agent therapy for metastatic breast and colorectal cancers,
with 10% of patients experiencing severe HFS (grade 3) (3).
The pharmacological mechanism of
capecitabine-associated HFS remains unclear. The elimination of the
eccrine gland system by capecitabine may result in HFS. Asgari
et al(4) previously
indicated that capecitabine affects the eccrine system due to
increased levels of thymidine phosphorylase in the skin
keratocytes, leading to capecitabine metabolite accumulation.
Furthermore, Mrozek-Orlowski et al(5) previously hypothesized that this
cytotoxic drug may be excreted in sweat, making the palms and soles
more prone to HFS. This is due to the large number of eccrine sweat
glands in these extremities, as areas that contain a high
concentration of apocrine sweat glands are affected.
Vascularization and increased pressure and temperature in the hands
and feet may perpetuate this effect (3).
Furthermore, previous studies have indicated that
heparin-binding epidermal growth factor-like growth factor (HB-EGF)
is also associated with chemotherapeutic resistance. Drug
resistance is one of the principal reasons for the failure of
chemotherapy. Chemotherapy also induces the elevated expression of
HB-EGF, and HB-EGF activation represents a critical mechanism for
the induction of chemotherapeutic resistance (6).
Topical retinoids are important therapeutic
anti-aging agents for the management of photodamaged skin.
Adapalene is a third-generation synthetic retinoid and a naphthoic
acid derivative that has specific pharmacological activities
similar to regular retinoids (7).
Adapalene also demonstrates a low potential for irritation and a
direct anti-inflammatory effect. Topical retinoids markedly
increase HB-EGF expression in human keratinocytes (8) and are considered to be important for
skin wound healing (9).
Therefore, the current report focused on the topical
retinoids that increase HB-EGF expression in the skin. We
hypothesized that topical retinoids induce local chemotherapeutic
resistance in the skin of patients receiving chemotherapy and
consequently, decrease the cutaneous side-effects of
chemotherapy.
Furthermore, it was considered that HFS may be
responsive to adapalene. This report presents a case of the
successful treatment of refractory HFS induced by capecitabine
using the topical application of adapalene. Written informed
consent was obtained from the patient.
Case report
A 75-year-old female was diagnosed with invasive
ductal carcinoma without metastasis (pT2N0M0). The patient was
treated with two courses of chemotherapy consisting of docetaxel
plus trastuzumab for one year, as the tumor cells were weakly
positive for estrogen receptors and positive for human epidermal
growth factor receptor type 2 (HER2). Following completion of the
chemotherapy, tamoxifen was administered. One year after the
initial surgery, axillary lymph node metastasis was detected and
the patient underwent axillary lymph node clearance surgery. The
patient was diagnosed with pleural metastasis ~5 months after the
second surgery, and oral capecitabine was initiated [capecitabine
(Xeloda) chemotherapy, at a daily dosage of 1,657 mg/m2
in divided doses]. Each cycle of therapy consisted of three weeks
of capecitabine administration, followed by a one-week resting
period. Pyridoxine (vitamin B6) was also administered for the
prophylaxis of HFS.
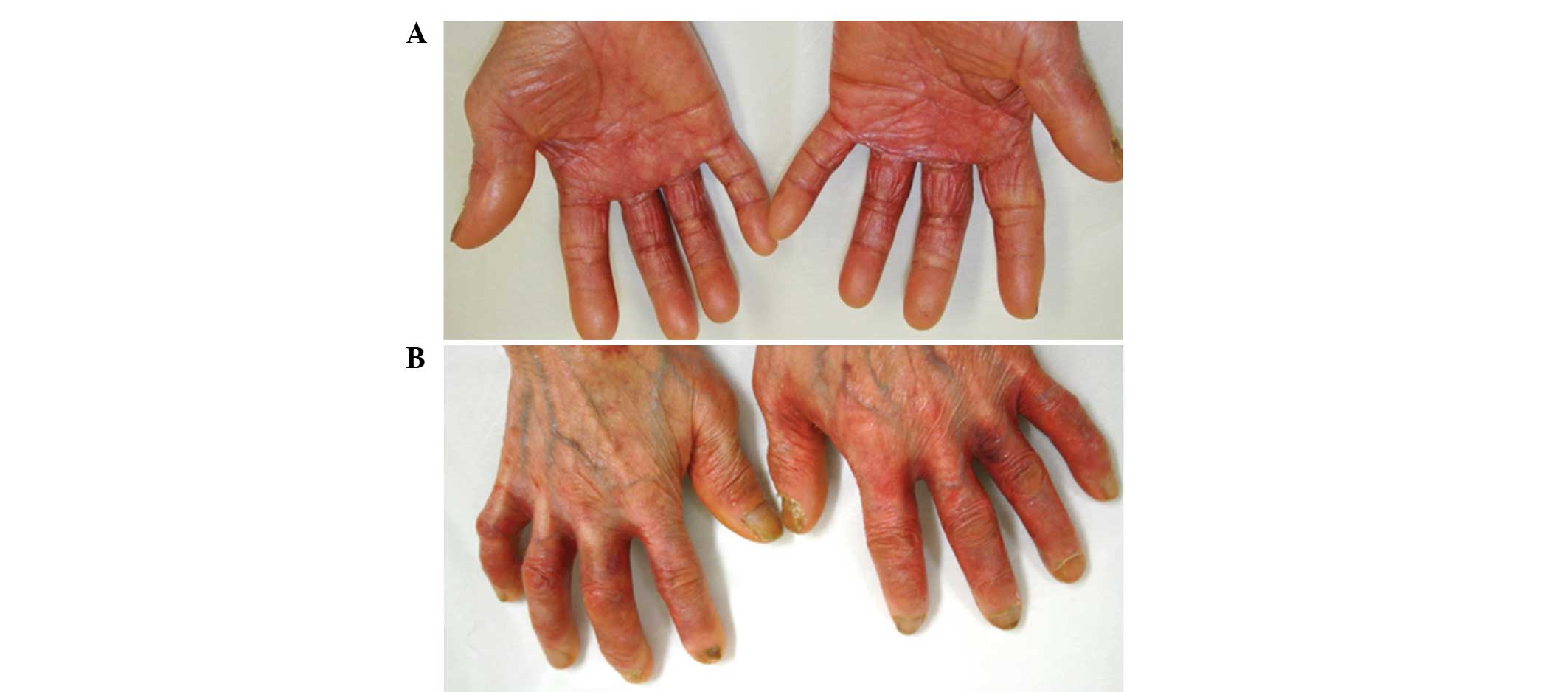
Treatment with capecitabine was effective, however,
following three courses, the patient complained of painful erythema
of the palms, fingers and soles of the feet. Furthermore, the
patient stated that the skin on the fingers and soles had peeled
off and that the underlying dermis felt extremely stiff and tight.
The patient was diagnosed with grade 3 HFS and was instructed to
apply clobetasol propionate (a superpotent steroid) to the hands
and feet following the cessation of capecitabine treatment. The
grade 3 HFS improved to grade 2, however, the areas remained
painful and the skin lesions continued to desquamate (Fig. 1). The patient was recommended to
discontinue the chemotherapy until they had recovered from HFS, but
the individual did not wish to discontinue capecitabine due to
cancer recurrence. Therefore, the application of 0.1% adapalene gel
was initiated to the affected areas on the hands twice daily. One
month later, the inflammation of the individual’s hands gradually
improved and the patient continued with the next course of
capecitabine. The application of adapalene was continued and a
marked reduction in HFS was observed, which resulted the reduction
of inflammation and pain to grade 0 within 3 months (Fig. 2). The topical steroid was
discontinued. The patient has remained in complete remission since
pleural recurrence 2 years previously.
Discussion
The current report presents a case with the
successful treatment of refractory HFS induced by capecitabine
using the topical application of adapalene. We hypothesized that
topical adapalene induced the local chemotherapeutic resistance of
the skin in the present patient receiving capecitabine,
consequently improving HFS.
There are various recommendations concerning topical
approaches or systemic therapy for HFS. However, the evidence of
their benefit is limited and controversial. With regard to systemic
strategies, in certain studies, pyridoxine (10,11)
has also been found to be beneficial as a therapy. In other
previous studies, however, pyridoxine therapy has been shown to
have no effect (12,13) and the precise mechanism of action
remains unknown. Cyclooxygenase-2 inhibitors have also been shown
to be effective as a systemic approach for the prophylaxis of
chemotherapy-associated HFS in patients with stage II and III
colorectal cancer (14). Local
therapy using mild emollient creams or gels in patients with grade
1 HFS may prevent mechanical irritation of the skin on the palms
and soles. Superpotent corticosteroids for blisters and erosions
have been found to have a sufficient anti-inflammatory effect. By
contrast, HFS may not be prevented using glucocorticoids (2). A sufficient effect has not been
previously demonstrated with dimethylsulfoxide or with a
urea/lactic-based topical keratolytic agent (15). Overall, the optimal therapy for the
prevention and treatment of HFS has not yet been established. In
the present case, topical clobetasol propionate and pyridoxine were
administered to the patient, but were also found to be ineffective.
The treatment for steroid-resistant HFS appears to be particularly
difficult.
Adapalene is a third-generation synthetic retinoid,
which contains a naphthoic acid backbone (7). In contrast to retinoic acid, adapalene
exhibits selectivity for the nuclear retinoic acid receptor (RAR
β/γ). Due to its receptor selectivity, it causes less skin
irritation. Topical retinoids have shown beneficial efficacy and
good safety profiles as therapeutic antiaging agents in the
management of photodamaged skin. Of all the topical retinoid
agents, only adapalene has been approved for acne treatment in
Japan. Topical retinoids have been found to show the following
clinical effects: i) Improvement of coarse wrinkling; ii) increased
skin smoothness and decreased roughness; and iii) improvements to
skin discoloration/dyschromia (16). One of the notable molecular
mechanisms of retinoid action is the increase in HB-EGF expression
in keratinocytes. HB-EGF is considered to be important for skin
wound healing, by accelerating keratinocyte migration (9).
HB-EGF is a member of the epidermal growth factor
family and is synthesized as a transmembrane protein that may
undergo proteolytic cleavage at the cell surface to release a
mature soluble 14–22-kDa N-terminal ectodomain (s-HB-EGF). This
s-HB-EGF subsequently binds to and activates its receptors, EGF
receptor (EGFR)/erbB1/HER1 or ErbB4/HER4. Following the binding of
s-HB-EGF, the heterodimerization or homodimerization of these
receptors drives signal transduction cascades, which have critical
roles in diverse cell fates, including development, proliferation,
differentiation and migration. EGFR activation leads to an
intracellular signaling cascade via Ras activation, which
stimulates the extracellular signal pathway-regulated
kinase/mitogen-activated protein kinase pathway. Suganuma et
al identified HB-EGF as a chemoresistance-related gene in
gastric cancer by cDNA microarray (17). It was observed that HB-EGF was
highly expressed in 5-FU and cisplatin-resistance groups.
Therefore, HB-EGF may also induce resistance to capecitabine, which
is metabolized to 5-FU. Chemotherapy induces an elevation in the
expression of HB-EGF, which is largely dependent on the activation
of chemoresistant genes, including activator protein-1 and nuclear
factor-κB, indicating that chemotherapy-induced HB-EGF activation
represents a critical mechanism for induced chemotherapeutic
resistance (6). Thus, HB-EGF has
emerged as a key molecule in the resistance to chemotherapeutic
agents. These observations demonstrate that HB-EGF is not only a
potent inducer of tumor growth, but also a predictor of response to
chemotherapy. HB-EGF contributes to tumor aggressiveness by
promoting invasion, metastasis and chemotherapeutic resistance.
Chemotherapy treatments increase HB-EGF levels, and
chemotherapy-induced HB-EGF activation may protect cells from
chemotherapy-induced cell death. Similarly, we hypothesized that
retinoid-induced HB-EGF may protect keratinocytes from
chemotherapy. In other words, topical retinoids that increase
HB-EGF expression are likely to induce local chemotherapeutic
resistance in the skin of patients receiving chemotherapy and
consequently, decrease the cutaneous side-effects of chemotherapy.
Topical adapalene, which has an anti-inflammatory effect and
increases HB-EGF in the keratinocyte, is predicted to be effective
for the treatment of HFS.
Rittié et al(8) reported that topical retinoid treatment
causes epidermal hyperplasia mediated by EGFR activation via
specific induction of its ligand, HB-EGF. Therefore, the topical
application of retinoids may be effective in controlling EGFR
inhibitor-induced skin reactions. Specific case reports have
indicated that acneiform eruption or periungual inflammation due to
EGFR inhibitors have been reduced by topical adapalene (18,19).
Lapatinib, a tyrosine kinase inhibitor of HER2 and EGFR, is
effective in combination with capecitabine in females with
HER2-positive metastatic breast cancer. According to a phase III
trial, in females with HER2-positive advanced breast cancer who
received lapatinib plus capecitabine or capecitabine alone, skin
rashes occurred more often in the group who received combination
therapy (20). A topical retinoid
may be more effective for skin reactions caused by lapatinib plus
capecitabine compared with capecitabine alone in patients with
metastatic breast cancer.
In the present case, the HFS of the patient improved
slowly over several months. We hypothesized that the topical
retinoid does not directly repair the damaged epidermis, but may
promote the skin cell turnover and result in the improvement of the
cutaneous adverse reactions. It normally takes 14 days for
post-mitotic epidermal cells to reach the stratum corneum (21) and therefore, the proliferation and
differentiation of keratinocytes by adapalene is likely to result
in the delayed appearance of skin improvement.
The most common and frequent adverse reaction of
topical retinoids is known as the ‘retinoid reaction’, which is
characterized by pruritus, a burning sensation at the application
sites, erythema and peeling, but these usually present with minimal
to mild intensity (22). In a
series of previous comparative trials, adapalene has been shown to
be significantly less irritating than various other retinoic acid
formulations. Furthermore, adapalene has been shown to be safe in
several previous studies (7). Due
to its chemical structure, the absorption of adapalene through the
human skin is low. However, avoidance of adapalene during early
pregnancy is firmly recommended. By contrast, in specific reviews
of EGFR inhibitor-induced skin reactions, the use of retinoids for
skin rash has not been generally recommended due to the lack of
comedones and the possible aggravation of xerosis and eczema
(12,23,24).
Adapalene is applied to facial acne, and as facial skin is more
sensitive than the skin of the hands or feet, the adapalene-related
skin problems of the hands or feet may be fewer than those of the
facial skin. In the present case, the application of adapalene to
the patient’s hands and feet did not produce any side-effects, such
as pruritus, erythema or xerosis.
In conclusion, topical retinoids may be effective
for the treatment of capecitabine-induced HFS, by increasing HB-EGF
expression and decreasing cutaneous side-effects. However, the
mechanism of action of this effect on HFS remains unclear. A pilot
trial designed to evaluate the potential efficacy and toxicities of
adapalene for preventing HFS is necessary. Such a trial is
currently underway at Kanazawa University to demonstrate clinical
efficacy. Further studies are required to establish the therapeutic
effect of topical retinoids on HFS.
Acknowledgements
The authors would like to thank the patient and the
patient’s family for their participation.
References
|
1
|
Shimma N, Umeda I, Arasaki M, et al: The
design and synthesis of a new tumor-selective fluoropyrimidine
carbamate, capecitabine. Bioorg Med Chem. 8:1697–1706. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Webster-Gandy JD, How C and Harrold K:
Palmar-plantar erythrodysesthesia (PPE): a literature review with
commentary on experience in a cancer centre. Eur J Oncol Nurs.
11:238–246. 2007. View Article : Google Scholar
|
|
3
|
Lassere Y and Hoff P: Management of
hand-foot syndrome in patients treated with capecitabine (Xeloda).
Eur J Oncol Nurs. 8(Suppl 1): S31–S40. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Asgari MM, Haggerty JG, McNiff JM,
Milstone LM and Schwartz PM: Expression and localization of
thymidine phosphorylase/platelet-derived endothelial cell growth
factor in skin and cutaneous tumors. J Cutan Pathol. 26:287–294.
1999. View Article : Google Scholar
|
|
5
|
Mrozek-Orlowski ME, Frye DK and Sanborn
HM: Capecitabine: nursing implications of a new oral
chemotherapeutic agent. Oncol Nurs Forum. 26:753–762.
1999.PubMed/NCBI
|
|
6
|
Wang F, Liu R, Lee SW, Sloss CM, Couget J
and Cusack JC: Heparin-binding EGF-like growth factor is an early
response gene to chemotherapy and contributes to chemotherapy
resistance. Oncogene. 26:2006–2016. 2007. View Article : Google Scholar
|
|
7
|
Piérard GE, Piérard-Franchimont C, Paquet
P and Quatresooz P: Spotlight on adapalene. Expert Opin Drug Metab
Toxicol. 5:1565–1575. 2009.
|
|
8
|
Rittié L, Varani J, Kang S, Voorhees JJ
and Fisher GJ: Retinoid-induced epidermal hyperplasia is mediated
by epidermal growth factor receptor activation via specific
induction of its ligands heparin-binding EGF and amphiregulin in
human skin in vivo. J Invest Dermatol. 126:732–739. 2006.
|
|
9
|
Shirakata Y, Kimura R, Nanba D, et al:
Heparin-binding EGF-like growth factor accelerates keratinocyte
migration and skin wound healing. J Cell Sci. 118:2363–2370. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fabian CJ, Molina R, Slavik M, Dahlberg S,
Giri S and Stephens R: Pyridoxine therapy for palmar-plantar
erythrodysesthesia associated with continuous 5-fluorouracil
infusion. Invest New Drugs. 8:57–63. 1990. View Article : Google Scholar
|
|
11
|
Yoshimoto N, Yamashita T, Fujita T, et al:
Impact of prophylactic pyridoxine on occurrence of hand-foot
syndrome in patients receiving capecitabine for advanced or
metastatic breast cancer. Breast Cancer. 17:298–302. 2010.
View Article : Google Scholar
|
|
12
|
Kang YK, Lee SY, Cheon YJ, et al:
Pyridoxine is not effective for the prevention of
capecitabine-induced hand foot syndrome (HFS): Results of a
randomized double-blind placebo-controlled study. Ann Oncol.
17:286–287. 2006.
|
|
13
|
Kang YK, Lee SS, Yoon DH, et al:
Pyridoxine is not effective to prevent hand-foot syndrome
associated with capecitabine therapy: results of a randomized,
double-blind, placebo-controlled study. J Clin Oncol. 28:3824–3829.
2010. View Article : Google Scholar
|
|
14
|
Zhang RX, Wu XJ, Wan DS, et al: Celecoxib
can prevent capecitabine-related hand-foot syndrome in stage II and
III colorectal cancer patients: result of a single-center,
prospective randomized phase III trial. Ann Oncol. 23:1348–1353.
2012. View Article : Google Scholar
|
|
15
|
Wolf SL, Qin R, Menon SP, et al; Cancer
Treatment Group Study N05C5. Placebo-controlled trial to determine
the effectiveness of a urea/lactic acid-based topical keratolytic
agent for prevention of capecitabine-induced hand-foot syndrome:
North Central Cancer Treatment Group Study N05C5. J Clin Oncol.
28:5182–5187. 2010. View Article : Google Scholar
|
|
16
|
Darlenski R, Surber C and Fluhr JW:
Topical retinoids in the management of photodamaged skin: from
theory to evidence-based practical approach. Br J Dermatol.
163:1157–1165. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Suganuma K, Kubota T, Saikawa Y, et al:
Possible chemoresistance-related genes for gastric cancer detected
by cDNA microarray. Cancer Sci. 94:355–359. 2003. View Article : Google Scholar
|
|
18
|
DeWitt CA, Siroy AE and Stone SP:
Acneiform eruptions associated with epidermal growth factor
receptor-targeted chemotherapy. J Am Acad Dermatol. 56:500–505.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hachisuka J, Doi K, Moroi Y and Furue M:
Successful treatment of epidermal growth factor receptor
inhibitor-induced periungual inflammation with adapalene. Case Rep
Dermatol. 3:130–136. 2011. View Article : Google Scholar
|
|
20
|
Geyer CE, Forster J, Lindquist D, et al:
Lapatinib plus capecitabine for HER2-positive advanced breast
cancer. N Engl J Med. 355:2733–2743. 2006. View Article : Google Scholar
|
|
21
|
Fuchs E and Raghavan S: Getting under the
skin of epidermal morphogenesis. Nat Rev Genet. 3:199–209. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mukherjee S, Date A, Patravale V, Korting
HC, Roeder A and Weindl G: Retinoids in the treatment of skin
aging: an overview of clinical efficacy and safety. Clin Interv
Aging. 1:327–348. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Segaert S and Van Cutsem E: Clinical
signs, pathophysiology and management of skin toxicity during
therapy with epidermal growth factor receptor inhibitors. Ann
Oncol. 16:1425–1433. 2005. View Article : Google Scholar
|
|
24
|
Lacouture ME: Mechanisms of cutaneous
toxicities to EGFR inhibitors. Nat Rev Cancer. 6:803–812. 2006.
View Article : Google Scholar : PubMed/NCBI
|