Introduction
Colorectal cancer (CRC) is the third most common
cancer and the fourth most frequent cause of cancer-related
mortality worldwide. Colon cancer does not exhibit a gender
preference, but rectal cancer is 20–50% more prevalent in males.
Despite advances in early diagnosis and treatment, 30–40% of
patients with CRC succumb to the disease (1). In Brazil, CRC is the fifth most
frequent cause of mortality in males and the third in females
(2).
A sequence of mutations in the genes involved in
cell cycle control and apoptosis transform normal mucosa cells into
malignant cells (3). Advances in
molecular biology techniques have permitted an improved
understanding of the genetic and molecular mechanisms involved in
cancer development. Nevertheless, the initial event responsible for
the malignant transformation of normal cells remains unclear. The
gene transcriptional effect is inhibited by hypermethylation in the
promoter region and oxidative damage to nuclear DNA, which are two
of the main mechanisms associated with the early stages of
colorectal carcinogenesis (4).
A single nucleotide polymorphism (SNP) is a genetic
change in a nucleotide of the DNA sequence that occurs in >1% of
the population (5). SNPs usually
occur in non-coding regions, however, SNPs that occur in the coding
regions of genes are frequently associated with various diseases,
including cancer (5). Numerous
genetic alterations, including polymorphisms in the cyclooxygenase
(COX)-2 and 5-lipoxygenase (LOX) genes, have been associated with
an increased risk of CRC and a poor prognosis, as well as a high
fat intake, which increases the risk of developing tumors due to
arachidonic acid metabolism that produces proinflammatory
substances (6). There are three
main enzymes involved in the metabolism of arachidonic acid, the
COXs, the LOXs and cytochrome P450 (7). These genes are involved in cell cycle
regulation, tumor growth and prostaglandin production. COX
catalyzes the transformation of arachidonic acid into endoperoxide,
which is enzymatically converted into substances that are involved
in platelet aggregation, blood vessel dilatation and chemotaxis
(8).
There are three isoforms of COX (COX-1, -2 and -3),
and COX-2 has been shown to play a role in the carcinogenesis of
colon, breast, prostate and esophageal cancer (?). In 2002, the
COX-2 gene -765G>C polymorphism was described by Papafili et
al and associated with a higher susceptibility to cancer
(9). Resistance to apoptosis
mediated by COX-2 is a central mechanism of tumorigenesis (10).
LOX, described in 1976 (11), is an additional enzyme involved in
the transformation of arachidonic acid into prostaglandins and
prostacyclins, and has been associated with a risk of CRC. Studies
investigating isoforms of LOX that play a role in the control
mechanisms of cancer emergence and progression have increased over
the past years (12). The synthesis
of proinflammatory mediators, such as leukotrienes, are catalyzed
by LOX. Leukotrienes stimulate colon cell proliferation and inhibit
apoptosis (13).
The aim of the present study was to analyze the
-765G>C polymorphism in the COX-2 gene and the -1708G>A
polymorphism in the 5-LOX gene in patients with CRC, and to
correlate these polymorphisms with lifestyle and dietary
habits.
Patients and methods
Patients and lifestyle habits
In a prospective sequential study, patients with CRC
were compared with a control group selected from subjects without
cancer or gastrointestinal symptoms observed during routine
examination at the Central Laboratory of the Federal University of
São Paulo (São Paulo, Brazil). All patients were born in Brazil and
were treated by the Oncology Group of the Paulista School of
Medicine (São Paulo, Brazil) between March 2009 and December 2010.
Colorectal adenocarcinoma of the colon or rectum was confirmed by
the pathologist. The study was approved by the Ethics Committee of
the Federal University of São Paulo and all patients signed an
informed written consent form.
Patients and controls answered a questionnaire
concerning lifestyle habits, including smoking status (non-smokers
and current and former smokers), alcohol consumption and physical
activity. The frequency of meat, fruit, vegetable and fat intake,
and the use of non-steroidal anti-inflammatory drugs (NSAIDs), was
also evaluated. The body mass index (BMI; kg/m2) was
calculated and the subjects were classified as malnourished,
well-nourished or overweight. Peripheral blood was collected for
extraction of genomic DNA.
Analysis of the COX-2 -765G>C
polymorphism
Leukocyte DNA was extracted from peripheral venous
blood collected with ethylenediaminetetraacetic acid using the
Qiagen® Spin Blood Mini kit (Qiagen, Hilden, Germany).
The COX-2 gene polymorphism was investigated by polymerase chain
reaction (PCR) and restriction fragment length polymorphism (RFLP)
analysis. The results were confirmed by genotyping in an ABI Prism
3100 genetic analyzer (Applied Biosystems, Carlsbad, CA, USA).
Genomic DNA was amplified using the following primers: Forward,
5′-ATTCTGGCCATCGCCGCTTC-3′ and reverse,
5′-CTCCTTGTTTCTTGGAAAGAGACG-3′.
Following amplification, the PCR products were
digested with Acil (New England Biolabs, Ipswich, MA, USA). The
digestion products were separated on 2% agarose gels stained with
ethidium bromide and visualized under ultraviolet light. The GG, GC
and CC genotypes were analyzed in the two groups.
Analysis of the 5-LOX 1708G>A
polymorphism
The following primers were used for amplification of
the 5-LOX 1708G>A polymorphism: Forward, 5′-GCACTGTATAGCATGTAC
ATTA-3′ and reverse, 5′-CGTGACCCATTTTGAGTTAG-3′. The PCR products
were purified using the QIAquick PCR Purification kit (Qiagen) and
sequenced in an ABI Prism 3100 genetic analyzer (Applied
Biosystems). The Sequence Scanner v1.0 program (Applied Biosystems)
was used for electropherogram analysis.
Statistical analysis
Statistical analyses were performed using SPSS
version 16.0 (SPSS Inc., Chicago, IL, USA). Student’s t-test was
used to compare ages between the groups. Differences in the
polymorphisms between the two groups were determined by the
χ2 test. This test was also used to compare clinical and
epidemiological variables between COX-2 and 5-LOX genotypes and
alleles in the group of cancer patients. Odds ratios (ORs) and 95%
confidence intervals (CIs) were calculated to evaluate the
association between the risk of developing cancer and these
variables. Multivariate logistic regression was performed to
identify risk factors. P<0.05 was considered to indicate a
statistically significant difference and a confidence interval of
95% were used.
Results
Patient analysis
A total of 185 patients with CRC and 146 control
subjects were studied. The mean age of patients with CRC was 62.7
years (standard deviation=13.1) and there were 99 females. No
difference in age, gender or BMI was observed between the groups.
The frequency of smokers (17%) and subjects practicing physical
activity (44%) was higher in the control group. No differences were
identified in the consumption of alcohol (P=0.391), fruits
(P=0.706), vegetables (P=0.577), cereals (P=0.935) or red meat
(P=0.495) between the groups. By contrast, fat intake was higher in
the cancer patients (P=0.05) (Table
I).
 | Table ICharacteristics of patients and OR for
CRC according to fat intake, physical activity and alcohol
consumption. |
Table I
Characteristics of patients and OR for
CRC according to fat intake, physical activity and alcohol
consumption.
| Variable | Cases, n (%) | Controls, n (%) | P-valuea | ORb (95% CI) | P-valuea | ORc (95% CI) |
|---|
| Gender |
| Male | 86 (46.5) | 71 (48.6) | 0.698 | | | |
| Female | 99 (53.5) | 75 (51.4) | | | | |
| Age, years |
| Male | 62.0±12.7 | 61.2±15.6 | 0.357d | | | |
| Female | 63.4±13.4 | 60.8±15.9 | 0.433d | | | |
| Total | 62.7±13.1 | 61.0±15.8 | 0.543d | | | |
| Fat intakee |
| Low | 10 (5.4) | 12 (8.2) | | 1.00 Reference | | 1.00 Reference |
| Medium | 90 (48.7) | 97 (66.4) | 0.699 | 1.23 (0.42–3.60) | 0.812 | 1.11 (0.46–2.70) |
| High | 85 (45.9) | 37 (25.4) | 0.050 | 3.11 (1.00–9.69) | 0.031 | 2.76 (1.09–6.64) |
| Physical
activity |
| Yes | 34 (18.4) | 66 (45.2) | | 1.00 Reference | | 1.00 Reference |
| No | 151 (81.6) | 80 (54.8) | <0.001 | 3.83 (2.08–7.06) | <0.001 | 3.66 (2.23–6.01) |
| NSAIDs use |
| Yes | 1 (0.5) | 44 (30.1) | | 1.00 Reference | | 1.00 Reference |
| No | 184 (99.5) | 102 (69.9) | <0.001 | 203
(17.8–2327) | <0.001 | 79.4
(10.8–584) |
| Alcohol
drinker |
| No | 134 (72.4) | 103 (70.6) | | 1.00 Reference | | 1.00 Reference |
| Yes | 51 (27.6) | 43 (29.4) | 0.706 | 0.47
(0.40–1.38) | 0.706 | 0.91
(0.56–1.47) |
| Cereals |
| Yes | 87 (47.1) | 68 (46.6) | | 1.00 Reference | | 1.00 Reference |
| No | 98 (52.9) | 78 (53.4) | 0.916 | 0.97(
0.57–1.67) | 0.935 | 0.98
(0.64–1.52) |
| Fruitse |
| High | 110 (59.4) | 83 (56.9) | | 1.00 Reference | | 1.00 Reference |
| Medium | 69 (37.2) | 61 (41.8) | 0.610 | 0.89
(0.56–1.41) | 0.487 | 0.85
(0.55–1.33) |
| Low | 6 (3.4) | 2 (1.4) | 0.178 | 3.13
(0.60–16.4) | 0.325 | 2.26
(0.45–11.5) |
| Vegetablese |
| High | 104 (56.2) | 80 (54.8) | | 1.00 Reference | | 1.00 Reference |
| Medium | 69 (37.3) | 61 (41.8) | 0.811 | 0.93
(0.54–1.62) | 0.546 | 0.87
(0.55–1.37) |
| Low | 12 (6.5) | 5 (3.4) | 0.095 | 3.33
(0.81–13.7) | 0.267 | 1.85
(0.62–5.45) |
| Cancer site |
| Colon | 103 (55.3) | | | | | |
| Rectum | 83 (44.7) | | | | | |
| Cancer stage |
| I | 28 (15.1) | | | | | |
| II | 84 (45.4) | | | | | |
| III | 52 (28.1) | | | | | |
| IV | 21 (11.4) | | | | | |
Genotype distribution
In the control group, the genotype distribution of
the two polymorphisms was in accordance with the Hardy-Weinberg
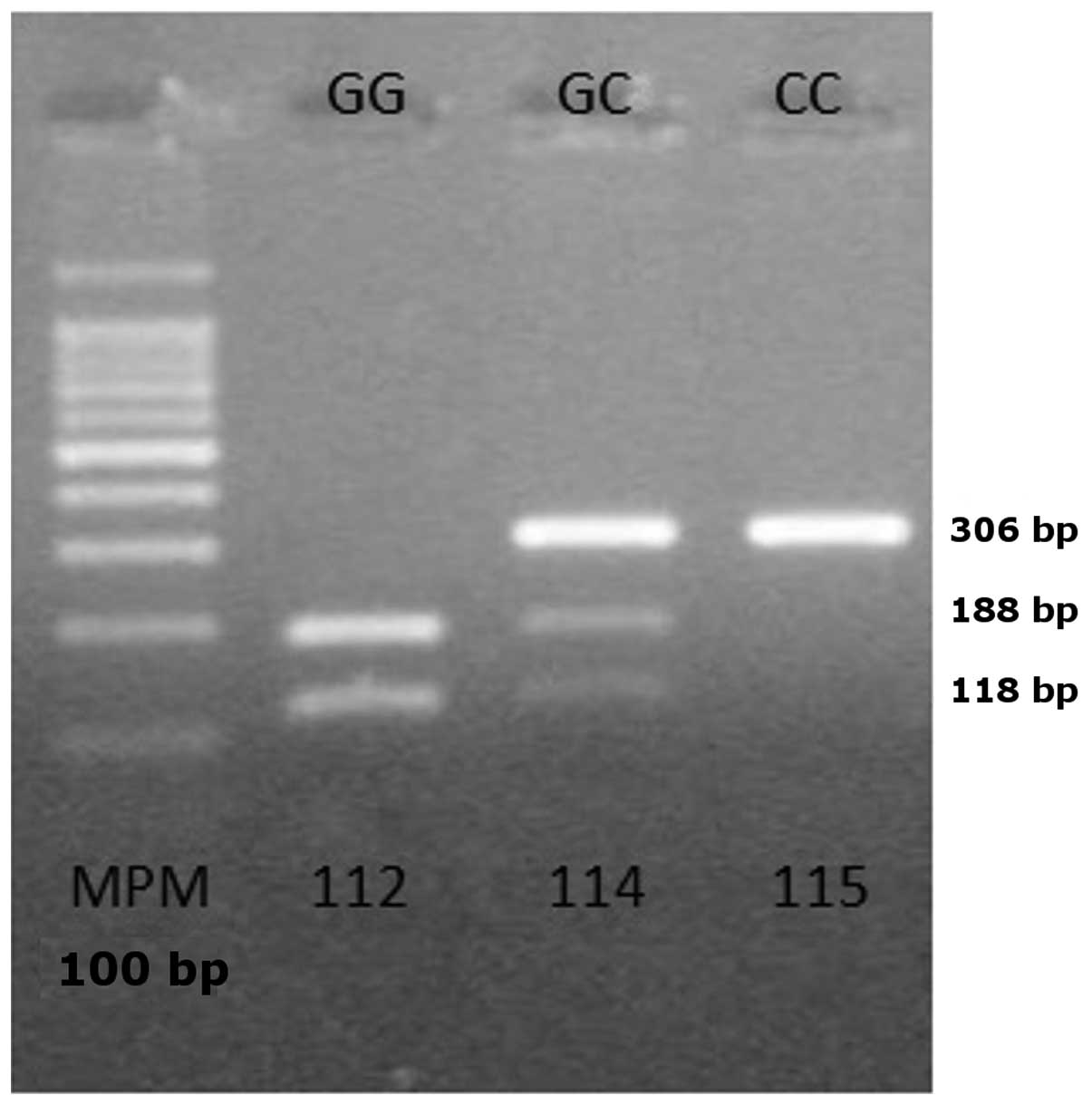
equilibrium (P>0.05). Analysis of the COX-2 polymorphism by
PCR-RFLP revealed two bands of 118 and 188 bp in subjects carrying
the homozygous wild-type genotype (GG), three bands of 306, 188 and
118 bp in subjects carrying the heterozygous genotype (GC) and one
band of 306 bp in subjects with the homozygous mutant genotype (CC)
(Fig. 1).
The heterozygous GC genotype was the most common in
the two groups. The CC genotype was more frequent in the cancer
group (P=0.013) and was associated with an increased risk of CRC
(OR, 2.20; 95% CI, 1.02–4.76). Allele C was also associated with a
higher risk of cancer (Table II).
The PCR-RFLP results of COX-2 were confirmed in specific samples by
sequencing (Fig. 2).
 | Table II5-LOX and COX-2 genotypes of patients
and OR for CCR. |
Table II
5-LOX and COX-2 genotypes of patients
and OR for CCR.
| Genotype | n (%) | n (%) | P-value | ORa (95% CI) | P-value | ORb (95% CI) |
|---|
| 5-LOX | 185 | 146 | | | | |
| G/G | 134 (72.4) | 104 (71.2) | 0.723c | 1.00 Reference | 0.970c | 1.00 Reference |
| G/A | 46 (24.9) | 38 (26.1) | 0.229 | 0.68
(0.36–1.28) | 0.807 | 0.94
(0.57–1.55) |
| A/A | 5 (2.7) | 4 (2.7) | 0.940 | 0.94
(0.19–4.75) | 0.965 | 0.97
(0.25–3.70) |
| COX-2 |
| G/G | 49 (26.5) | 56 (38.4) | 0.004c | 1.00 Reference | 0.050c | 1.00 Reference |
| G/C | 111 (60.0) | 77 (52.7) | 0.015 | 2.11
(1.16–3.83) | 0.042 | 1.65
(1.02–2.67) |
| C/C | 25 (13.5) | 13 (8.9) | 0.013 | 3.63
(1.31–10.1) | 0.046 | 2.20
(1.02–4.76) |
| G Allele | 209 (65.5) | 189 (70.5) | | 1.00 Reference | | 1.00 Reference |
| C Allele | 161 (43.5) | 103 (29.5) | <0.001 | 1.94
(1.14–3.31) | <0.05 | 1.41
(1.03–1.94) |
| G/C + C/C | 136 (73.5) | 90 (61.6) | | 1.00 Reference | | 1.00 Reference |
| G/G | 49 (26.5) | 56 (38.4) | 0.006 | 0.44
(0.25–0.79) | 0.021 | 0.58
(0.36–0.92) |
| G/C + G/G | 160 (86.5) | 133 (91.1) | | 1.00 Reference | | 1.00 Reference |
| C/C | 25 (13.5) | 13 (8.9) | 0.093 | 2.21
(0.88–5.56) | 0.191 | 1.60
(0.79–3.25) |
With regard to the 5-LOX polymorphism (Fig. 3), 72.4% of patients with CRC and
71.2% of control subjects were homozygous for the wild-type. No
difference was observed between groups (Table II).
Statistical analysis
Multivariate analysis showed an increased risk of
cancer in subjects who did not practice physical exercise or who
consumed fat ≥3 times/week. This analysis confirmed the CC genotype
as an independent risk factor for developing cancer (Table III).
 | Table IIIMultivariate logistic regression
analysis stratified by the selected variables. |
Table III
Multivariate logistic regression
analysis stratified by the selected variables.
| Variable | Cases, n (%) | Controls (n=146), n
(%) | P-value | ORa (95% CI) |
|---|
| Fat intakeb |
| Low | 10 (5.4) | 12 (8.2) | | 1.00 Reference |
| Medium | 90 (48.7) | 97 (66.4) | 0.952 | 1.04
(0.34–3.16) |
| High | 85 (45.9) | 37 (25.4) | 0.149 | 2.40
(0.73–7.89) |
| Physical
activity |
| Yes | 34 (18.4) | 66 (45.2) | | 1.00 Reference |
| No | 151 (81.6) | 80 (54.8) | <0.001 | 3.90
(2.04–7.43) |
| COX-2
genotypes |
| G/G | 49 (26.5) | 56 (38.4) | | 1.00 Reference |
| G/C | 111 (60.0) | 77 (52.7) | 0.010 | 2.30
(1.21–4.34) |
| C/C | 25 (13.5) | 13 (8.9) | 0.003 | 5.03
(1.74–14.6) |
Discussion
The present study investigated whether polymorphisms
of COX-2 and 5-LOX are associated with the risk of CRC, in addition
to the correlation between these polymorphisms and lifestyle.
A high intake of fats causes changes in the
intestinal flora that increase the concentration of bile acids,
cell proliferation and prostaglandin production and thus, the
inflammatory process (14). The
consumption of red meat has been associated with an increased
production of free radicals, which cause oxidative damage to
epithelial cells and genetic mutations (15). The patients of the current study
reported a high frequency of fat intake and a low frequency of
physical activity.
It is known that a sedentary lifestyle has been
associated with an increased cancer risk, and low levels of COX-2
expression have been observed in rats submitted to physical
exercise (16).
In the present study, the use of NSAIDs was more
common among the control subjects, possibly due to the high
prevalence of patients with cardiovascular disease in this group.
Previous epidemiological studies have shown that acetylsalicylic
acid is able to reduce the incidence of CRC by 40–50%. Among the
mechanisms of action of NSAIDs, four inhibitors of the receptor
activation of peroxisome γ into cellular DNA may be cited,
inactivating the genes responsible for development, metabolism,
cell growth and differentiation (17). Notably, in the present study,
smokers were more common in the control group, but no statistically
significant correlation was identified between smoking and the risk
of CRC. This may be explained by the method of collecting the
patient history, which depended on the questioning of patients on
their smoking habits and not the smoking index rate. Previously,
Pandey et al(18) found no
correlation between smoking and COX-2 polymorphisms in patients
with cancer of the cervix.
Although certain studies have previously reported an
association between alcohol, low vitamin intake and cancer, no
consensus exists in the literature (19). In the present study, no correlation
was identified between alcohol consumption and cancer risk.
COX-2 polymorphisms have been associated with an
increased risk of cancer. Among the 10 COX-2 polymorphisms that
have been associated with cancer risk, −765G>C and −1195A>G
are the most common in CRC (20,21).
The COX-2 gene −765G>C polymorphism has been shown to modify the
gene transcription that may cause an alteration in the binding
capacity of specificity protein 1 and consequently, an increased
expression of COX-2. The homozygous mutant CC genotype has been
associated with a high incidence of leukemia (22) and bladder (23), breast (24) and esophageal cancer (25). In the present study, the risk of CRC
was higher among subjects carrying the CC genotype even after
control for the other variables studied. In addition, Biramijamal
et al found a correlation between this polymorphism and an
increased risk of CRC (OR, 7.1; 95% CI, 1.03–5.26) (26). By contrast, a decreased risk of
developing hepatocarcinoma was observed in patients with the CC
genotype (27). A higher risk of
CRC and gastric cancer (28) has
been described for subjects carrying the wild-type GG genotype, but
this association has not been confirmed by others. Kristinsson
et al, studying COX-2 polymorphisms (765G>C and
1195A>G), observed that the GG/GG haplotype was more frequent in
esophageal adenocarcinoma (29). No
association of these polymorphisms was observed in the oral and
pharyngeal cancer risk (30).
In the current study, the mutant CC genotype of the
COX-2 gene polymorphism increased the risk of CRC by ~2-fold
(Table II) and by ~5-fold when
combined with physical inactivity and consumption of fried
foods.
Racial and ethnic differences between the
populations studied may explain these contradictory results since
the distribution of COX-2 polymorphisms differs considerably
between the populations.
The polymorphism in the 5-LOX gene, resulting in a
change of guanine to adenine at position 1,708, has been studied in
CRC (31) and lung, kidney,
bladder, pancreas, esophageal and prostate cancer. 5-LOX is an
enzyme that is involved in the control of cell proliferation and
apoptosis inhibition, and has been associated with CRC risk.
Previously, Jiang et al(32)
observed that the levels of 12- and 5-LOX were particularly high in
the tumors of patients who succumbed to breast cancer. In CRC
(33) and pancreatic cancer
(6), 5-LOX expression studied by
immunohistochemistry and RT-PCR was found to correlate with tumor
expansion and invasion of blood vessels.
Wang et al(34) previously reported an increased risk
of breast cancer in females with a high intake of n-6
polyunsaturated fats and the 1700G>A polymorphism in the 5-LOX
gene. In addition, Shen et al(35) described an increased risk of lung
cancer in the sputum of patients carrying the 12-LOX (GA)
polymorphism.
Although no association was identified between CRC
and the 5-LOX polymorphism in the present study, patients with
genotypes GA/AA exhibited a 2.5-fold higher chance of developing
cancer when they consumed meat >3 times per week. These
genotypes (GA/AA) were also associated with an increased CRC risk
in patients with a high intake of fats upon univariate
analysis.
In conclusion, the current study identified a
significant difference in the distribution of the COX-2 gene −765CC
polymorphism between patients with CRC and controls. The presence
of the −765CC genotype was associated with an increased risk of
CRC. The CC genotype mutated COX-2 increased the risk of CRC by ~2
times and was not associated with physical activity. In addition,
the intake of fried food increased the risk of CRC by 5 times.
However, future studies are required to investigate the possible
association of this polymorphism with the prognosis of CRC.
Acknowledgements
The present study was supported by a grant from the
São Paulo Research Foundation (no. 09/14388-5). The authors would
like to thank the volunteers who were involved in this
cross-sectional study.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Globocan 2008: Cancer incidence and
mortality worldwide. IARC CancerBase No. 10 Lyon, France: 2010,
http://globocan.iarc.fr.
Accessed August 31, 2011
|
|
2
|
Estimates 2012: Cancer incidence in
Brazil. INCA (National Cancer Institute); Rio de Janiero, Brazil:
pp. 34–35. 2012
|
|
3
|
Fearon ER and Vogelstein B: A genetic
model for colorectal tumorigenesis. Cell. 61:759–767. 1990.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fleisher AS, Esteller M, Harpaz N, et al:
Microsatellite instability in inflammatory bowel disease-associated
neoplastic lesions is associated with hypermethylation and
diminished expression of the DNA mismatch repair gene, hMLH1.
Cancer Res. 60:4864–4868. 2000.
|
|
5
|
Lodish H, Berk A, Zipursky SL, Matsudaira
P, Baltimore D and Darnell J: Genetic analysis in molecular
biology. Cal and Molecular Biology. Nader HB: Rio de Janeiro:
Revinter; pp. 255–293. 2002
|
|
6
|
Hennig R, Ding XZ, Tong WG, Schneider MB,
Standop J, Friess H, Büchler MW, Pour PM and Adrian TE:
5-Lipoxygenase and leukotriene B(4) receptor are expressed in human
pancreatic cancers but not in pancreatic ducts in normal tissue. Am
J Pathol. 161:421–428. 2002. View Article : Google Scholar
|
|
7
|
Williams CS, Mann M and DuBois RN: The
role of cyclooxygense in inflammation, cancer, and development.
Oncogene. 18:7908–7916. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Steele VE, Holmes CA, Hawk ET, Kopelovich
L, Lubet RA, Crowell JA, Sigman CC and Kelloff GJ: Lipoxygenase
inhibitors as potential cancer chemopreventives. Cancer Epidemol
Biomarkers Prev. 8:467–483. 1999.PubMed/NCBI
|
|
9
|
Papafili A, Hill MR, Brull DJ, McAnulty
RJ, Marshall RP, Humphries SE and Laurent GJ: Common promoter
variant in cyclooxygenase-2 represses gene expression: evidence of
role in acute-phase inflammatory response. Arterioscler Thromb Vasc
Biol. 22:1631–1636. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zhang X, Miao X, Tan W, Ning B, Liu Z,
Hong Y, Song W, Guo Y, Zhang X, Shen Y, Qiang B, Kadluabar FF and
Lin D: Identification of functional genetic variants in
cyclooxygenase-2 and their association with risk of esophageal
cancer. Gastroenterology. 129:565–576. 2005.PubMed/NCBI
|
|
11
|
Borgeat P, Hamberg M and Samuelsson B:
Transformation of arachidonic acid homo-gamma-linoleic acid by
rabbit polymorphonuclear leuckocytes. Monohydroxy acids from novel
lipoxygenases. J Biol Chem. 251:7816–7820. 1976.
|
|
12
|
Pidgeon GP, Lysaght J, Krishnamoorthy S,
Reynolds JV, O’Byrne K, Nie D and Honn KV: Lipoxygenase metabolism:
roles in tumor progression and survival. Cancer Metastasis Rev.
26:503–524. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Goel A and Boland CR: Recent insights into
the pathogenesis of colorectal cancer. Curr Opin Gastroenterol.
26:47–52. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fitzpatrick FA: Inflammation,
carcinogenesis, and cancer. Int Immunopharmacol. 1:1651–1667. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zell JA, Ziogas A, Bernstein L, Clarke CA,
Deapen D, Largent JA, Neuhausen SL, Stram DO, Ursin G and
Anton-Culver H: Meat consumption, nonsteroidal anti-inflamatory
drug use, and mortality among colorectal cancer patients in the
California Teachers Study. Cancer Prev Res. 3:865–875. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Demarzo MM, Martins LV, Fernandes CR,
Herrero FA, Perez SE, Turatti A and Garcia SB: Exercise reduces
inflammation and cell proliferation in rat colon carcinogenesis.
Med Sci Sports Exerc. 40:618–621. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Baron JA, Cole BF, Sandler RS, et al: A
randomized trial of aspirin to prevent colorectal adenomas. N Engl
J Med. 348:891–898. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Pandey S, Mittal RD, Srivastava M,
Srivastava K and Mittal B: Cyclooxygenase-2 gene polymorphisms and
risk of cervical cancer in a North Indian population. Int J Gynecol
Cancer. 20:625–630. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Hirano T: Alcohol consumption and
oxidative DNA damage. Int J Environ Res Public Health. 8:2895–2906.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hoff JH, te Morsche RH, Roelofs HM, van
der Logt EM, Nagengast FM and Peters WH: COX-2 polymorphisms
−765G-->C and −1195A-->G and colorectal cancer risk. World J
Gastroenterol. 15:4561–4565. 2009.
|
|
21
|
Xing LL, Wang ZN, Jiang L, Zhang Y, Xu YY,
Li J, Luo Y and Zhang X: Cyclooxygenase 2 polymorphism and
colorectal cancer: −765G>C variant modifies risk associated with
smoking and body mass index. World J Gastroenterol. 14:1785–1789.
2008.
|
|
22
|
Wang CH, Wu KH, Yang YL, Peng CT, Wang RF,
Tsai CW, Tsai RY, Lin DT, Tsai FJ and Bau DT: Association study of
cyclooxygenase 2 single nucleotide polymorphisms and childhood
acute lymphoblastic leukemia in Taiwan. Anticancer Res.
30:3649–3653. 2010.PubMed/NCBI
|
|
23
|
Gangwar R, Mandhani A, Mittal RD and
Pradesh U: Functional polymorphisms of cyclooxygenase-2 (COX-2)
gene and risk for urinary bladder cancer in North India. Surgery.
149:126–134. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Piranda DN, Festa-Vasconcellos JS, Amaral
LM, Bergmann A and Vianna-Jorge R: Polymorphisms in regulatory
regions of Cyclooxygenase-2 gene and breast cancer risk in
Brazilians: a case-control study. BMC Cancer. 10:6132010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Liang Y, Liu JL, Wu Y, Zhang ZY and Wu R:
Cyclooxygenase-2 polymorphisms and susceptibility to esophageal
cancer: a meta-analysis. Tohoku J Exp Med. 223:137–144. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Biramijamal F, Basatvat S, Hossein-Nezhad
A, Soltani MS, Akbari Noghabi K, Irvanloo G and Shamimi K:
Association of COX-2 promoter polymorphism with gastrointestinal
tract cancer in Iran. Biochem Genet. 48:915–923. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Akkız H, Bayram SI, Bekar A, Akgöllü E and
Ülger Y: Functional polymorphisms of cyclooxygenase-2 gene and risk
for hepatocellular carcinoma. Mol Cell Biochem. 347:201–208.
2011.PubMed/NCBI
|
|
28
|
Sitarz R, Leguit RJ, de Leng WW, Polak M,
Morsink FM, Bakker O, Maciejewski R, Offerhaus GJ and Milne AN: The
COX-2 promoter polymorphism −765 G>C is associated with
early-onset, conventional and stump gastric cancers. Mod Pathol.
21:685–690. 2008.
|
|
29
|
Kristinsson JO, van Westerveld P, te
Morsche RH, Roelofs HM, Wobbes T, Witteman BJ, Tan AC, van Oijen
MG, Jansen JB and Peters WH: Cyclooxygenase-2 polymorphisms and the
risk of esophageal adeno- or squamous cell carcinoma. World J
Gastroenterol. 15:3493–3497. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Peters WH, Lacko M, Te Morsche RH, Voogd
AC, Oude Ophuis MB and Manni JJ: COX-2 polymorphisms and the risk
for head and neck cancer in white patients. Head Neck. 31:938–943.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
In KH, Asano K, Beier D, Grobholz J, et
al: Naturally occurring mutations in the human 5-lipoxygenase gene
promoter that modify transcription factor binding and reporter gene
transcriptions. J Clin Invest. 99:1130–1137. 1997. View Article : Google Scholar
|
|
32
|
Jiang WG, Douglas-Jones A and Mansel RE:
Levels of expression of lipoxygenases and cyclooxygenase-2 in human
breast cancer. Prostaglandins Leukot Essent Fatty Acids.
69:275–281. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Soumaoro LT, Iida S, Uetake H, Ishiguro M,
Takagi Y, Higuchi T, Yasuno M, Enomoto M and Sugihara K: Expression
of 5-Lipoxygenase in human colorectal cancer. World J
Gastroenterol. 39:6355–6360. 2006.PubMed/NCBI
|
|
34
|
Wang J, John EM and Ingles SA:
5-lipoxygenase and 5-lipoxygenase-activating protein gene
polymorphisms, dietary linoleic acid, and risk for breast cancer.
Cancer Epidemiol Biomarkers Prev. 17:2748–2754. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Shen M, Vermeulen R, Rajaraman P, Menashe
I, He X, Chapman RS, Yeager M, Thomas G, Burdett L, Hutchinson A,
Yuenger J, Chanock S and Lan Q: Polymorphisms in innate immunity
genes and lung cancer risk in Xuanwei, China. Environ Mol Mutagen.
50:285–290. 2009. View
Article : Google Scholar : PubMed/NCBI
|