Introduction
Gene therapy has been extensively used for the
treatment of various inherited or acquired diseases and has been
commonly considered to be the most promising method for cancer
therapy over the past decade. The successful manipulation of gene
therapy relies on delivery vectors and the selection of functional
genes. However, among various considerations for the development of
gene therapy techniques that are able to fulfill the requirements
of current demands, the lack of safe and efficient gene delivery
vectors is a key limiting factor (1). Non-viral vectors, including cationic
polymer, have gained increasing interest as alternatives to viral
vectors due to their low immune response, low carcinogenic risk and
ease of synthesis. However, the greatest disadvantages of non-viral
vectors are low efficiency and low specific targeting ability
(2). The most attractive strategy
to improve transfection efficiency is targeted delivery, where
therapeutic genes are delivered to selected cells of interest. A
wide range of targeting ligands for cancer gene therapy have been
incorporated into non-viral vectors, such as folate, Asn-Gly-Arg
and Arg-Gly-Asp (RGD) peptides, transferrins and certain
antibodies. Due to the low molecular weight, high specificity and
easy availability of peptides, linking peptides may efficiently
combine the corresponding receptors on cell surfaces, and it
remains an outstanding strategy for improving the delivery
efficiency and targeting ability (3). Proteins containing an RGD sequence,
together with the integrins that serve as receptors for the
sequence, constitute a major recognition system for cell adhesion.
The RGD motif has high affinity for αv integrins on tumor cells and
has been used for the purpose of actively targeting vectors to
deliver drugs or genes into tumor cells (4). Certain studies have adopted
RGD-modified polymers or lipids as non-viral vectors for the
targeted delivery of genetic material to achieve efficient cancer
gene therapy, including antiangiogenic therapy (2,5–7).
However, due to the complexity of the characteristics of non-viral
vectors, no ideal vectors have been obtained that may have
potential for clinical application. The present study attempted to
constitute a novel type of vector based on polyethylenimine (PEI;
Mw=25 kDa) with conjugation of novel synthesized CP9 peptide
(CYGGRGDTP), which exhibits high combination activity with the
integrins on tumor cells. The physicochemical characteristics of
the novel vector and the biological profiles were studied. The
observations of the present study suggested that the targeted
delivery of RGD peptides modifies PEI complexes and may be useful
for cancer gene therapy.
Materials and methods
CP9-PEI synthesis
A total of 5.6 ml (4.5 mg/ml; 1.0×10−6
mol resolved in saline solution) PEI solution was reacted with 1.25
mg N-succinimidyl-3-(2-pyridyldithio) propionate
(4.0×10−6 mol; 1 ml saline and dimethyl sulfoxide
solution) at room temperature for 1 h under nitrogen atmosphere
protection in the dark with continuous stirring. Once the reaction
had finished, 2.8 mg CP9 peptide was slowly dropped into the
reaction system for an increased reaction for 2–3 h with the
nitrogen protection. The ultimate products were dialyzed against
pure running water with dialysis membrane (Mw cut-off, 10,000) for
48 h and then lyophilized for an additional 48 h. The final
desiccated white products were stored at −80°C for following
experiments.
1H-nuclear magnetic resonance
(NMR) analysis
Synthesized CP9-PEI was dissolved in D2O
and the 1H-NMR spectroscopic data were obtained using a
Bruker 400 MHz (Fällanden, Switzerland) spectrometer with eight
scans at room temperature.
In total, ~1 mg CP9-PEI was dissolved in pure
H2O and ultraviolet (UV) detection was performed using a
Hitachi U-3400 ultraviolet spectrophotometer (Hitachi, Ltd., Tokyo,
Japan).
Fourier transform infrared spectroscopy
(FTIR) detection
A total of ~1 mg CP9-PEI was prepared by dispersing
with potassium bromide and the complexes were compressed into
disks. The FTIR detection was conducted on a FTIR spectrometer
(Spectrum 2000; Perkin-Elmer, Waltham, MA, USA). Overall, 16 scans
were signal averaged to a resolution of 2 cm−1 at room
temperature.
Gel retardation assay
Electrophoretic mobility of the polymer
CP9-PEI/plasmid DNA (pDNA) polyplexes was measured using an agarose
gel electrophoresis system (Invitrogen, Carlsbad, CA, USA). An
appropriate amount of CP9-PEI was added to an equal volume of pDNA
solution to achieve the desired polymer/pDNA ratio (N/P ratio). In
total, 180 μl CP9-PEI/pDNA solutions (N/P ratios equal to 0, 0.5,
1, 2, 3, 4 and 5) were loaded into the loading wells of the agarose
gel. The gel electrophoresis was performed at room temperature in
Tris-acetate-EDTA buffer in 1% (w/w) agarose gel at 80 V for 45
min. The DNA bands were visualized by an UV illuminator (IS-1000;
Alpha Innotech, San Leandro, CA, USA).
Transmission electron microscope
observation
The CP9-PEI/pDNA solution was prepared (N/P ratio of
10) using saline as the solvent for transmission electron
microscope observation. The mixture solution was vortexed for 1 min
and left standing for 20 min, then ~1 ml solution was dropped on
the copper net. The samples were dried and the observation of the
morphology of the polyplexes was conducted under a JEM-2010
transmission electron microscope (JEOL, Tokyo, Japan).
As with the analysis of particle size, a series of
CP9-PEI/pDNA solutions (N/P ratios of 5, 10, 20 and 30) were
prepared in saline and analysis was performed using a 90 Plus
particle size analyzer (Brookhaven Instruments Corporation,
Holtsville, NY, USA) at 25°C. Scattering light was detected at 90°
and the wavelength was 670 nm.
In vitro gene delivery
The HepG2 cells (ATCC, Rockville, MD, USA) were
seeded in 48-well plates at a density of 2.5×104/well
with 850 μl Dulbecco’s modified Eagle’s medium (DMEM) containing
10% fetal calf serum (FCS) at 37°C for 24 h culture. When the
confluence of the cells had reached 70–80%, the culture medium was
replaced with 800 μl serum-free DMEM and the polyplexes of
CP9-PEI/pDNA with various N/P ratios (5, 10, 20 and 30) containing
1 μg pCMV-luc were dropped into each well (PEI was used as the
polymer control). The polyplexes were incubated with the cells for
6 h at 37°C, followed by supplementation with DMEM containing 10%
FCS for an additional 36 h. Later, the incubation medium were
removed and the cells were rinsed with phosphate-buffered saline
(PBS) and frozen-thawed in 200 μl PBS at −80°C. The luciferase
activity of the cell extracts was measured by a luciferase assay
system (Promega Corporation, Madison, WI, USA). The quantity of
total protein per well of cell extracts was determined by protein
assay kit (BCA; Pierce Biotechnology, Inc., Rockford, IL, USA).
Inhibition effect
The gene delivery process was similar to that of
in vitro gene delivery. However, in this step, prior to the
addition of polyplexes, the free CP9 peptide at different
concentrations (10, 50 and 100 nmol/l) was first added into the
culture system for 2 h of co-culture with the cells [peptide
containing RGE sequence (CYGGRGETP) acted as control group] and
then the gene delivery process was continued.
Statistical analysis
Unless noted otherwise, results from in vitro
experiments are represented by at least three independent
experiments. All data are expressed as the mean ± standard
deviation. Statistical analysis was performed using one-way
analysis of variance and Fisher’s least significant difference
test. Analysis was performed using SPSS 12.0 (SPSS, Inc., Chicago,
IL, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
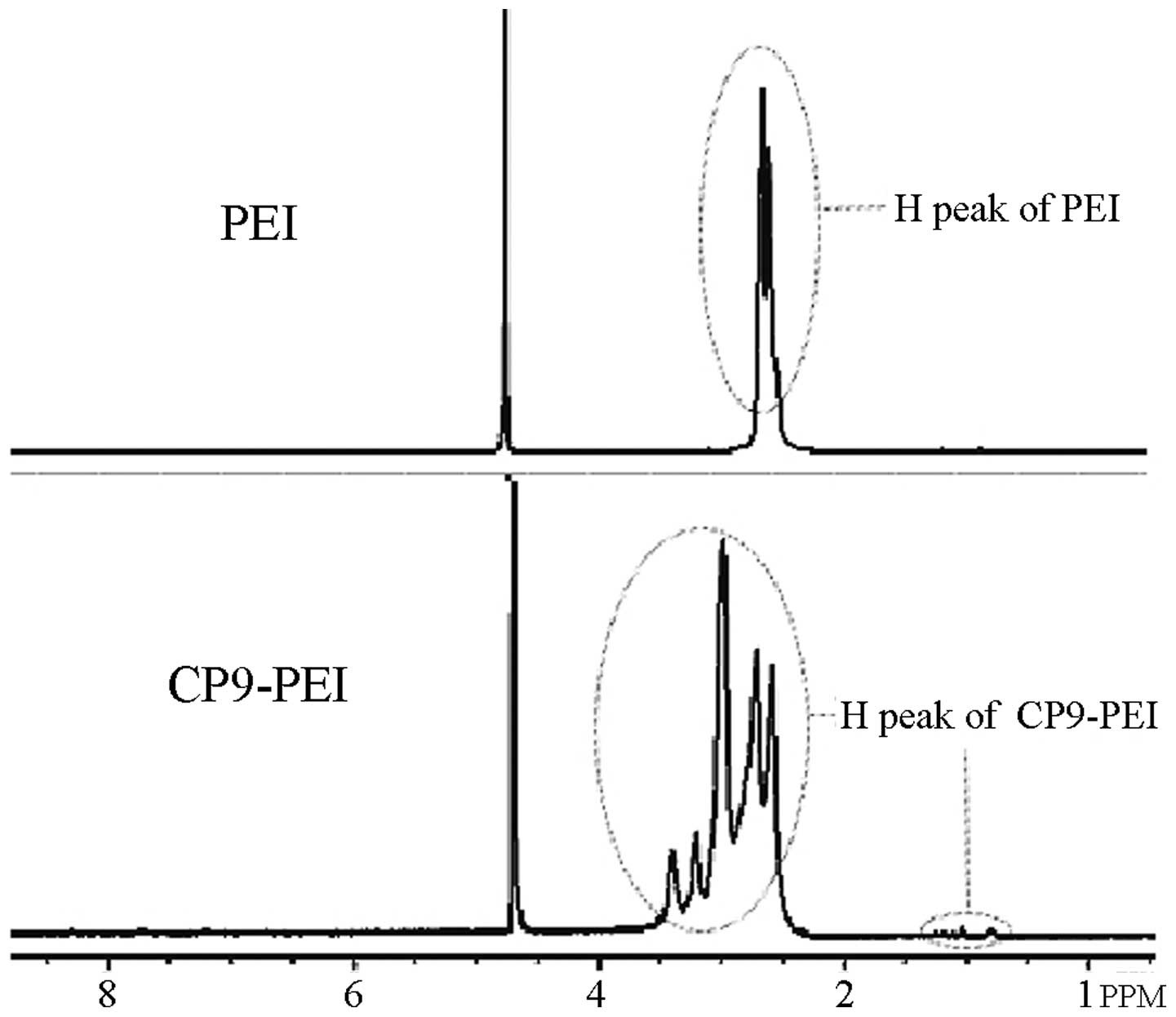
1H-NMR analysis
The results of the 1H-NMR analysis
(Fig. 1) showed that as with PEI,
the main three chemical displacements of H proton were located
between 2.1 and 3.0 ppm, which correspond with the three H protons
in the structure of PEI. However, when the CP9 peptide was
conjugated onto PEI, new H proton waves were identified between the
displacements of 3.0 and 3.5 ppm, and the superposition waves
appeared between the range of 2.1 and 3.0 ppm.
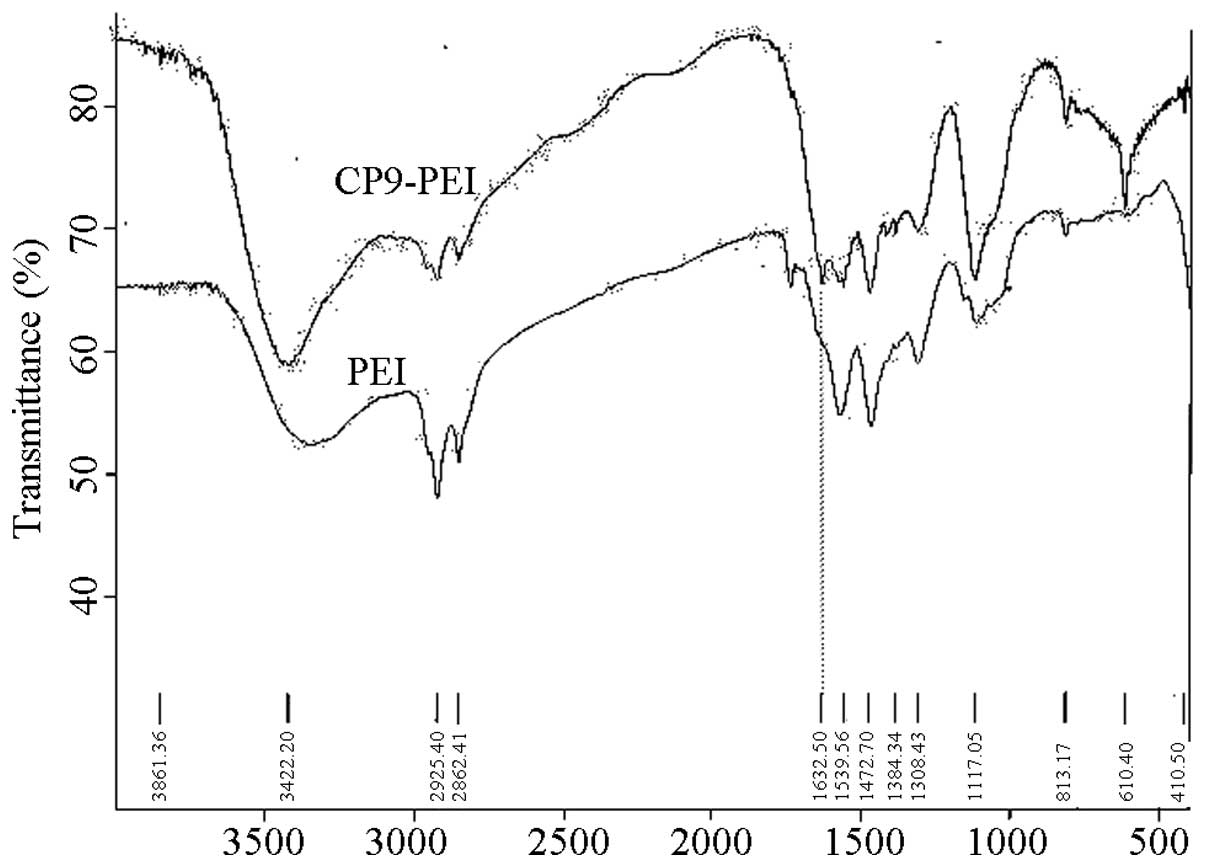
FTIR detection
The FTIR detection (Fig.
2) showed that at wavelengths of 3,420, 2,925 and 2,852
cm−1, PEI and CP9-PEI exhibited absorbance peaks that
showed the radical of -NH or -CH2 in their chemical
structures. However, a new absorbance peak at 1,630 cm−1
appeared, which suggested carbonyl group (C=O) formation in the
synthesized CP9-PEI.
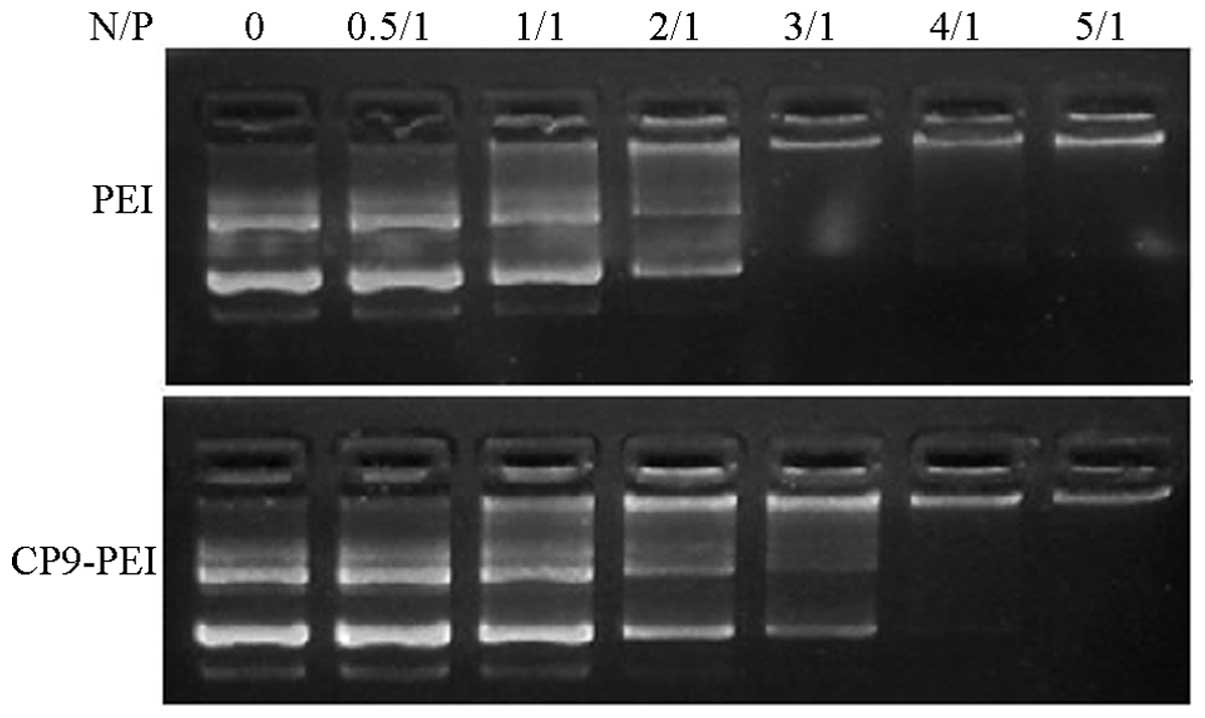
Gel retardation action
The gel retardation assay showed that CP9-PEI may
completely retard the mobility of the plasmids in the agarose gel
at the N/P ratio of 4; while in PEI, the N/P ratio was 3 (Fig. 3).
Polyplex particle size and
morphology
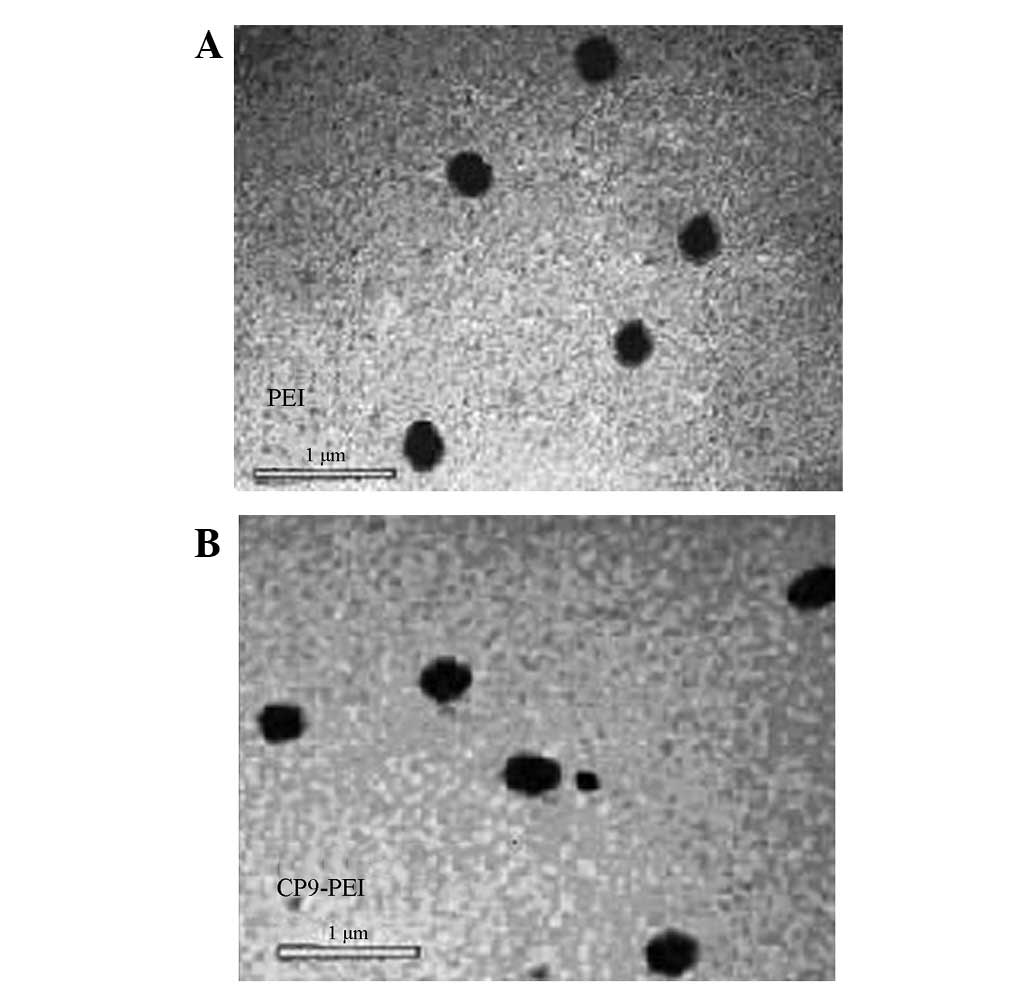
By transmission electron microscopy (Fig. 4), the nanoparticles comprised with
CP9-PEI or PEI plasmids showed round or round-like compact
particles with diameters of ~200 nm when the N/P ratio was 10.
Further detection (Fig. 5)
exhibited that in CP9-PEI or PEI plasmids, the diameter of the
particles decreased with an increased N/P ratio from 5 to 30.
However, at an N/P ratio of 10, the diameter of the
CP9-PEI/pCMV-luc polyplexes was ~187.54±13.14 nm, which was similar
to that of the PEI/pCMV-luc polyplexes (201.01±11.22 nm;
P=0.248).
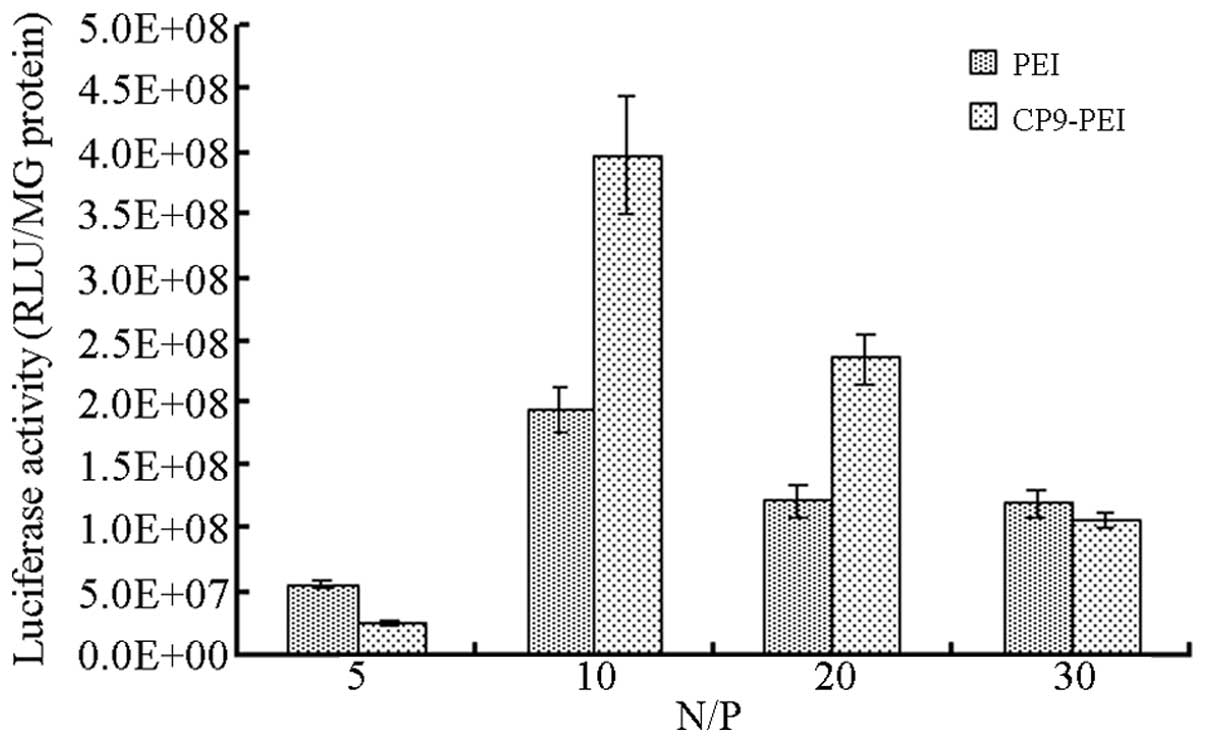
Gene delivery
The outcome of the gene delivery of the vectors in
HepG2 cells (Fig. 6) suggested that
CP9-PEI and PEI reached the highest delivery efficiency at an N/P
ratio of 10. However, under that condition, the efficiency of
CP9-PEI was 3.98×108 RLU/mg protein, but that of PEI was
only 1.95×108 RLU/mg protein.
Inhibition effect
At an N/P ratio of 10, the inhibition experiments
showed that with the increasing concentration of free CP9 peptides
(from 0 to 100 nmol/l), the delivery efficiency of CP9-PEI
decreased from 3.98×108 to 1.80×108 RLU/mg
protein. However, as with the control peptides containing RGE
sequence, no such inhibition effect was identified.
Discussion
Numerous developments in the field of gene therapy
have been made and >1,800 programs have entered clinical tests
(8). Among these, viral vectors
were predominantly employed. However, the viral vectors exhibit a
number of shortcomings, such as low hereditary material carrying
capability, high immunogenicity and safety considerations,
including carcinogenesis. Previous studies of non-viral vectors,
including liposomes and cationic polymers, have recently appeared
and have obtained great achievements (1,8).
PEI, a chemical with multiple amine structures, is
currently a ‘golden standard’ in the field of non-viral gene
delivery for its outstanding characteristics, including high
delivery efficiency, simple structure, ease of preparation, good
value and safety. In 1995, Boussif et al developed the gene
delivery function of PEI (9) and it
has since become the focus for research. Certain strategies have
been developed to modify PEI to improve its efficiency and avoid
the disadvantages, such as non-specific cell targeting ability
(10). Some factors may affect the
delivery profiles of PEI, including the molecular weight, branch
structures, menstruum system and the parameters of the delivery
process (11). The majority of
previous studies has shown that the 25-kDA branched PEI is a rather
prospective non-viral delivery reagent in gene therapy (12–14).
The present study adopted the 25-kDa branched PEI as the backbone
for constructing the novel vector to endow more beneficial
characteristics.
The current study employed the CP9 ligand peptides,
which contain the RGD sequence and may combine efficiently with the
integrins on the majority of tumor cells, to construct the novel
vector. Since the ligand-receptor integration mechanism can switch
on the receptor-mediated delivery pathway, such construction
strategy must endow a high-efficiency vector and theoretically, a
tumor cell-targeting function (15).
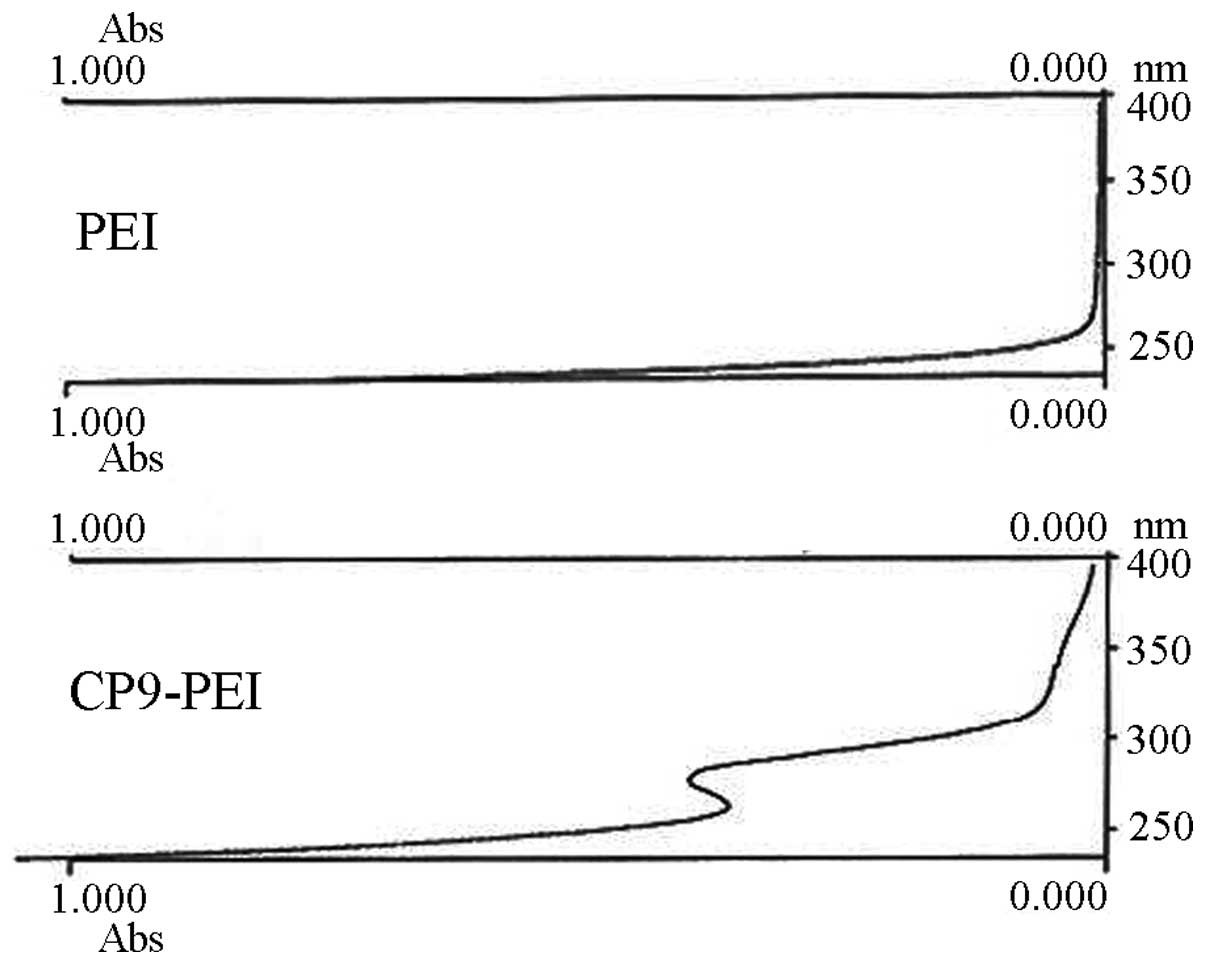
In the current study, 1H-NMR, UV
detection and FTIR methods were first utilized to confirm the
successful synthesis of CP9-PEI. Only the CP9 moiety was
victoriously conjugated onto the primary amines of PEI and new H
proton peaks appeared on the spectrum of 1H-NMR, peptide
absorbance emerged at ~270 nm on the UV detection and new carbonyl
coupling (C=O) produced a peak of 1,630 cm−1 on the
spectrum of FTIR (Figs. 1–3). Evidently, the 1,630 cm−1
peak was affected by the existence of abundant amine groups in PEI
(16). However, the that peak
formed at 1,630 cm−1 was small, which must be imputed to
the low conjugation ratio of CP9 to PEI.
One the most critical steps of non-viral vector
delivery function is its plasmid condensing ability (17). Only when nanoparticles of the
polymer/plasmids form may the vectors enter the cells and nucleus,
releasing the hereditary material (18). The results of the present study
showed that CP9-PEI may completely condense the plasmids at a N/P
ratio of 4 (Fig. 4). However, in
PEI, the responding N/P ratio was 3, which suggested that with
conjugation, the moiety of CP9 and the condensing ability were
impaired. However, such impairment had little influence, since at a
N/P ratio of 10, CP9-PEI condensed the plasmids into nanoparticles
with diameters of ~200 nm (Fig. 5).
The condensing process was affected by certain factors, including
the types of polymers, molecular weight, modification of polymers
and menstruum system (19).
Considering the limitation of endocytosis of the vector, the Brown
movement and the precipitation of the nanoparticles onto the cells
surface, it is generally considered that the optimal diameter of
polyplexes for gene delivery is 100–300 nm (20). In the present study, the synthesized
CP9-PEI showed optimal characteristics, which have been determined
as prospective profiles for excellent vectors (Fig. 6). Other factors, including molecular
weight, modification of polymers and menstruum system, remain under
investigation.
The ligand conjugation strategy may initiate the
receptor-mediated gene delivery process, which replaces part of the
static electricity-mediated pathway between polymers and cells
(21). Such a replacement effect
may endow the vector-specific receptors targeting capability and
reduce non-specific contact delivery function. Certain types of
conjugation ligands have been previously studied, such as epidermal
growth factor, transferrin and monoclonal antibodies (2). In general, the conjugation of ligands
effectively improves the level of delivery efficiency of the vector
through switching on the receptor-mediated delivery pathway and
also renders the vector more advantageous with targeting
properties. The αvβ3/αvβ5 types of integrin are excellent targets
for cancer gene therapy, as they exhibit markedly higher expression
levels in numerous types of cancer cells, such as HepG2, and in
endothelial cells in tumor angiogenesis (22). The main function of integrin is its
involvement in the procedure of cancer cell adherence, invasion and
metastasis. Commonly, the short peptides containing the RGD
sequence (RGDS) have been considered to exhibit great combination
activity with integrin, for example, the RGDS peptides have been
confirmed to simulate the characteristics of the natural ligand of
integrin (23). The results of the
current study (Fig. 7) showed that
while conjugated with the CP9 peptides, PEI exhibited more than
two-fold the delivery efficiency compared with that of pure PEI.
Furthermore, the free peptide inhibition tests, including the RGE
sequence-containing peptide tests, suggested that the improved
effect was due to the RGD core sequence (Fig. 8). Evidently, the CP9 moiety improved
the efficiency and simultaneously led to the integrin targeting
capability. Integrin was widely expressed in the majority of types
of cancer cells at a higher level and it may be speculated that the
CP9-PEI vector has vast prospects in cancer gene therapy.
The main aim of the present study of non-viral
vectors was to investigate their potential for application in
vivo. Currently, the majority of studies analyzing PEI have
focused on the local application, since the positive charge of the
polyplex remains a great obstacle for its systemic employment.
Certain strategies, including PEGylation, have been previously
studied (24). In conclusion, the
new CP9-PEI vector synthesized in the current study exhibited
optimal characteristics, enhanced gene delivery efficiency and
tumor cell-targeting capability. It has been considered that with
increased modification, such as PEGylation, the vector may be
developed into an ideal carrier for gene therapy.
Acknowledgements
The authors would like to thank the financial
support of grants from the Chinese National Natural Science
Foundation (nos. 81001034 and 30872945) and the Zhejiang Medicines
Health Science and Technology Program (nos. 2009B087 and
2011RCB030).
References
|
1
|
Pack DW, Hoffman AS, Pun S and Stayton PS:
Design and development of polymers for gene delivery. Nat Rev Drug
Discov. 4:581–593. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Park J, Singha K, Son S, et al: A review
of RGD-functionalized nonviral gene delivery vectors for cancer
therapy. Cancer Gene Ther. 19:741–748. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Guo XD, Wiradharma N, Liu SQ, et al:
Oligomerized alpha-helical KALA peptides with pendant arms bearing
cell-adhesion, DNA-binding and endosome-buffering domains as
efficient gene transfection vectors. Biomaterials. 33:6284–6291.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Katayama K, Furuki R, Yokoyama H, et al:
Enhanced in vivo gene transfer into the placenta using RGD
fiber-mutant adenovirus vector. Biomaterials. 32:4185–4193. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Matsui H, Sakurai F, Katayama K, et al:
Enhanced transduction efficiency of fiber-substituted adenovirus
vectors by the incorporation of RGD peptides in two distinct
regions of the adenovirus serotype 35 fiber knob. Virus Res.
155:48–54. 2011. View Article : Google Scholar
|
|
6
|
Nie Y, Schaffert D, Rödl W, Ogris M,
Wagner E and Günther M: Dual-targeted polyplexes: one step towards
a synthetic virus for cancer gene therapy. J Control Release.
152:127–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
O’Neill AM, Smith AN, Spangler EA, et al:
Resistance of canine lymphoma cells to adenoviral infection due to
reduced cell surface RGD binding integrins. Cancer Biol Ther.
11:651–658. 2011.PubMed/NCBI
|
|
8
|
Ginn SL, Alexander IE, Edelstein ML, Abedi
MR and Wixon J: Gene therapy clinical trials worldwide to 2012 - an
update. J Gene Med. 15:65–77. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boussif O, Lezoualc’h F, Zanta MA, et al:
A versatile vector for gene and oligonucleotide transfer into cells
in culture and in vivo: polyethylenimine. Proc Natl Acad Sci.
92:7297–7301. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Huntosova V, Buzova D, Petrovajova D, et
al: Development of a new LDL-based transport system for
hydrophobic/amphiphilic drug delivery to cancer cells. Int J Pharm.
436:463–471. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Doane TL and Burda C: The unique role of
nanoparticles in nanomedicine: imaging, drug delivery and therapy.
Chem Soc Rev. 41:2885–2911. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zheng M and Zhong Z, Zhou L, Meng F, Peng
R and Zhong Z: Poly (ethylene oxide) grafted with short
polyethylenimine gives DNA polyplexes with superior colloidal
stability, low cytotoxicity, and potent in vitro gene transfection
under serum conditions. Biomacromolecules. 13:881–888. 2012.
View Article : Google Scholar
|
|
13
|
Nimesh S: Polyethylenimine as a promising
vector for targeted iRNA delivery. Curr Clin Pharmacol. 7:121–130.
2012. View Article : Google Scholar
|
|
14
|
Patnaik S and Gupta KC: Novel
polyethylenimine-derived nanoparticles for in vivo gene delivery.
Expert Opin Drug Deliv. 10:215–228. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jain K, Kesharwani P, Gupta U and Jain NK:
A review of glycosylated carriers for drug delivery. Biomaterials.
33:4166–4186. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jiang Q, Shi P, Li C, et al: (Coixan
polysaccharide)-graft-polyethylenimine folate for tumor-targeted
gene delivery. Macromol Biosci. 11:435–444. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Shin JY, Suh D, Kim JM, et al: Low
molecular weight polyethylenimine for efficient transfection of
human hematopoietic and umbilical cord blood-derived CD34+ cells.
Biochim Biophys Acta. 1725:377–384. 2005.PubMed/NCBI
|
|
18
|
Hu Q, Wang J, Shen J, et al: Intracellular
pathways and nuclear localization signal peptide-mediated gene
transfection by cationic polymeric nanovectors. Biomaterials.
33:1135–1145. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sawant RR, Sriraman SK, Navarro G, Biswas
S, Dalvi RA and Torchilin VP: Polyethyleneimine-lipid
conjugate-based pH-sensitive micellar carrier for gene delivery.
Biomaterials. 33:3942–3951. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Munier S, Messai I, Delair T, Verrier B
and Ataman-Onal Y: Cationic PLA nanoparticles for DNA delivery:
Comparison of three surface polycations for DNA binding, protection
and transfection properties. Colloids Surf B Biointerfaces.
43:163–173. 2005. View Article : Google Scholar
|
|
21
|
Stoneham CA, Hollinshead M and Hajitou A:
Clathrin-mediated endocytosis and subsequent endo-lysosomal
trafficking of adeno-associated virus/phage. J Biol Chem.
287:35849–35859. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kagaya H, Oba M, Miura Y, et al: Impact of
polyplex micelles installed with cyclic RGD peptide as ligand on
gene delivery to vascular lesions. Gene Ther. 19:61–69. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kim J, Nam HY, Kim TI, et al: Active
targeting of RGD-conjugated bioreducible polymer for delivery of
oncolytic adenovirus expressing shRNA against IL-8 mRNA.
Biomaterials. 32:5158–5166. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Wu ZW, Chien CT, Liu CY, Yan JY and Lin
SY: Recent progress in copolymer-mediated siRNA delivery. J Drug
Target. 20:551–560. 2012. View Article : Google Scholar : PubMed/NCBI
|