Introduction
In recent decades, lung cancer has become the
leading cause of cancer-related mortality in China. In 2004,
66.7/100,000 individuals succumbed to lung cancer in China
(1). Non-small cell lung cancer
(NSCLC) is the principal type of lung cancer, with a 5-year
survival rate of <15% (2).
Therefore, the identification of specific biomarkers associated
with occurrence, development and prognosis of NSCLC is
essential.
In 1971, Folkman reported that new angiogenesis
maintains tumor growth when the tumor is >2 mm and predicted
that the presence of a tumor angiogenesis factor leads to the
formation of these vessels (3). In
this process, vascular endothelial growth factor (VEGF) families
are the most important molecules, such as VEGF-A, -B, -C and -D and
the placenta growth factor. VEGF-A, which is also called VEGF, is
of great importance in angiogenesis (4).
In cancer, degradation of the extracellular matrix
results in tumor cell growth, proliferation and metastasis. The
matrix metalloproteinases (MMPs) represent the most critical family
of endopeptidases associated with tumorigenesis. MMP-9 is a member
of the MMP family and plays an evident role in the angiogenesis of
tumors as well as regulating the bioavailability of VEGF (5).
The FLEXMAP 3D™ system is a multiplex platform based
on suspension assay technology. The prominent advantage of this
technology is that it can detect ≤500 types of analytes
simultaneously in a single well. Its multiplexed function and
enhanced sensitivity have been the cause of its wide use in recent
years (6). In the present study,
the serum levels of VEGF and MMP-9 were detected using Luminex
multiplex technology, and their diagnostic and prognostic values
were explored in NSCLC.
Materials and methods
Patients
In total, 332 cases of histopathologically
confirmed NSCLC and 91 cases of confirmed benign lung disease were
enrolled from the Affiliated Jiangsu Cancer Hospital, Nanjing
Medical University (Nanjing, China) between February 2009 and
November 2012. Of the NSCLC patients, 272 were classified as the
pretreatment group and the remainders as the postoperative group.
Initially, all the patients in the pretreatment group had been
pathologically diagnosed with NSCLC and had not received any prior
treatment. However, the patients in the postoperative group had
received lung surgery in the previous month. The characteristics of
the pretreatment group are shown in Table I; the median age of the patients was
61 years (range, 30–84 years) and all cases were staged according
to the latest TNM staging issued in 2009 by the International Union
Against Cancer. Of the 91 cases with benign lung diseases, 64 were
pulmonary hamartomas, 17 were pulmonary inflammatory pseudotumor,
six were pulmonary fibromas and four were pulmonary chondromas. The
median age of the patients with benign lung dieseases was 42 years
(range, 32–69 years). In addition, 120 healthy controls (without
any abnormalities following a comprehensive examination) were
enrolled, with a median age of 59 years (range, 35–79 years). A
total of 155 inoperable NSCLC (stages IIIb and IV) patients were
successfully followed up and the median survival time was 8 months
(range, 1–20 months).
 | Table ICharacteristics of the pretreatment
group of NSCLC. |
Table I
Characteristics of the pretreatment
group of NSCLC.
| Clinicopathological
characteristics | Patients, n (%) |
|---|
| Gender |
| Male | 196 (72.1) |
| Female | 76 (27.9) |
| Age, years |
| >60 | 159 (58.5) |
| ≤60 | 113 (41.5) |
| Tumor location |
| Left lung | 122 (44.9) |
| Right lung | 148 (54.4) |
| Whole lung | 2 (0.7) |
| TNM stage |
| I | 35 (12.9) |
| II | 27 (9.9) |
| IIIa | 32 (11.8) |
| IIIb | 43 (15.8) |
| IV | 135 (49.6) |
| Lymph node
metastasis |
| Yes | 214 (78.7) |
| No | 58 (21.3) |
| Grading |
| 1 | 18 (6.6) |
| 2 | 128 (47.1) |
| 3 | 126 (46.3) |
| Histological type
(NSCLC) |
| Squamous
carcinoma | 77 (28.3) |
| Adenocarcinoma | 190 (69.9) |
| Adenosquamous
carcinoma | 4 (1.5) |
| Sarcoma | 1 (0.3) |
Collection and preservation of blood
samples
In total, 3 ml venous blood was extracted from the
fasting patients and healthy controls. The blood samples were
placed into the endotoxin- and pyrogen-free test tubes immediately.
The whole blood specimens were then shaken three times and left to
coagulate for 30 min at room temperature. Finally, the blood
samples were centrifuged at 1,000 × g for 10 min, and the serum was
removed and stored at −80°C prior to use. The serum of the
participants was obtained following approval by the Ethics
Committee of Jiangsu Cancer Hospital (Nanjing, China). Written
informed consent was obtained from the patients.
Luminex multiplex technology for VEGF and
MMP-9
Luminex multiplex technology was used to conduct the
present study. The FLEXMAP 3D system was supplied by Luminex
Corporation (Austin, TX, USA). The serum levels of VEGF and MMP-9
were determined using human cytokine/chemokine panel (cat. no.
MPXHCYTO-60K) and human cardiovascular disease panel 1 (cat. no.
HCVD1-67AK) from Millipore (Billerica, MA, USA), respectively. For
the main immunoassay procedure for VEGF and MMP-9, all reagents
were allowed to warm to room temperature (20–25°C) prior to use.
The placement of standards [0 (background), 3.2, 16, 80, 400, 2,000
and 10,000 pg/ml for VEGF; and 0 (background), 0.016, 0.08, 0.4,
2.0, 10.0, 50.0 ng/ml for MMP-9], controls 1 and 2 and samples on
the Well Map Worksheet were then diagrammed in a vertical
configuration. Subsequently, the filter plate was prewetted by
pipetting 200 μl assay buffer into each well of the microtiter
filter plate and was sealed and mixed on a plate shaker for 10 min
at room temperature (20–25°C). Assay buffer was then removed by
vacuum and 25 μl each standard or control was added into the
appropriate wells. Assay buffer was used as the 0 pg/ml standard
(background); 25 μl assay buffer was added to the sample well and
25 μl serum matrix solution was added to the background, standard
and control wells. Next, 25 μl sample was added to the appropriate
wells, the mixing bottle was vortexed and 25 μl beads from the kit
were added to each well. Following incubation with agitation on a
plate shaker overnight at 4°C, the samples were washed twice with
200 μl per well of wash buffer and 25 μl detection antibody was
added to each well. After incubation with agitation on a plate
shaker for 1 h at room temperature (20–25°C), 2 μl
streptavidin-phycoerythrin was added to each well. The plate was
then further incubated for 30 min, washed twice with 200 μl/well
wash buffer and 150 μl (100 μl for MMP-9) sheath fluid was added to
each well. The plate was run on the FLEXMAP 3D™system and the
median fluorescence intensity results were saved and analyzed using
a weighted five-parameter logistic method for calculating the
concentration of samples. The assay was performed in duplicate.
Statistical analysis
SPSS 21.0 (SPSS, Inc., Chicago, IL, USA) was used
for the statistical analysis. The experimental data had a skewed
distribution; therefore, non-parametric analysis was conducted. The
Mann-Whitney U test was used to compare the variables between two
groups, while the Kruskal-Wallis test was used to compare variables
between more than two groups. The correlation between VEGF and
MMP-9 was analyzed by Spearman’s rank correlation. The area under
the receiver operating characteristic curve (AUROC) was calculated
and the logistic regression analysis was used to evaluate the
diagnostic value of the two predictors. The overall survival time
was calculated as the duration between the date of the initial
pathological diagnosis and mortality or the latest follow-up. The
median serum levels of VEGF and MMP-9 were used as the cut-off
points. The overall survival rates were estimated using the
Kaplan-Meier survival curves and, simultaneously, the log-rank test
was used to compare the survival distributions. Univariate and
multivariate Cox regression models were used to evaluate the effect
of clinicopathological parameters on patient prognosis. The median
and 25–75% quartiles were used for statistical description, and
P<0.05 was considered to indicate a statistically significant
difference.
Results
Serum levels of VEGF and MMP-9 in the
pretreatment group of NSCLC, patients with benign lung diseases and
healthy controls
The concentration of VEGF and MMP-9 in the serum of
the pretreatment group was significantly higher than that of the
patients with benign lung diseases and the healthy controls [VEGF:
81 pg/ml (range, 47–152.5 pg/ml) vs. 59 pg/ml (range, 21–118 pg/ml)
and 62.5 pg/ml (range, 25.25–116.5 pg/ml), respectively
(P<0.0001); and MMP-9: 1,092.5 ng/ml (range, 660.5–1,825 ng/ml)
vs. 326 ng/ml (range, 169–509 ng/ml) and 259 ng/ml (range,
122.25–531 ng/ml), respectively (P<0.0001)]. However, a
statistically significant difference was not observed between the
patients with benign lung diseases and the healthy controls in
terms of VEGF and MMP-9 serum levels (P>0.05) (Fig. 1).
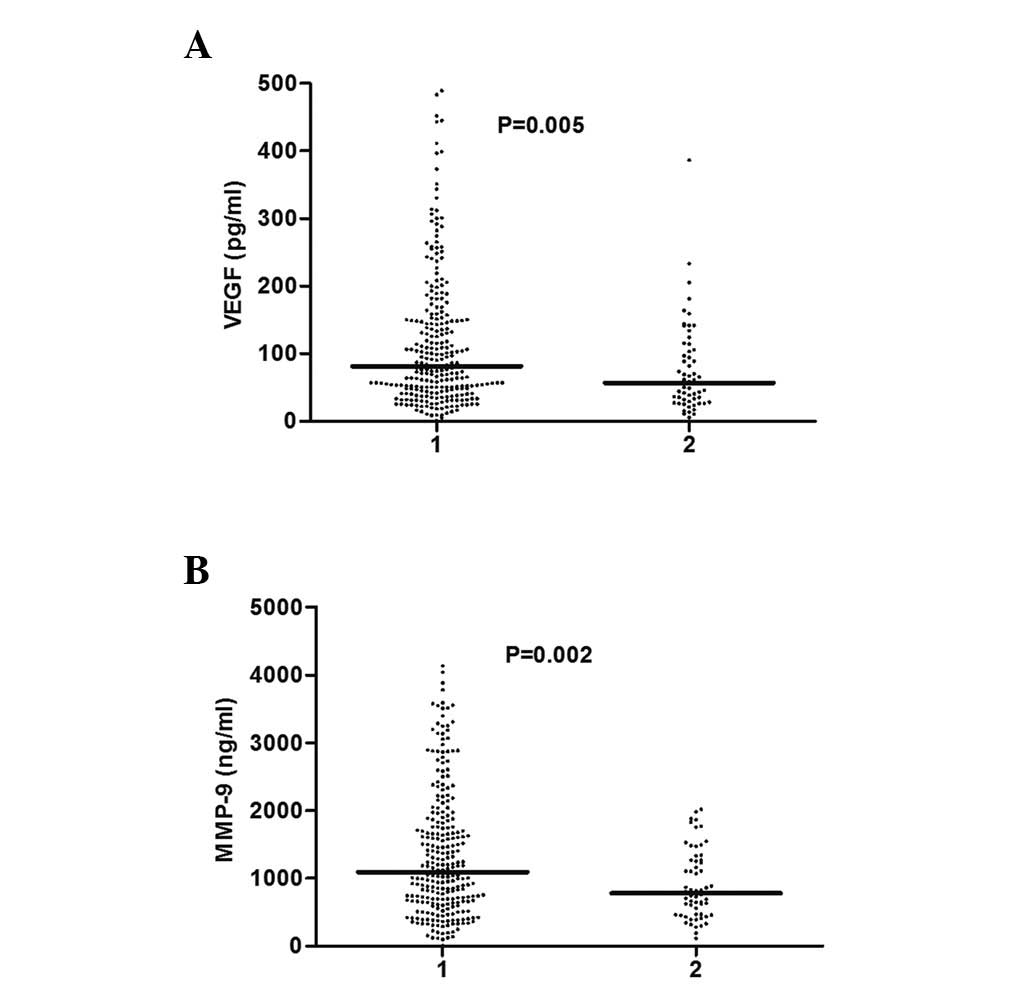
Serum levels of VEGF and MMP-9 in the
pretreatment and postoperative groups of NSCLC
Compared with the pretreatment group, the serum
levels of VEGF and MMP-9 were significantly decreased following the
pneumonectomy [VEGF: 81 pg/ml (range, 47–152.5 pg/ml) vs. 56.5
pg/ml (range, 29.75–111.75 pg/ml), respectively (P=0.005); and
MMP-9: 1,092.5 ng/ml (range, 660.5–1,825 ng/ml) vs. 778.5 ng/ml
(range, 461.75–1,266.25 ng/ml), respectively (P=0.002)] (Fig. 2).
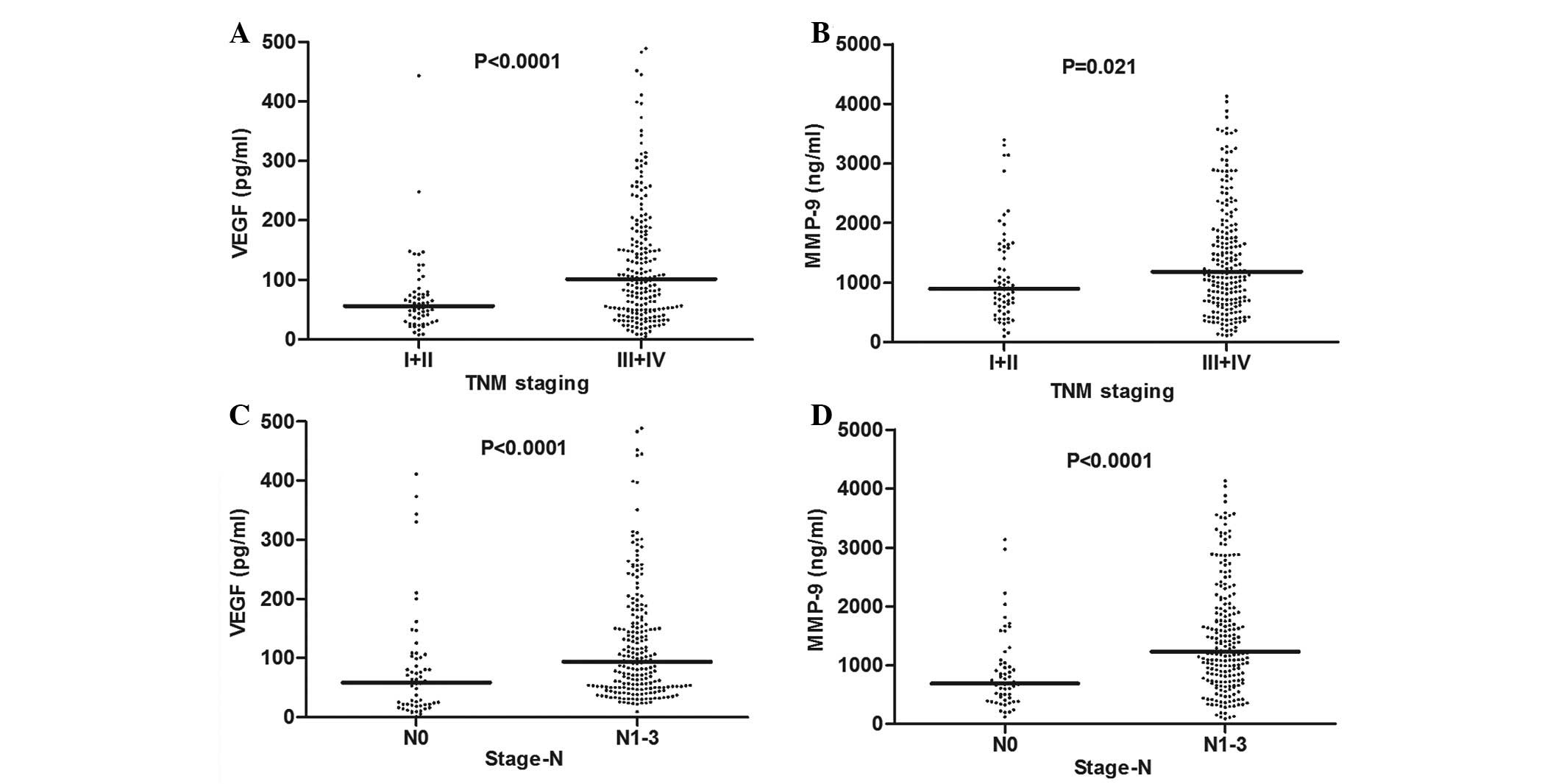
Correlation between serum levels of VEGF
and MMP-9 and clinicopathological parameters in the pretreatment
group of NSCLC
As shown in Table
II, the concentration of VEGF in patients with TNM stages III
and IV was higher than that in patients with stages I and II [101.5
pg/ml (range, 50–183.25 pg/ml) vs. 56.5 pg/ml (range, 34–76 pg/ml),
respectively; P<0.0001] (Fig.
3A). The serum levels of VEGF were significantly higher in
patients with lymph node metastasis compared with those without
lymph node metastasis [94 pg/ml (range, 50–170 pg/ml) vs. 58 pg/ml
(range, 20.75–101.5 pg/ml), respectively; P<0.0001] (Fig. 3C). Moreover, the levels of MMP-9 in
serum was found to significantly correlate with TNM staging [1,182
ng/ml (range, 692–1,932.25 ng/ml) vs. 894 ng/ml (572–1,586.25
ng/ml), respectively; P=0.021] (Fig.
3B) and lymph node metastasis [1,299.5 ng/ml (range,
740.25–1,994.5 ng/ml), vs. 691.5 ng/ml (range, 404.75–1,028
ng/ml), respectively; P<0.0001] (Fig. 3D). However, no differences were
identified between the serum levels of the two factors and other
clinicopathological parameters (P>0.05).
 | Table IICorrelation between serum VEGF and
MMP-9 levels and clinicopathological parameters in the NSCLC
pretreatment group. |
Table II
Correlation between serum VEGF and
MMP-9 levels and clinicopathological parameters in the NSCLC
pretreatment group.
| VEGF, pg/ml | MMP-9, ng/ml |
|---|
|
|
|
|---|
| Variable | Median, n | Q1–Q3, n | P-value | Median, n | Q1–Q3, n | P-value |
|---|
| Gender |
| Male | 86 | 49.25–167.75 | 0.055 | 1159 | 693.5–1950.75 | 0.075 |
| Female | 70 | 34–131.25 | | 939.5 | 607–1622.5 | |
| Age, years |
| >60 | 82 | 47–159 | 0.981 | 1098 | 660–1758 | 0.911 |
| ≤60 | 78 | 46.5–147 | | 1086 | 665.5–1918 | |
| Tumor location |
| Left lung | 79 | 43.5–147.25 | 0.203 | 1035.5 | 661.5–1699 | 0.150 |
| Right lung | 84 | 48–167.75 | | 1160 | 637–1847 | |
| Whole lung | 36 | 22–50 | | 2774 | 2036–3512 | |
| TNM stage |
| I and II | 56.5 | 34–76 | <0.0001 | 894 | 572–1586.25 | 0.021 |
| III and IV | 101.5 | 50–183.25 | | 1182 | 692–1932.25 | |
| Lymph node
metastasis |
| Yes | 94 | 50–170 | <0.0001 | 1299.5 | 740.25–1994.5 | <0.0001 |
| No | 58 | 20.75–101.5 | | 691.5 | 404.75–1028 | |
| Grading |
| 1 | 154 | 69.25–221 | 0.077 | 998.5 | 587.25–3576 | 0.892 |
| 2 | 83 | 47.25–172 | | 1043 | 636.75–2206.25 | |
| 3 | 73.5 | 43.5–131.25 | | 1186 | 710.5–1603.75 | |
| Histological
type |
| Squamous
carcinoma | 78 | 48–153.5 | 0.988 | 1098 | 725.5–2088 | 0.403 |
|
Adenocarcinoma | 83.5 | 42–151.5 | | 1092 | 640.25–1739.25 | |
| Adenosquamous
carcinoma | 114.4 | 32.5–237 | | 1308.5 | 736.25–1686.5 | |
| Sarcomaa | 74 | | | 391 | | |
Correlation between the serum levels of
VEGF and MMP-9 in NSCLC patients
As shown in Fig. 4,
Spearman’s rank correlation was used to analyze the correlation
between the serum levels of VEGF and MMP-9 in the pretreatment
group of NSCLC. The results indicated that the expression of VEGF
was found to significantly correlate with the expression of MMP-9
(r=0.159; P=0.009) (Fig. 4A).
However, no statistically significant difference was identified
between the serum levels of VEGF and MMP-9 in the postoperative
group (r=0.073; P=0.578) (Fig.
4B).
Diagnostic value of VEGF and MMP-9 in the
pretreatment group of NSCLC
The receiver operating characteristic curves and the
logistic regression models were used to assess the diagnostic value
of the serum levels of VEGF and MMP-9 in the pretreatment group of
NSCLC. The AUROC of the serum levels of VEGF, differentiating the
pretreatment group from the patients with benign lung diseases, was
0.632 (95% CI, 0.566–0.698) compared with 0.885 (95% CI,
0.852–0.919) for MMP-9. The AUROC of VEGF and MMP-9 was 0.624 (95%
CI, 0.565–0.683) and 0.88 (95% CI, 0.845–0.914), respectively, for
differentiating the pretreatment group from the healthy controls.
In addition, the AUROC of VEGF for distinguishing between the
pretreatment group and patients with benign lung diseases and
healthy controls was 0.627 (95% CI, 0.578–0.677), while for MMP-9,
the AUROC was 0.882 (95% CI, 0.853–0.911). The AUROC of MMP-9 was
found to be higher than that of VEGF in the various groups. The
logistic regression analysis was used to estimate the diagnostic
value of the two factors and the results showed that only MMP-9
remained significant (P<0.05) (Fig.
5A–F).
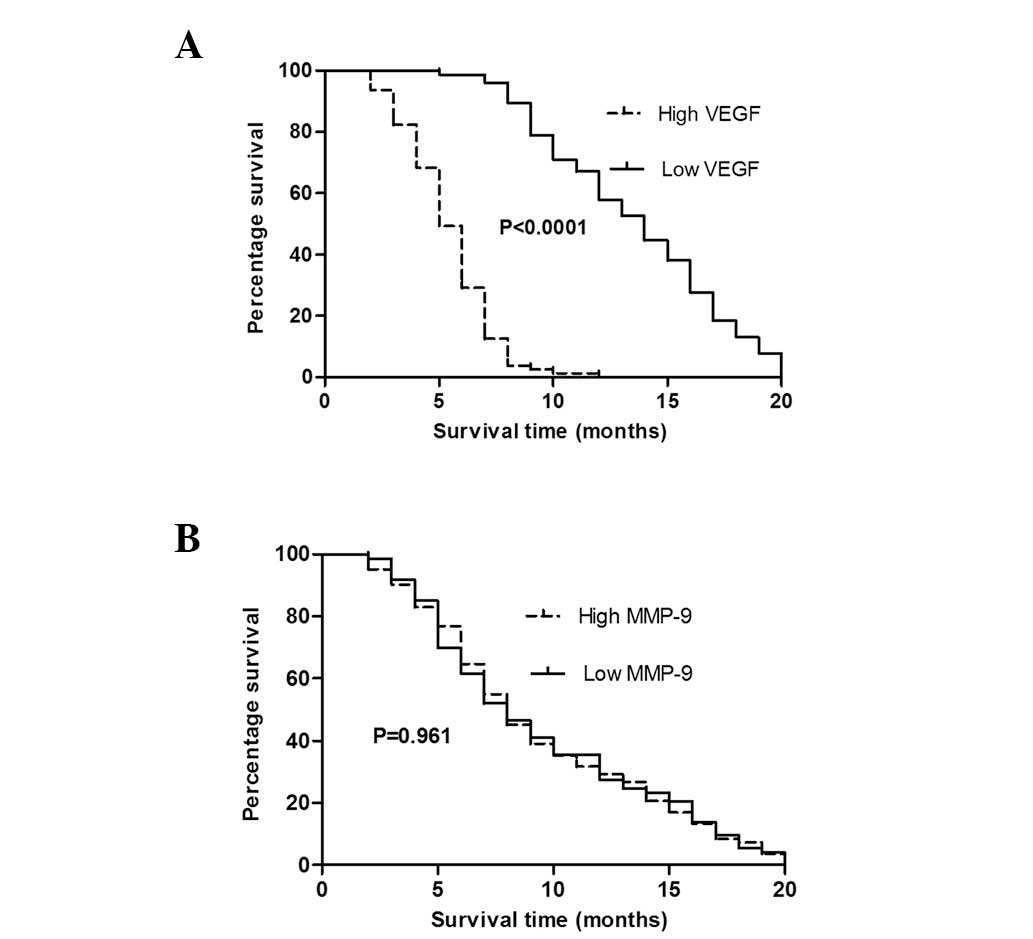
Prognostic value of VEGF and MMP-9 in the
inoperable NSCLC patients
Kaplan-Meier survival curves and the log-rank test
were used for statistical analysis in patients with inoperable
NSCLC. Patients with low levels of VEGF (<101.5 pg/ml) were
found to exhibit a significantly longer overall survival time than
those with high levels of VEGF (>101.5 pg/ml) (P<0.0001).
However, no statistically significant difference was identified in
the overall survival time between the high (>1,182 ng/ml) and
low levels (<1,182 ng/ml) of MMP-9 (P>0.05), as shown
in Fig. 6. Cox regression models
were used to analyze the effect of clinicopathological parameters
on patient survival time, and the parameters included age, gender,
levels of VEGF and MMP-9, histological type, grading, lymph node
metastasis and tumor location (Table
III). In the univariate Cox regression, lymph node metastasis
(P=0.001) and the levels of VEGF (P<0.0001) in serum were found
to closely correlate with patient survival time. The two variables
were analyzed using multivariable Cox regression and the results
indicated that lymph node metastasis (P=0.027) and the
concentration of VEGF (P<0.0001) in serum remained significant.
Therefore, the serum levels of VEGF and lymph node metastasis have
been identified as independent prognostic factors in the inoperable
NSCLC.
 | Table IIIUnivariate and multivariate Cox
regression analysis of inoperable NSCLC patients. |
Table III
Univariate and multivariate Cox
regression analysis of inoperable NSCLC patients.
| Univariate | Multivariate |
|---|
|
|
|
|---|
| Variable | P-value | HR, 95% CI | Exp (B) | P-value |
|---|
| Age | 0.299 | | | |
| Gender | 0.133 | | | |
| VEGF | <0.0001 | 1.005–1.008 | 1.006 | <0.0001 |
| MMP-9 | 0.106 | | | |
| Histological
type | 0.576 | | | |
| Differentiated
degree | 0.232 | | | |
| Lymph node
metastasis | 0.001 | 1.066–2.861 | 1.746 | 0.027 |
| Tumor location | 0.971 | | | |
Discussion
Neoplasm metastasis is the leading cause of
mortality in patients with lung cancer. Angiogenesis and basement
membrane degradation are essential in the process of the growth and
invasion of tumor lesions (3,5). VEGF
is important for angiogenesis, while MMP-9 is involved in basement
membrane degradation. Recent studies have confirmed that VEGF
stimulates the proliferation of endothelial cells by activating
MMPs and, accordingly, inducing the angiogenesis of tumors
(7,8). MMP-9 is critical in the invasion and
metastasis of tumors and is also considered as a type of tumor
angiogenic factor involved in the signaling system of VEGF-VEGF
receptor (9,10). The objective of the current study
was to analyze the correlation between the serum levels of VEGF and
MMP-9, as well as their diagnostic and prognostic values in
NSCLC.
Compared with immunohistochemical staining, the
prominent advantages of measuring angiogenic factors in the blood
are high availability and reduced bias (11). Luminex multiplex assays were used to
detect the levels of VEGF and MMP-9 in the serum. The serum levels
of VEGF and MMP-9 were found to be significantly increased in the
pretreatment group of NSCLC compared with the patients with benign
lung diseases and healthy controls. These results are consistent
with a number of previous observations (12,13). A
significant decrease in the serum levels of VEGF and MMP-9 was
observed in the postoperative group compared with the pretreatment
group of NSCLC patients. This result may be due to the decrease of
the tumor load and suggests that the two factors are associated
with the outcome of NSCLC patients.
In the present study, the serum levels of VEGF and
MMP-9 were found to significantly correlate with TNM staging in the
pretreatment group of NSCLC. Compared with stages I and II, the
concentration of VEGF and MMP-9 in the patients with stages III and
IV was significantly elevated. Currently, the expression of the two
factors is detected by various methods, such as RT-PCR,
immunohistochemistry and ELISA. Previous studies have shown that
the expression of VEGF increases as NSCLC staging progresses
(14). The expression of MMP-9 is
also associated with clinicopathological stage in the serum of
NSCLC (15). These hypotheses are
consistent with the results of the current study. By contrast, a
previous study found no correlation between the expression of the
two factors and TNM staging in 91 patients with NSCLC (16).
In the present study, the serum levels of VEGF and
MMP-9 in the pretreatment group of NSCLC with lymph node metastasis
were significantly higher than in those without lymph node
metastasis. A number of previous studies have found that the high
expression of VEGF and MMP-9 closely correlate with lymph node
metastasis (15,17). No significant differences were
identified between the levels of the two factors and age, gender,
degree of differentiation, histological type and tumor location.
This result is consistent with a number of previous domestic and
foreign studies (16,18–20).
However, a previous study reported that the expression of VEGF and
MMP-9 were found to closely correlate with the degree of
differentiation, using immunohistochemical detection in 136
patients with NSCLC (21). The
differential expression in the serum and tissue, as well as the use
of various detection methods may account for this result. All the
abovementioned studies indicate that the serum levels of VEGF and
MMP-9 significantly correlate with the progression and metastasis
of NSCLC.
In the current study, the correlation between VEGF
and MMP-9 was analyzed and the expression of the two proteins was
found to positively correlate in the pretreatment group. This
result may prompt a synergistic effect of the two factors in tumor
angiogenesis and lymph node metastasis in the pretreatment group of
NSCLC. Previous studies have detected a significant correlation
between the serum levels of VEGF and MMP-9 in patients who did not
undergo surgery (7,8,22).
However, no correlation between the serum levels of VEGF and MMP-9
was identified in the postoperative group of the current study.
This may be due to the significant decrease in the concentration of
the two indicators following tumor resection.
In the present study, it was found that the
diagnostic accuracy of MMP-9 was improved compared with that of
VEGF in the different groups. In the logistic regression models,
only MMP-9 remained significant. The diagnostic values of VEGF and
MMP-9 have remained unclear in previous studies (23,24),
but certain subsidiary diagnostic values of the two indicators
function to confirm NSCLC in patients who are difficult to
diagnose.
Finally, the overall survival time was analyzed in
the inoperable NSCLC patients of the present study. Patients with
low levels of VEGF were found to exhibit a significantly longer
overall survival time than those with high levels of VEGF. A large
number of previous studies have reported the prognostic value of
VEGF in patients with NSCLC; a large amount of data have supported
the prognostic value of the expression of VEGF and have confirmed
that the overexpression of VEGF is associated with poor prognosis
in NSCLC (25). By contrast, a
certain study was unable to identify the prognostic value of VEGF
in NSCLC using quantitative immunoassay (26).
In the current study, no statistically significant
difference was identified in survival time between the high and low
levels of MMP-9. A previous study indicated that there was no
prognostic value of the expression of MMP-9 in 90 cases of NSCLC
using immunohistochemistry (27).
An additional study found no correlation between the expression of
MMP-9 and the overall survival time of patients with NSCLC
(16). Conversely, it has been
demonstrated that MMP-9 is an independent adverse prognostic factor
in the tissues and serum of lung cancer (15,28).
Currently, the prognostic values of VEGF and MMP-9 remain
controversial.
In addition, lymph node metastasis and the
pretreatment serum levels of VEGF were identified as independent
prognostic variables in a multivariate analysis of the current
study. Therefore, the detection of serum VEGF levels may predict
the progression of tumors and aid the treatment of inoperable NSCLC
patients.
Overall, the present study showed that the levels of
VEGF and MMP-9 were closely correlated with the progression and
metastasis of NSCLC, and that MMP-9 may be a potential diagnostic
indicator of NSCLC. The pretreatment serum levels of VEGF may be an
independent prognostic marker for inoperable NSCLC, and therefore
may be identified as an indicator to predict the outcome of
patients with inoperable NSCLC. However, there were a number of
inadequacies in the current study. Firstly, the sample size was not
large, therefore, future studies must be conducted in larger
populations. Secondly, the follow-up was available only for
inoperable patients, but not for all patients with NSCLC. All NSCLC
patients must be followed up for the next five years or even
decades. Finally, the evaluation of the expression of VEGF and
MMP-9 was not evaluated in situ. An increased number of
future in-depth studies are required to confirm the diagnostic and
prognostic values of VEGF and MMP-9 in NSCLC.
References
|
1
|
Zhao P, Dai M, Chen W and Li N: Cancer
trends in China. Jpn J Clin Oncol. 40:281–285. 2010. View Article : Google Scholar
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
3
|
Folkman J: Tumor angiogenesis: therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kerbel RS: Tumor angiogenesis. N Engl J
Med. 358:2039–2049. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kessenbrock K, Plaks V and Werb Z: Matrix
metalloproteinases: regulators of the tumor microenvironment. Cell.
141:52–67. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hsu HY, Joos TO and Koga H: Multiplex
microsphere-based flow cytometric platforms for protein analysis
and their application in clinical proteomics-from assays to
results. Electrophoresis. 30:4008–4019. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hiratsuka S: Vasculogenensis, angiogenesis
and special features of tumor blood vessels. Front Biosci (Landmark
Ed). 16:1413–1427. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang Y, Yu LK and Xia N: Evaluation of
serum and pleural levels of endostatin and vascular epithelial
growth factor in lung cancer patients with pleural effusion. Asian
Pac J Trop Med. 5:239–242. 2012. View Article : Google Scholar
|
|
9
|
Muramatsu M, Yamamoto S, Osawa T and
Shibuya M: Vascular endothelial growth factor receptor-1 signaling
promotes mobilization of macrophage lineage cells from bone marrow
and stimulates solid tumor growth. Cancer Res. 70:8211–8221. 2010.
View Article : Google Scholar
|
|
10
|
Hiratsuka S, Nakamura K, Iwai S, et al:
MMP9 induction by vascular endothelial growth factor receptor-1 is
involved in lung-specific metastasis. Cancer Cell. 2:289–300. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bremnes RM, Camps C and Sirera R:
Angiogenesis in non-small cell lung cancer: the prognostic impact
of neoangiogenesis and the cytokines VEGF and bFGF in tumours and
blood. Lung Cancer. 51:143–158. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Tas F, Duranyildiz D, Oguz H, Camlica H,
Yasasever V and Topuz E: Serum vascular endothelial growth factor
(VEGF) and bcl-2 levels in advanced stage non-small cell lung
cancer. Cancer Invest. 24:576–580. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Jumper C, Cobos E and Lox C: Determination
of the serum matrix metalloproteinase-9 (MMP-9) and tissue
inhibitor of matrix metalloproteinase-1 (TIMP-1) in patients with
either advanced small-cell lung cancer or non-small-cell lung
cancer prior to treatment. Resp Med. 98:173–177. 2004. View Article : Google Scholar
|
|
14
|
Katsabeki-Katsafli A, Kerenidi T, Kostikas
K, et al: Serum vascular endothelial growth factor is related to
systemic oxidative stress in patients with lung cancer. Lung
Cancer. 60:271–276. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zheng S, Chang Y, Hodges KB, et al:
Expression of KISS1 and MMP-9 in non-small cell lung cancer and
their relations to metastasis and survival. Anticancer Res.
30:713–718. 2010.PubMed/NCBI
|
|
16
|
Yurdakul A, Akyürek N, Yilmaz Ş, Karakaya
J, Memış L and Öztürk C: Prognostic impact of matrix
metalloproteinases (MMP-9 and MMP-2) and vascular endothelial
growth factor expression in non-small cell lung cancer. Turk J Med
Sci. 42:281–288. 2012.
|
|
17
|
Tamura M, Oda M, Matsumoto I, et al: The
combination assay with circulating vascular endothelial growth
factor (VEGF)-C, matrix metalloproteinase-9, and VEGF for
diagnosing lymph node metastasis in patients with non-small cell
lung cancer. Ann Surg Oncol. 11:928–933. 2004. View Article : Google Scholar
|
|
18
|
Laack E, Köhler A, Kugler C, et al:
Pretreatment serum levels of matrix metalloproteinase-9 and
vascular endothelial growth factor in non-small-cell lung cancer.
Ann Oncol. 13:1550–1557. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shimanuki Y, Takahashi K, Cui R, Hori S,
Takahashi F, Miyamoto H and Fukurchi Y: Role of serum vascular
endothelial growth factor in the prediction of angiogenesis and
prognosis for non-small cell lung cancer. Lung. 183:29–42. 2005.
View Article : Google Scholar
|
|
20
|
Cox G, Jones JL and O’Byrne KJ: Matrix
metalloproteinase 9 and the epidermal growth factor signal pathway
in operable non-small cell lung cancer. Clin Cancer Res.
6:2349–2355. 2000.PubMed/NCBI
|
|
21
|
Jin Y, Li JP, Tang LY, et al: Protein
expression and significance of VEGF, EGFR and MMP-9 in non-small
cell lung carcinomas. Asian Pac J Cancer Prev. 12:1473–1476.
2011.PubMed/NCBI
|
|
22
|
Hawinkels LJ, Zuidwijk K, Verspaget HW, et
al: VEGF release by MMP-9 mediated heparansulphate cleavage induces
colorectal cancer angiogenesis. Eur J Cancer. 44:1904–1913. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jantus-Lewintre E, Sanmartín E, Sirera R,
et al: Combined VEGF-A and VEGFR-2 concentrations in plasma:
diagnostic and prognostic implications in patients with advanced
NSCLC. Lung Cancer. 74:326–331. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Atkinson JM, Pennington CJ, Martin SW, et
al: Membrane type matrix metalloproteinases (MMPs) show
differential expression in non-small cell lung cancer (NSCLC)
compared to normal lung: correlation of MMP-14 mRNA expression and
proteolytic activity. Eur J Cancer. 43:1764–1771. 2007. View Article : Google Scholar
|
|
25
|
Farhat FS, Tfayli A, Fakhruddin N, et al:
Expression, prognostic and predictive impact of VEGF and bFGF in
non-small cell lung cancer. Crit Rev Oncol Hematol. 84:149–160.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chakra M, Pujol JL, Lamy PJ, et al:
Circulating serum vascular endothelial growth factor is not a
prognostic factor of non-small cell lung cancer. J Thorac Oncol.
3:1119–1126. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Fujise N, Nanashima A, Taniguchi Y, et al:
Prognostic impact of cathepsin B and matrix metalloproteinase-9 in
pulmonary adenocarcinomas by immunohistochemical study. Lung
Cancer. 27:19–26. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Martins SJ, Takagaki TY, Silva AGP, et al:
Prognostic relevance of TTF-1 and MMP-9 expression in advanced lung
adenocarcinoma. Lung Cancer. 64:105–109. 2009. View Article : Google Scholar : PubMed/NCBI
|