Introduction
Colorectal cancer is the third most common cancer
and accounts for 9.4% of cancer cases worldwide (1,2). Its
mortality rate among all types of cancer is only below that of lung
cancer. Currently, surgery is the only efficient approach to cure
colorectal cancer. The cure rate is ≤90% if surgery is performed at
the early stage. However, the definitive diagnosis is usually made
at the late stage. Late diagnosis and treatment lead to the
five-year survival rate of only 40% and ~50% of patients succumb to
distant metastasis (1). Thus, in
order to improve the cure rate and prognosis of colorectal cancer,
it is significantly important for medical researchers to further
understand the pathogenic and metastatic mechanisms, and to
investigate efficient early diagnosis indicators and new therapy
methods.
MicroRNAs (miRNAs) are endogenous non-coding
single-stranded RNAs, containing ~22 nt. miRNAs complementarily
bind to multiple sites in the 3′ untranslated region of the target
mRNA for cleavage or translational repression, and regulate gene
expression at the post-transcriptional level. miRNAs are involved
in a wide range of physiological and pathophysiological processes,
such as cell proliferation, differentiation, apoptosis and
development. The dysfunction of miRNAs can cause various diseases,
including tumorigenesis. Different miRNAs exert different effects,
for example as oncogenes or tumor suppressors, in various types of
cancer (3). miRNAs are not only
involved in pathogenesis, but are also implicated in the early
diagnosis and, therefore, prognosis evaluation of human cancer
(4).
microRNA-449a (miR-449a) is an miRNA that has been
previously identified in cancer studies. miR-449a is downregulated
and shows tumor suppressive effects in various types of cancer,
including prostatic carcinoma (5,6); lung
(7), liver (8) and gastric (9) cancer; oophoroma (10); and breast (11) and bladder (12) carcinoma. In normal conditions, high
levels of miR-449a have been found in testis, lung and trachea
tissue (13). In normal colon
tissue, a high expression of miR-449a has also been identified,
although, the level was relatively lower than that in the other
three tissues (13). The expression
of miR-449a has also been found in colon cancer-derived cell lines
(13,14). However, the expression and clinical
significance of miR-449a in colorectal cancer tissues remain
unknown.
The present study investigated the expression of
miR-449a in human colorectal carcinoma tissues. miR-449a levels
were found to be increased in carcinoma tissues, compared with the
adjacent non-tumor tissues. The increase of miR-449a expression
mainly appeared in patients with normal serum carcinoembryonic
antigen (CEA) values, but not in patients with elevated CEA values.
The results suggested that miR-449a is an important regulatory
factor and potential prognosis indicator in colorectal
carcinoma.
Subjects and methods
Subjects
In total, 24 patients who received colorectal
carcinoma surgery at the Third Xiangya Hospital (Changsha, China)
between April 2012 and October 2012 were selected. The diagnosis of
colorectal carcinoma was confirmed pathologically. These patients
did not receive chemotherapy or radiotherapy. All patients provided
signed written informed consent. The study was in strict accordance
with National Institutes of Health guidelines and was approved by
the ethics committee of The Third Xiangya Hospital of Central South
University. Biopsies were obtained from colorectal carcinoma
tissues and the adjacent intestinal mucosa (5 cm from the tumor
tissues) during surgery. Some tissues were used for pathology to
confirm the diagnosis, and a number were used for mRNA extraction
and real-time polymerase chain reaction (qPCR).
Cell cultures
Colorectal carcinoma cells, HT29, SW480, SW620 and
HCT116, were purchased from the American Type Culture Collection
(Manassas, VA, USA). Normal colonic epithelial cell line, NCM460,
was purchased from Incell Corporation, LLC (San Antonio, TX, USA).
Cells were grown in RPMI-1640 medium (HyClone; Thermo Fisher
Scientific, Waltham, MA, USA), supplemented with heat-inactivated
10% FBS (v/v), 100 U/ml penicillin, 100 μg/ml streptomycin and 1%
non-essential amino acids (v/v) (Invitrogen Life Technologies,
Carlsbad, CA, USA) and cultured at 37°C in an atmosphere of 5%
CO2 with a relative humidity of 95%.
RNA extraction and qPCR
Total RNA was extracted from human tissues or cells
using TRIzol reagent (Invitrogen Life Technologies) and the
quantity of RNA was measured. The samples, with an A260/A280 ratio
of 1.8/2.0, were considered good quality and kept for further use.
Total RNA (4 μg) was then reversely transcribed into cDNA using a
reverse transcription reagent kit (Invitrogen Life Technologies).
The resulting cDNA was detected using an ABI 7500 Real-Time PCR
system (Applied Biosystems, Inc., Foster City, CA, USA) with SYBR
Green. The small nuclear RNA, U6, was used as an internal control
for miR-449a detection (15). The
primer sequences for qPCR were as follows: Sense,
CTCGCTGGCAGTGTATTGTTAG and antisense, TATCGTTGTACTCCAGACCAAGAC for
miR-449a; and sense, CTCGCTTCGGCAGCACA and antisense,
AACGCTTCACGAATTTGCGT for U6. After an initial denaturation at 95°C
for 3 min, the PCR cycling was performed as follows: 95°C for 12
sec and 65°C for 50 sec. Amplification was performed for 40 cycles
and all samples were performed in triplicate. Results were
presented as the levels of expression following normalization to U6
using the 2−ΔCt method.
Serum CEA measurement
Serum CEA levels were measured using ELISA according
to the manufacturer’s instructions (no. ab99992; Abcam, Cambridge,
UK). The assay employed anti-human CEA antibody coated onto a
96-well plate. Standard or serum samples were pipetted into the
wells and CEA present in samples was bound to the wells by the
immobilized antibody. The wells were washed and biotinylated
anti-human CEA antibody was added. Following the washing of unbound
biotinylated antibody, horseradish peroxidase-conjugated
streptavidin was pipetted into the wells. The wells were washed
again, TMB substrate solution was added and color developed in
proportion to the amount of bound CEA. Absorbance value was
measured at 450 nm. CEA concentration in serum was achieved
according to standard curves.
Statistical analysis
Data are presented as the mean ± standard error of
the mean. Statistical analysis was performed using SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). The comparison between the
two groups was performed by the paired t-test. P<0.05 was
considered to indicate a statistically significant difference.
Results
PCR primer specificity
Various sequences and sizes of PCR product exhibit
different melting temperatures, which produce different melting
curves at the end of qPCR. A single peak on the melting curve
usually indicates a single reaction product. The melting curves for
miR-449a and U6 showed single peaks (Fig. 1), which suggested good specificity
for the two primers.
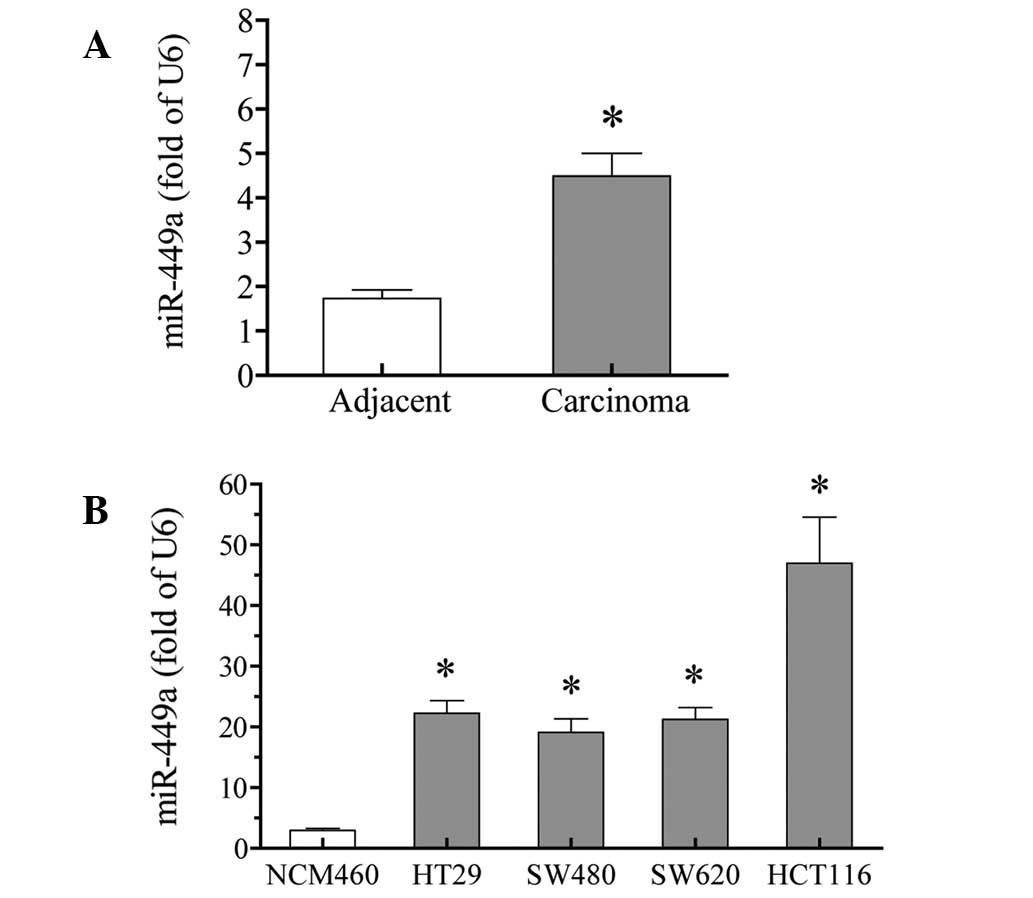
miR-449a is upregulated in colorectal
carcinoma tissues and cells
miR-449a expression was tested in colorectal
carcinoma tissues using qPCR. The results showed that the miR-449a
expression was significantly increased in carcinoma tissues,
compared with that in adjacent non-tumor tissues. The increase was
≤2.6 fold (P<0.01; Fig. 2A).
To further confirm these results, the expression of
miR-449a was examined in cultured colorectal carcinoma cells. All
four tested colorectal carcinoma cells, HT29, SW480, SW620 and
HCT116, expressed higher levels of miR-449a, compared with the
normal colorectal NCM460 cells (P<0.01). Among the four
colorectal carcinoma cells, HCT116 cells exhibited the highest
expression of miR-449a (>15-fold higher than in NCM460 cells)
(Fig. 2B).
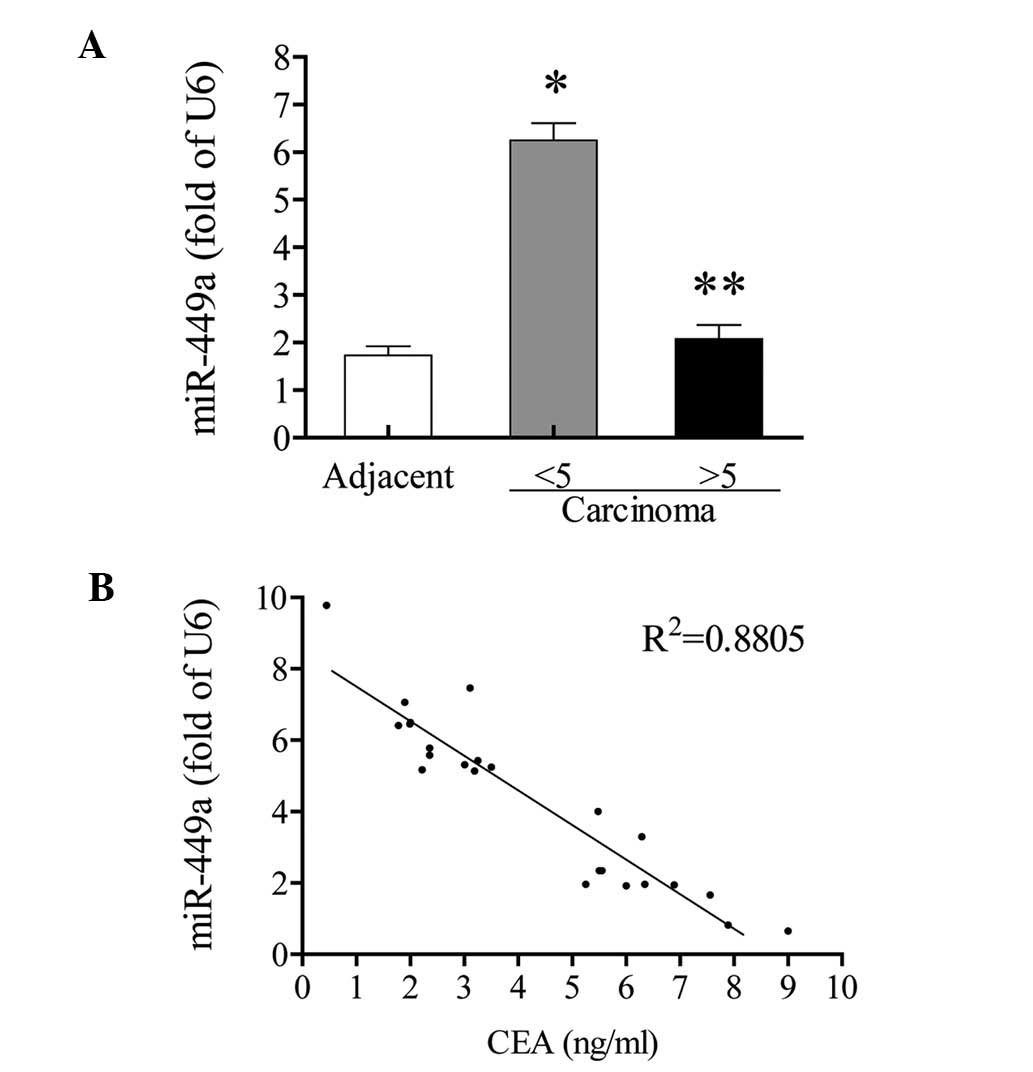
miR-449a negatively correlates with the
serum CEA level
Among all 24 patients, 11 patients exhibited
abnormal serum CEA levels (>5 ng/ml), while the other 13
patients exhibited normal serum CEA levels (<5 ng/ml). The
expression of miR-449a in adjacent non-tumor tissues was not
significantly different between patients with normal and abnormal
serum CEA levels (data not shown). However, in carcinoma tissues,
the increase of miR-449a only appeared in those with normal CEA
levels (3.6 fold; P<0.05, vs. the adjacent non-tumor tissues).
In patients with abnormal CEA levels, the miR-449a expression was
not significantly increased, compared with the adjacent non-tumor
tissues (P>0.05; Fig. 3A). In
the correlation analysis, the expression of miR-449a in carcinoma
tissues showed an inverse correlation with CEA value
(R2=0.88; Fig. 3B).
miR-449a expression is independent of TNM
stage
miR-4409a expression was investigated to identify
whether it was found to correlate with TNM stage. The results
showed no significant differences between TNM stage II and stages
III and IV in the carcinoma tissues and adjacent non-tumor tissues.
The increased expression of miR-449a in carcinoma tissues appeared
at early TNM stage II and remained at a similarly high level at
stages III and IV (Fig. 4). No
significant differences were observed among various ages, genders,
locations and differentiation degrees (Table I).
 | Table ImiR-449a expression in colorectal
carcinoma tissues of various genders, ages, locations and
differentiations. |
Table I
miR-449a expression in colorectal
carcinoma tissues of various genders, ages, locations and
differentiations.
| Groups | miR-449a expression
(fold of U6) | P-value |
|---|
| Gender |
| Male (n=13) | 4.76±0.83 | 0.575 |
| Female (n=11) | 4.17±0.56 | |
| Age, years |
| ≤60 (n=13) | 4.80±0.78 | 0.530 |
| >60 (n=11) | 4.13±0.65 | |
| Location |
| Colon (n=15) | 4.84±0.65 | 0.391 |
| Rectum (n=9) | 3.91±0.83 | |
| Differentiation |
| Poor and moderate
(n=16) | 4.17±0.56 | 0.377 |
| Well (n=8) | 5.15±1.06 | |
Discussion
miRNA-449a has been previously reported to be
downregulated in various types of cancer tissues and may play a
tumor-suppressive role. Controversial to these reports, the results
of the present study showed that miR-449a was significantly
increased in human colorectal carcinoma tissues and cultured
colorectal carcinoma cells.
The few previous studies involving miR-449a showed
that colon cancer cell lines (V9m, V855, V410 and V478 cells)
exhibit a 2-fold higher expression of miR-449 than the normal colon
tissues (14). The current study
directly compared the expression of miR-449a in colorectal
carcinoma tissues and adjacent non-tumor tissues. To the best of
our knowledge, the present study is the first to report the
elevated expression of miR-449a in human colorectal carcinoma
tissues.
miRNAs are a family of non-coding small molecules,
which are important in differentiation, proliferation and
apoptosis. In addition, miRNAs are important members involved in
the tumorigenesis and development of various tumors. As gene
silencing factors, miRNAs reveal a complex functional effect.
Different miRNAs may exert reverse effects, acting as oncogenes or
tumor suppressors in various types of cancer (3,16,17).
One miRNA, miR-96, acts as an oncogene in prostate cancer (18) and a tumor suppressor in pancreatic
cancer (19). The current study
observed the increased expression of miR-449a in colorectal
carcinoma tissues and cells. To investigate the possible role of
miR-449a in colorectal carcinoma, the correlation between miR-449a
expression and serum CEA levels, an important prognosis factor in
cancer (including colorectal carcinoma), was compared.
In the current study it was notable that the
increased expression of miR-449a in carcinoma tissues only existed
in patients with normal serum CEA levels, but not in those with
elevated levels of serum CEA. The expression of miR-449a in
carcinoma tissues revealed a marked inverse correlation with serum
CEA levels. Moreover, with increased CEA levels, the miR-449a was
found to decrease towards the levels found in adjacent non-tumor
tissues.
A large number of previous studies have shown that
higher serum CEA levels predict a worse prognosis in cancer. In
rectal cancer, the patients with preoperative serum CEA levels
within the normal range have been shown to exhibit a significantly
improved prognosis with five-year survival rates of 75.8%, compared
with patients with elevated levels who exhibited five-year survival
rates of 46.5% (20). In patients
with liver and lung metastases of colorectal carcinoma,
preoperatively elevated CEA levels are an independent risk factor
for low survival (21). Thus, the
increase of miR-449a in colorectal carcinoma tissues, as identified
in the present study, may indicate a good prognosis.
A previous study in ovarian cancer showed that
miR-449a expression was inversely correlated with TNM stage
(10). In the present study, the
increased miR-449a expression detected at TNM stage II was not
significantly different from the expression at stages III and IV.
The overexpression of miR-449a may highlight a possible diagnosis
candidate for early colorectal carcinoma.
In summary, miR-449a expression is increased in
colorectal carcinoma tissues. Its inverse correlation with serum
CEA levels suggests that it is a good prognosis indicator. However,
its role in colorectal carcinoma (serving as a suppressor/promoter
or not) and possible targets, such as CDK6 (11) and HDAC1 (5), require further investigation.
Acknowledgements
The present study was supported by the ‘1.2.5’
Talents Project of The Third Xiangya Hospital of Central South
University (Changsha, China).
References
|
1
|
Corté H, Manceau G, Blons H and
Laurent-Puig P: MicroRNA and colorectal cancer. Dig Liver Dis.
44:195–200. 2012.
|
|
2
|
Boyle P and Levin B: Colorectal cancer.
World Cancer Report 2008. IARC Press; Lyon: pp. 374–378. 2008
|
|
3
|
Farazi TA, Hoell JI, Morozov P and Tuschl
T: MicroRNAs in Human Cancer. Adv Exp Med Biol. 774:1–20. 2013.
View Article : Google Scholar
|
|
4
|
Zhu W, Liu X, He J, Chen D, Hunag Y and
Zhang YK: Overexpression of members of the microRNA-183 family is a
risk factor for lung cancer: a case control study. BMC Cancer.
11:3932011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Noonan EJ, Place RF, Pookot D, Basak S,
Whitson JM, Hirata H, Giardina C and Dahiya R: miR-449a targets
HDAC-1 and induces growth arrest in prostate cancer. Oncogene.
28:1714–1724. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Noonan EJ, Place RF, Basak S, Pookot D and
Li LC: miR-449a causes Rb-dependent cell cycle arrest and
senescence in prostate cancer cells. Oncotarget. 1:349–358.
2010.PubMed/NCBI
|
|
7
|
Jeon HS, Lee SY, Lee EJ, Yun SC, Cha EJ,
Choi E, Na MJ, Park JY, Kang J and Son JW: Combining
microRNA-449a/b with a HDAC inhibitor has a synergistic effect on
growth arrest in lung cancer. Lung Cancer. 76:171–176. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Buurman R, Gürlevik E, Schäffer V, Eilers
M, Sandbothe M, Kreipe H, Wilkens L, Schlegelberger B, Kühnel F and
Skawran B: Histone deacetylases activate hepatocyte growth factor
signaling by repressing microRNA-449 in hepatocellular carcinoma
cells. Gastroenterology. 143:811–820. 2012. View Article : Google Scholar
|
|
9
|
Bou Kheir T, Futoma-Kazmierczak E,
Jacobsen A, Krogh A, Bardram L, Hother C, Grønbæk K, Federspiel B,
Lund AH and Friis-Hansen L: miR-449 inhibits cell proliferation and
is down-regulated in gastric cancer. Mol Cancer.
18;10:292011.PubMed/NCBI
|
|
10
|
Zhang Q, He XJ, Ma LP, Li N, Yang J, Cheng
YX and Cui H: Expression and significance of microRNAs in the p53
pathway in ovarian cancer cells and serous ovarian cancer tissues.
Zhonghua Zhong Liu Za Zhi. 33:885–890. 2011.(In Chinese).
|
|
11
|
Yang X, Feng M, Jiang X, Wu Z, Li Z, Aau M
and Yu Q: miR-449a and miR-449b are direct transcriptional targets
of E2F1 and negatively regulate pRb-E2F1 activity through a
feedback loop by targeting CDK6 and CDC25A. Genes Dev.
23:2388–2393. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Chen H, Lin YW, Mao YQ, Wu J, Liu YF,
Zheng XY and Xie LP: MicroRNA-449a acts as a tumor suppressor in
human bladder cancer through the regulation of pocket proteins.
Cancer Lett. 320:40–47. 2012. View Article : Google Scholar
|
|
13
|
Lizé M, Pilarski S and Dobbelstein M:
E2F1-inducible microRNA 449a/b suppresses cell proliferation and
promotes apoptosis. Cell Death Differ. 17:452–458. 2010.PubMed/NCBI
|
|
14
|
Guo C, Sah JF, Beard L, Willson JK,
Markowitz SD and Guda K: The noncoding RNA, miR-126, suppresses the
growth of neoplastic cells by targeting phosphatidylinositol
3-kinase signaling and is frequently lost in colon cancers. Genes
Chromosomes Cancer. 47:939–946. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lardizábal MN, Nocito AL, Daniele SM,
Ornella LA, Palatnik JF and Veggi LM: Reference genes for real-time
PCR quantification of microRNAs and messenger RNAs in rat models of
hepatotoxicity. PLoS One. 7:e363232012.PubMed/NCBI
|
|
16
|
Liu M, Tang Q, Qiu M, Lang N, Li M, Zheng
Y and Bi F: miR-21 targets the tumor suppressor RhoB and regulates
proliferation, invasion and apoptosis in colorectal cancer cells.
FEBS Lett. 585:2998–3005. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Watahiki A and Wang Y, Morris J, Dennis K,
O’Dwyer HM, Gleave M, Gout PW and Wang Y: MicroRNAs associated with
metastatic prostate cancer. PLoS One. 6:e249502011. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mihelich BL, Khramtsova EA, Arva N,
Vaishnav A, Johnson DN, Giangreco AA, Martens-Uzunova E, Bagasra O,
Kajdacsy-Balla A and Nonn L: miR-183-96-182 cluster is
overexpressed in prostate tissue and regulates zinc homeostasis in
prostate cells. J Biol Chem. 286:44503–44511. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Yu S, Lu Z, Liu C, Meng Y, Ma Y, Zhao W,
Liu J, Yu J and Chen J: miRNA-96 suppresses KRAS and functions as a
tumor suppressor gene in pancreatic cancer. Cancer Res.
70:6015–6025. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Boras Z, Kondza G, Sisljagić V, Busić Z,
Gmajnić R and Istvanić T: Prognostic factors of local recurrence
and survival after curative rectal cancer surgery: a single
institution experience. Coll Antropol. 36:1355–1361. 2012.
|
|
21
|
Meimarakis G, Angele M, Conrad C, Schauer
R, Weidenhagen R, Crispin A, Giessen C, Preissler G, Wiedemann M,
Jauch KW, et al: Combined resection of colorectal hepatic-pulmonary
metastases shows improved outcome over chemotherapy alone.
Langenbecks Arch Surg. 398:265–276. 2013. View Article : Google Scholar
|