Introduction
Colorectal carcinoma (CRC) is one of the most common
types of digestive tract malignant tumor in humans. It is the forth
and third most common type of cancer in males and females
worldwide, respectively (1), and is
one of the major causes of cancer-related mortality (2). CRC incidence rates have continued to
increase in economically transitioning countries, which may reflect
the adoption of western lifestyles and behaviors, including the
consumption of high-fat diets, physical inactivity and smoking
(3). The cancer stem cell (CSC)
theory was first proposed by Hamburger and Salmon (4) who demonstrated that only a small
percentage of tumor cells are able to form colonies in soft agar.
According to the CSC hypothesis, cancer originates from uncommon
cells, stem cells (SCs), which show pluripotency and self-renewal
(5). These self-renewing CSCs may
constitute only a small fraction of the tumor cells, with the bulk
of the tumor composed of differentiated cells that lack
self-renewal capacity (6). These
CSCs are hypothesized to cause the initiation, progression and
recurrence of cancer. Markers of CSCs may be used to identify CSCs
and study their role in the cause of tumorigenesis.
Aldehyde dehydrogenase 1 (ALDH1), a detoxifying
enzyme responsible for the oxidation of intracellular aldehydes
(7), is one of the common markers
of CSCs and SCs. Immunohistochemistry (IHC) results have previously
demonstrated that ALDH1 expression and enzyme activity were higher
in breast, lung or colon cancer, in which ALDH1 expression was
limited in the normal tissue, but was significantly increased in
malignant tissue (8–10). The aim of the present study was to
identify whether ALDH1 may serve as a valuable marker in CRC
patients and whether the detection of ALDH1 may be useful for
distinguishing between colorectal carcinogenesis and normal
colorectal tissues.
CD133 (also known as Prominin-1 or AC133) is a
transmembrane glycoprotein of 865 amino acid, with a total
molecular weight of 120 kDa, which is expressed on the surface of
apical plasma membrane protrusions of embryonic epithelial
structures (11). In a number of
previous studies, investigators have used monoclonal antibodies
against CD133 for the identification and isolation of a putative
CSC population from malignant tumors of the brain (12), prostate (13), liver (14), pancreas (15), lung (16) and colon (17–19).
Furthermore, CD133 appears to be the most important colon CSC
marker, since subpopulations of CD133+ colon cancer
cells have demonstrated increased tumorigenic potential in
transplantation studies in vivo and in vitro(20). Other previous studies have also
shown that overexpression of CD133 is associated with poor
prognosis and distant metastasis in primary colon cancer (20,21).
In the current study, immunohistochemical
examination of ALDH1 and CD133 expression was performed to identify
whether ALDH1 and CD133 expression was present in patients with
CRC. In addition, the correlation between the expression of ALDH1
and CD133 was investigated to understand their role in neoplasia
and patient prognosis.
Materials and methods
Patients and tissue specimens
The tissues of primary CRCs were obtained from the
Department of Pathology at the Red Flag Hospital Affiliated to
Mudanjiang Medical College (Mudanjiang, China) between January 2005
and January 2007. Prior to surgery, no patients had received any
type of therapy, such as radiation or chemotherapy. Of the total 60
cancer specimens, there were 20 well-differentiated, 20 moderately
differentiated and 20 poorly differentiated adenocarcinomas. Normal
mucosal specimens ≥5 cm distant from the primary CRCs were obtained
from patients with CRC. All tissue samples were fixed in formalin,
embedded in paraffin and deparaffinized for IHC staining. All
protocols were reviewed and approved by the Ethical Committee of
Mudanjiang Medical College (Mudanjiang, China) and written informed
consent was obtained from all participating patients. All tumor
histology and grades were determined by diagnostic evaluation by
two pathologists.
Follow-up
Clinical and pathological records of all patients
involved in the study were reviewed periodically. Patients were
followed up regularly for five years at the Red Flag Hospital
Affiliated to Mudanjiang Medical College. All patients were
followed up from January 1, 2005 to mortality or the study closing
date (February 1, 2012). The overall survival (OS) of each case was
the assessment used for prognostic analyses.
IHC
IHC was performed as previously described (22,23).
Formalin-fixed, paraffin-embedded human CRC tissue blocks were
sectioned at 4-μm thickness. All slides were deparaffinized with
xylene and rehydrated with alcohol. Antigen retrieval was achieved
by pressure-cooking with target retrieval solution (EarthOx, LLC,
San Francisco, CA, USA) for 8 min. The sections were rinsed with
TBS and blocked in buffer for 30 min in a wet box at 37°C. For
ALDH1 and CD133 staining, sections were respectively incubated at
4°C overnight with anti-ALDH1 and -CD133 solution (1:400; EarthOx,
LLC). Slides were then incubated with secondary antibody and,
following incubation, sections were rinsed three times with TBS-T.
Next, DAB solution was used to colorize the specimens, prior to
dehydrating, clearing and mounting with neutral gums. Finally, the
samples were examined by microscopy (ECL1 PSE 80i; Nikon, Tokyo,
Japan).
Evaluation of labeling
Imaging analysis of the colorectal tumors for ALDH1
and CD133 expression was performed in three to seven randomly
selected high-power fields (magnification, ×200) per case. Staining
intensity was scored as follows: 0, negative; 1, weak; 2, moderate;
and 3, strong. The positively stained area (distribution) was
expressed as the percentage of the whole area under evaluation and
scored as follows: 0, no staining; 1, 1–20% positive cells; 2,
21–50% positive cells; 3, 51–80% positive cells; and 4, 81–100%
positive cells. It was assured that >20% of tumor cells showing
ALDH1 or CD133 staining were positive. Immunohistochemical
evaluation of ALDH1 or CD133 expression was performed independently
by two pathologists blinded to the patients’ clinical and
pathological information. Discrepancies between the pathologists
were resolved by consensus.
Statistical analysis
All data were analyzed using SPSS software for
Windows, version 19.0 (SPSS Inc., Chicago, IL, USA). The
correlation between the immunohistochemical staining of the markers
(ALDH1 or CD133) and the clinicopathological parameters was
evaluated by the χ2 test, and P<0.05 was considered
to indicate a statistically significant difference. The correlation
between the expression of ALDH1 and CD133 was assessed by
Spearman’s rank test, and P<0.001 was considered to indicate a
statistically significant difference. The Kaplan-Meier method was
used to estimate the OS of patients, and the correlation between
survival differences and expression of ALDH1 or CD133 was analyzed
by the log-rank test.
Results
Patient characteristics
A total of 60 patients were enrolled in the present
study, with 50% males (30 out of 60) and 50% females (30 out of
60). The median age of the patients was 51.6 years (range, 32–68
years) and the male to female ratio was 1:1. Of all patients, 25
(41.7%) were ≥60 years old and 35 (58.3%) were <60 years old.
The diagnosis of all patients was colorectal adenocarcinoma.
Patients were classified with well-, moderately or poorly
differentiated tumor cells. Out of the 60 patients, there were 20
(33.3%) with well-differentiated, 20 (33.3%) with moderately
differentiated and 20 (33.3%) with poorly differentiated
adenocarcinoma. In total, 24 patients (40%) were at Dukes’ stages A
and B, while 36 patients (60%) were at Dukes’ stages C and D. In
addition, there were eight (13.3%) TNM stage I, 16 (26.7%) TNM
stage II, 28 (46.7%) TNM stage III and eight (13.3%) TNM stage IV
patients. Lymph node metastases were present in 34 patients (56.7%)
and absent in 26 patients (43.3%) (Table I).
 | Table IPatient characteristics. |
Table I
Patient characteristics.
| Clinical data | Value |
|---|
| Patients, n (%) | 60 (100.0) |
| Male | 30 (50.0) |
| Female | 30 (50.0) |
| Age, years |
| ≥60, n (%) | 25 (41.7) |
| <60, n (%) | 35 (58.3) |
| Median (range) | 51.6 (32–68) |
| Differentiation, n
(%) |
| Well | 20 (33.3) |
| Moderate | 20 (33.3) |
| Poor | 20 (33.3 |
| Dukes’ stage, n
(%) |
| A and B | 24 (40.0) |
| C and D | 36 (60.0) |
| TNM stage, n (%) |
| I | 8 (13.3) |
| II | 16 (26.7) |
| III | 28 (46.7) |
| IV | 8 (13.3) |
| Lymph node
metastasis, n (%) |
| Positive | 34 (56.7) |
| Negative | 26 (43.3) |
Expression of ALDH1 and CD133 in normal
colorectal and CRC tissue
CRC tissue exhibited significantly higher levels of
ALDH1 and CD133 protein expression compared with normal colorectal
tissue (P<0.05). ALDH1 and CD133 were detected in the cytoplasm
of CRC tissue. In the 60 normal controls, ALDH1 reactivity was
demonstrated in ~10% of the epithelial cells and the CD133
expression rate was <20% of the total cells. Of the 60 CRC
specimens, high levels of staining were identified in the samples
(≥20%). Patients exhibiting >20% positive cells were classified
as ALDH1- or CD133-positive and the remainder as negative. The
results of immunostaining for ALDH1 and CD133 in the normal
colorectal and CRC tissues are shown in Table II.
 | Table IIComparison of ALDH1- and
CD133-positive expression between colorectal cancer and normal
colorectal tissues. |
Table II
Comparison of ALDH1- and
CD133-positive expression between colorectal cancer and normal
colorectal tissues.
| Colorectal
tissue | n | ALDH1-positive, n
(%) | χ2 | P-value | CD133-positive, n
(%) | χ2 | P-value |
|---|
| Cancer | 60 | 31 (51.7) | | | 28 (46.7) | | |
| Normal | 60 | 15 (25.0) | 4.51 | <0.05 | 13 (21.7) | 4.168 | <0.05 |
Correlation between the positive
expression of ALDH1 and CD133 and clinicopathological
characteristics of CRC
No significant correlation was identified between
ALDH1 and CD133 expression and patient age, gender and tumor size.
ALDH1 expression was closely associated with tumor cell
differentiation and Dukes’ and TNM staging. It was found that with
an improved degree of differentiation, the tumor cells exhibited a
lower rate of ALDH1 staining. A marked positive correlation was
observed between the degree of differentiation and proportion of
ALDH1 immunostaining in tumor cells (χ2=8.918;
P<0.05) among the tested samples. A higher ALDH1 band intensity
was detected in poorly differentiated malignant tumor cells
compared with the well- or moderately differentiated tumor cells
(Figs. 1A and B). ALDH1 expression
was noted in 5 (25%) of the 20 well-differentiated cases, 12 (60%)
of the 20 moderately differentiated cases and 14 (70%) of the 20
poorly differentiated cases. Patients with Dukes’ stages C and D
showed a higher expression rate of ALDH1 (24/36; 66.7%) compared
with those with Dukes’ stages A and B (7/24; 29.2%) (P<0.05).
Patients with TNM stages III and IV exhibited greater positive
staining for ALDH1 compared with those with TNM stages I and II,
similar to Dukes’ stages C and D compared with Dukes’ stages A and
B (Figs. 1C and D). No association
was identified between lymph node metastasis and ALDH1 expression.
By contrast, CD133 expression exhibited no association with tumor
cell differentiation, but its expression was closely associated
with lymph node metastasis. Positive lymph node metastasis was
identified in 20 of the 34 CD133-positive cases (58.8%); however,
negative lymph node metastasis was identified in only eight of the
26 CD133-positive cases (30.8%; P<0.05). Furthermore, CD133
expression exhibited a correlation with Dukes’ and TNM staging. The
expression of CD133 in Dukes’ stage C and D or TNM stage III and IV
was evidently higher compared with that in Dukes’ stage A and B or
TNM stage I and II adenocarcinomas (χ2=4.92; P<0.05;
Fig. 2). The correlation between
the ALDH1 and CD133 proteins and clinicopathological features of
CRC patients are summarized in Table
III.
 | Table IIICorrelation between the positive
expression of ALDH1 and CD133 and clinicopathological
characteristics of human colorectal cancer. |
Table III
Correlation between the positive
expression of ALDH1 and CD133 and clinicopathological
characteristics of human colorectal cancer.
| Variables | n | ALDH1-positive,
n | χ2 | P-value | CD133-positive,
n | χ2 | P-value |
|---|
| Gender |
| Male | 30 | 17 | 0.6000 | >0.05 | 15 | 0.2678 | >0.05 |
| Female | 30 | 14 | | | 13 | | |
| Age, years |
| ≥60 | 25 | 15 | | | 13 | | |
| <60 | 35 | 16 | 1.1910 | >0.05 | 15 | 0.4898 | >0.05 |
| Tumor size, cm |
| ≤3 | 22 | 10 | | | 13 | | |
| >3 | 38 | 21 | 0.5370 | >0.05 | 15 | 2.1500 | >0.05 |
|
Differentiation |
| Well | 20 | 5 | | | 12 | | |
| Moderate | 20 | 12 | 8.9180 | <0.05 | 10 | 3.7500 | >0.05 |
| Poor | 20 | 14 | | | 6 | | |
| Dukes’ stage |
| A and B | 24 | 7 | | | 7 | | |
| C and D | 36 | 24 | 8.1000 | <0.05 | 21 | 4.9200 | <0.05 |
| TNM stage |
| I and II | 24 | 7 | | | 7 | | |
| III and IV | 36 | 24 | 8.1000 | <0.05 | 21 | 4.9200 | <0.05 |
| Lymph node
metastasis |
| Positive | 34 | 17 | | | 20 | | |
| Negative | 26 | 14 | 0.0873 | >0.05 | 8 | 4.659 | <0.05 |
Correlation between ALDH1 and CD133
expression in human CRC
Of the total 60 human specimens, ALDH1- and
CD133-positive expression was identified in 12 cases, while
negative expression was identified in 15 cases. In addition, 19
patients were identified as ALDH1-positive, but CD133-negative. By
contrast, 14 patients were identified as ALDH1-negative and
CD133-positive. Spearman’s rank correlation analysis showed that
ALDH1 expression and CD133 expression in CRC are significantly
positively correlated (r=0.241; P=0.0322; Table IV).
 | Table IVCorrelation between ALDH1 and CD133
expression and colorectal cancer. |
Table IV
Correlation between ALDH1 and CD133
expression and colorectal cancer.
| CD133 | | |
|---|
|
| | |
|---|
| ALDH1 | Positive | Negative | r value | P-value |
|---|
| Positive | 12 | 19 | | |
| Negative | 14 | 15 | 0.241 | 0.0322 |
| Total | 26 | 34 | | |
Kaplan-Meier survival analysis
Among the 60 study patients, ALDH1-positive patients
showed shorter survival times compared with ALDH1-negative patients
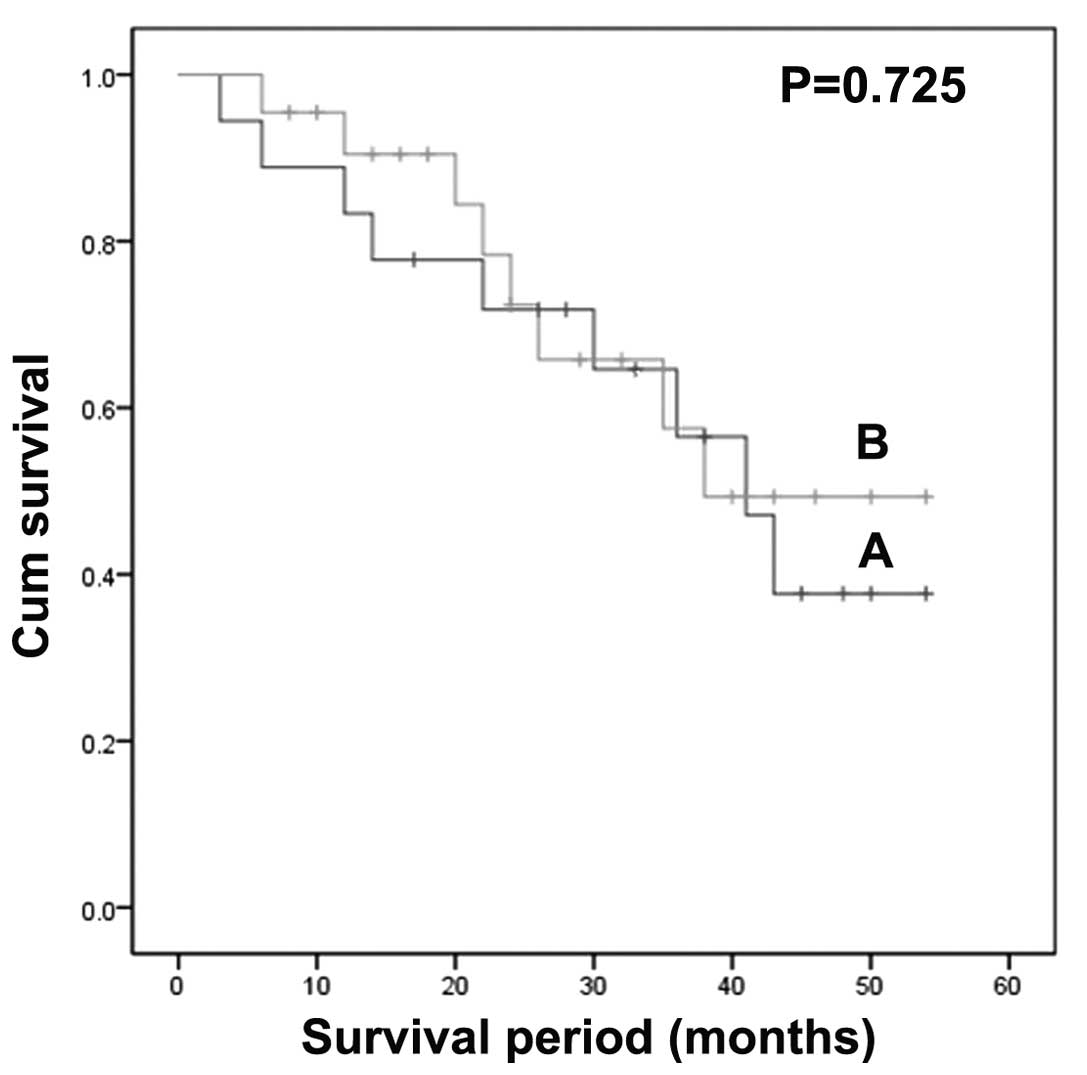
(log-rank test; χ2=4.34; P=0.037; Fig. 3). However, by contrast,
CD133-positive patients did not have significantly higher survival
times than CD133-negative patients (log-rank test;
χ2=0.124; P=0.725; Fig.
4).
Discussion
CRC is one of the most common types of malignant
tumor worldwide (1). Furthermore,
it is a complicated and multifactorial process. Hamburger and
Salmon first proposed the CSC theory in 1977 (4) and demonstrated that only a small
percentage of tumor cells have the capability to form colonies in
soft agar. Therefore, therapies are only required to target CSCs.
According to the CSC hypothesis, cancer originates from uncommon
cells, SCs, which show pluripotency and self-renewal (5). CSCs are hypothesized to cause the
initiation, progression and recurrence of cancer. Fractionation of
cancer cells on the basis of displayed CSC surface markers has
yielded subpopulations of neoplastic cells with a greatly enhanced
ability, relative to the majority of corresponding populations, to
seed new tumors upon implantation in immunodeficient mice.
ALDH1 is a common expression marker of CSCs and SCs
(8). The present study found that
compared with normal colorectal tissues, tumor tissues expressed a
high ALDH1 staining rate (≥20%; P<0.05). Additionally, a marked
correlation between the differentiation degree and expression of
ALDH1 was demonstrated. Through IHC staining, it was demonstrated
that as the differentiation degree worsened (from well- to poorly
differentiated), the ALDH1 staining rate increased
(χ2=8.918; P<0.05). These results are consistent with
a previous study by Huang et al, who confirmed that the
overall proliferative cell population (SCs and rapidly
proliferating cells) increases during colorectal tumorigenesis
(8). An additional important
observation of the present study was that low-grade tumors
exhibited a higher expression of ALDH1 staining compared with
high-grade tumors (P<0.05). This result was consistent with
Dukes’ staging in the current study. It was also demonstrated that
the ALDH1-positive patients exhibited shorter survival times than
ALDH1-negative patients (P=0.034). These results imply that ALDH1
may serve as a marker for CSCs to distinguish colorectal
carcinogenesis from normal colorectal tissues and to anticipate
patient prognosis.
In the present study, CD133 was found to be
infrequently expressed in normal colorectal tissues compared with
the tumor tissues. This result is consistent with that of a
previous study by O’Brien et al(17). In addition, the present study
identified that age, gender, tumor size and histological grade were
independent of CD133 expression levels, consistent with a previous
study by Horst et al(24).
However, it was found that CD133 closely correlates with tumor
stage or Dukes’ stage; the higher the stage, the higher the rate of
CD133 staining. Specimens positive for lymph node metastasis
demonstrated higher expression rates of CD133 compared with
negative cases. Although, through survival analysis, no significant
correlation was found between the expression of CD133 and patient
survival period. This result contradicts that of a previous study
by Ieta et al(20)
demonstrating that overexpression of CD133 was correlated with a
poor prognosis. However, the results of the present study show that
CD133-negative expression also affected patient survival times,
which is consistent with Schmelkov et al, who previously
demonstrated that CD133-negative colon cancer cells also have the
ability to initiate tumors (25).
In conclusion, ALDH1 and CD133 as markers of CSCs
are found in CRC tissues. Their expression is closely associated
with the prognosis and clinicopathological characteristics of
patients with CRC. However, whether the use of a single CSC marker
to identify CSCs is sufficient, remains an important question.
Therefore, the joint detection of CSC marker expression in patients
is likely to be useful to improve predictions for the prognosis of
the disease and understanding of the clinicopathological
characteristics. Future studies are required to conduct further
studies on larger samples, since few CSCs cause tumor relapse and
metastasis. The specific markers of CSCs not only accurately detect
‘real’ CSCs, but also develop a novel treatment strategy targeting
CSCs in human CRC.
Acknowledgements
The authors would like to thank Dr Ying Wu of Red
Flag Hospital Affiliated to Mudanjiang Medical College for
contributing to the experiments and results, and Ms. Jia-Ning Qiu
of Ji Lin University for contributing to the language. The present
study was supported by project grants from the Science and
Technology Department of Heilongjiang Province in China (no.
D201259).
References
|
1
|
Center MM, Jemal A and Ward E:
International trends in colorectal cancer incidence rates. Cancer
Epidemiol Biomarkers Prev. 18:1688–1694. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jemal A, Siegel R, Xu J and Ward E: Cancer
statistics, 2010. CA Cancer J Clin. 60:277–300. 2010. View Article : Google Scholar
|
|
3
|
Peppone LJ, Mahoney MC, Cummings KM,
Michalek AM, Reid ME, Moysich KB and Hyland A: Colorectal cancer
earlier in those exposed to tobacco smoke: implication for
screening. J Cancer Res Clin Oncol. 134:743–751. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hamburger AW and Salmon SE: Primary
bioassay of human tumor stem cells. Science. 197:461–463. 1977.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Boman BM and Wicha MS: Cancer stem cells:
a step toward the cure. J Clin Oncol. 26:2795–2799. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Abdul Khalek FJ, Gallicano GI and Mishra
L: Colon cancer stem cells. Gastrointest Cancer Res. Nov;(Suppl 1):
S16–S23. 2010.
|
|
7
|
Sophos NA and Vasiliou V: Aldehyde
dehydrogenase gene superfamily: the 2002 update. Chem Biol
Interact. 143–144:5–22. 2003.PubMed/NCBI
|
|
8
|
Huang EH, Hynes MJ, Zhang T, Ginestier C,
Dontu G, Appelman H, et al: Aldehyde dehydrogenase 1 is a marker
for normal and malignant human colonic stem cells (SC) and tracks
SC overpopulation during colon tumorigenesis. Cancer Res.
69:3382–3389. 2009. View Article : Google Scholar
|
|
9
|
Patel M, Lu L, Zander DS, Sreerama L, Coco
D and Moreb JS: ALDH1A1 and ALDH3A1 expression in lung cancers:
correlation with histologic type and potential precursors. Lung
Cancer. 59:340–349. 2008. View Article : Google Scholar
|
|
10
|
Nogami T, Shien T, Tanaka T, Nishiyama K,
Mizoo T, Iwamto T, et al: Expression of ALDH1 in axillary lymph
node metastases is a prognostic factor of poor clinical outcome in
breast cancer patients with 1–3 lymph node metastases. Breast
Cancer. Mar 10–2012.(Epub ahead of print).
|
|
11
|
Corbeil D, Roper K, Hellwig A, Tavian M,
Miraglia S, Watt SM, et al: The human AC133 hematopoietic stem cell
antigen is also expressed in epithelial cells and targeted to
plasma membrane protrusions. J Biol Chem. 275:5512–5520. 2000.
View Article : Google Scholar
|
|
12
|
Singh SK, Hawkins C, Clarke ID, Squire JA,
Bayani J, Hide T, et al: Identification of human brain tumor
initiating cells. Nature. 432:396–401. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Collins AT, Berry PA, Hyde C, Stower MJ
and Maitland NJ: Prospective identification of tumorigenic prostate
cancer stem cells. Cancer Res. 65:10946–10951. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yin S, Li J, Hu C, Chen X, Yao M, Yan M,
et al: CD133 positive hepatocellular carcinoma cells possess high
capacity for tumorigenicity. Int J Cancer. 120:1444–1450. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hermann PC, Huber SI, Herrler T, Aicher A,
Ellwart JW, Guba M, et al: Distinct populations of cancer stem
cells determine tumor growth and metastatic activity in human
pancreatic cancer. Cell Stem Cell. 1:313–323. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Eramo A, Lotti F, Sette G, Pilozzi E,
Biffoni M, Di Virgilio A, et al: Identification and expansion of
the tumorigenic lung cancer stem cell population. Cell Death
Differ. 15:504–514. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
O’Brien CA, Pollett A, Gallinger S and
Dick JE: A human colon cancer cell capable of initiating tumor
growth in immunodeficient mice. Nature. 445:106–110.
2007.PubMed/NCBI
|
|
18
|
Ricci-Vitiani L, Lombardi DG, Pilozzi E,
Biffoni M, Todaro M, Peschle C and De Maria R: Identification and
expansion of human colon-cancer-initiating cells. Nature.
445:111–115. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Todaro M, Alea MP, Di Stefano AB,
Cammareri P, Vermeulen L, Lovino F, et al: Colon cancer stem cells
dictate tumor growth and resist cell death by production of
interleukin-4. Cell Stem Cell. 1:389–402. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ieta K, Tanaka F, Haraguchi N, Kita Y,
Sakashita H, Mimori K, et al: Biological and genetic
characteristics of tumor-initiating cells in colon cancer. Ann Surg
Oncol. 15:638–648. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Horst D, Kriegl L, Engel J, Kirchner T and
Jung A: CD133 expression is an independent prognostic marker for
low survival in colorectal cancer. Br J Cancer. 99:1285–1289. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang T, Otevrel T, Gao Z, Gao Z, Ehrlich
SM, Fields JZ and Boman BM: Evidence that APC regulates surviving
expression: a possible mechanism contributing to the stem cell
origin of colon cancer. Cancer Res. 61:8664–8667. 2001.
|
|
23
|
Boman BM, Walters R, Fields JZ, Kovatich
AJ, Zhang T, Isenberg GA, et al: Colonic crypt changes during
adenoma development in familial adenomatous polyposis:
immunohistochemical evidence for expansion of the crypt base cell
population. Am J Pathol. 165:1489–1498. 2004. View Article : Google Scholar
|
|
24
|
Horst D, Sheel SK, Liebmann S, Neumann J,
Maatz S, Kirchner T and Jung A: The cancer stem cell marker CD133
has high prognostic impact but unknown functional relevance for the
metastasis of human colon cancer. J Pathol. 219:427–434. 2009.
View Article : Google Scholar
|
|
25
|
Shmelkov SV, Butler JM, Hooper AT, Hormigo
A, Kushner J, Milde T, St Clair R, et al: CD133 expression is not
restricted to stem cells and both CD133+ and
CD133− metastatic colon cancer cells initiate tumors. J
Clin Invest. 118:2111–2120. 2008.PubMed/NCBI
|