Introduction
Interdigitating dendritic cell sarcoma (IDCS)
originates from the dendritic cell, a type of professional
antigen-presenting cell that participates in innate and adaptive
immune response (1). IDCS is an
extremely rare tumor, which mostly occurs in the lymph node. Only a
small proportion of IDCS invades extranodal sites, such as the
liver, spleen, lung, intestine and bone marrow (2–5).
Primary retroperitoneal leiomyosarcoma originates from
retroperitoneal smooth muscle tissue, such as vascular smooth
muscle, smooth muscle in retroperitoneal potential gap and residual
embryonic smooth muscle (6).
Retroperitoneal leiomyosarcoma is also a rare disease. The current
report presents a unique case of IDCS presenting in the kidney
combined with retroperitoneal leiomyosarcoma in the Department of
Urology, The First Affiliated Hospital of Nanjing Medical
University (Nanjing, China). Written informed consent was obtained
from the patient.
Case report
A 46-year-old male was found to exhibit a left renal
mass combined with a periprostatic mass by computed tomography (CT)
scan during a health check in December 2011. The patient exhibited
no systemic symptoms, such as fever, sweating, weight loss and
fatigue. On admission, the patient's physical examination and
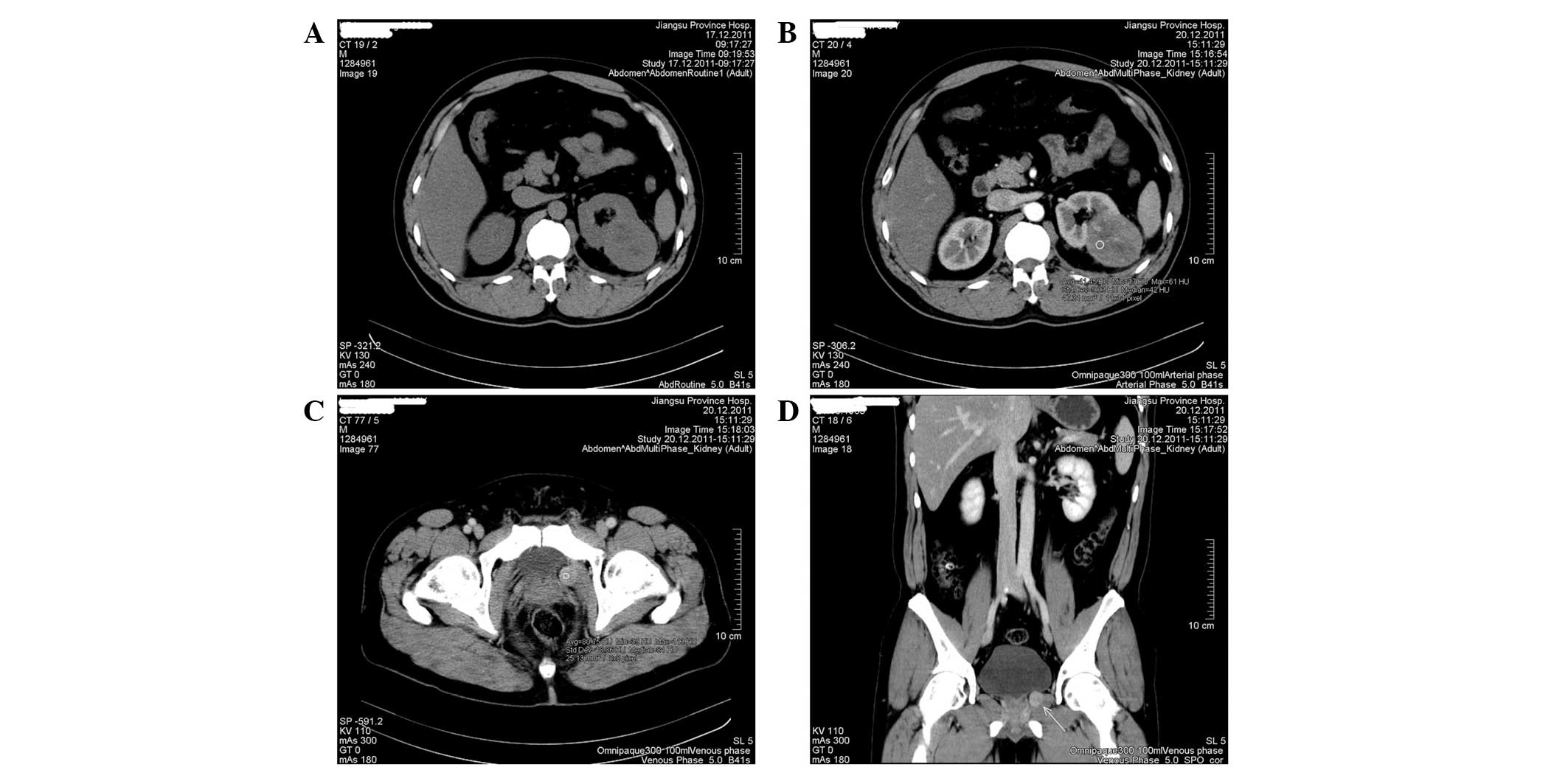
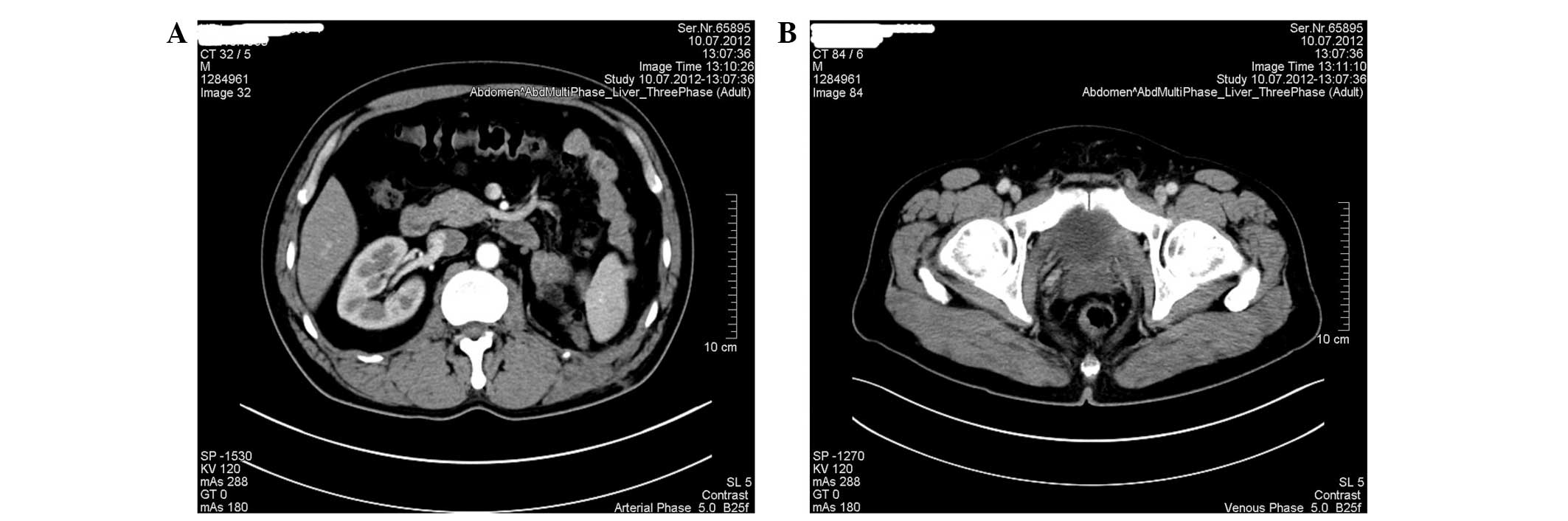
laboratory results were normal. Abdominal CT scan showed a roughly
circular, well-demarcated mass with apparent enhancement effect
(5.4×4.5 cm2 in maximum diameter) in the lower pole of
the left kidney (Fig. 1A and B). An
additional 1.5×1.0-cm2 oval-shaped mass was identified
on the left side of the prostate (Fig.
1C and D). Primary renal carcinoma and likely metastasis in the
periprostatic site were considered, according to unitary
theory.
The patient underwent radical nephrectomy. During
the surgery, the tumor was found to be closely adhered to the left
kidney, with no definite capsule. The left kidney and mass,
measuring 6×5×4 cm3, were completely resected. The tumor
exhibited a hard texture and off-white cut surface.
Microscopically, routine pathological examination suggested a
malignant tumor of the left kidney. The cutting edge of the ureter,
renal pelvis and renal capsule were not invaded by the tumor. The
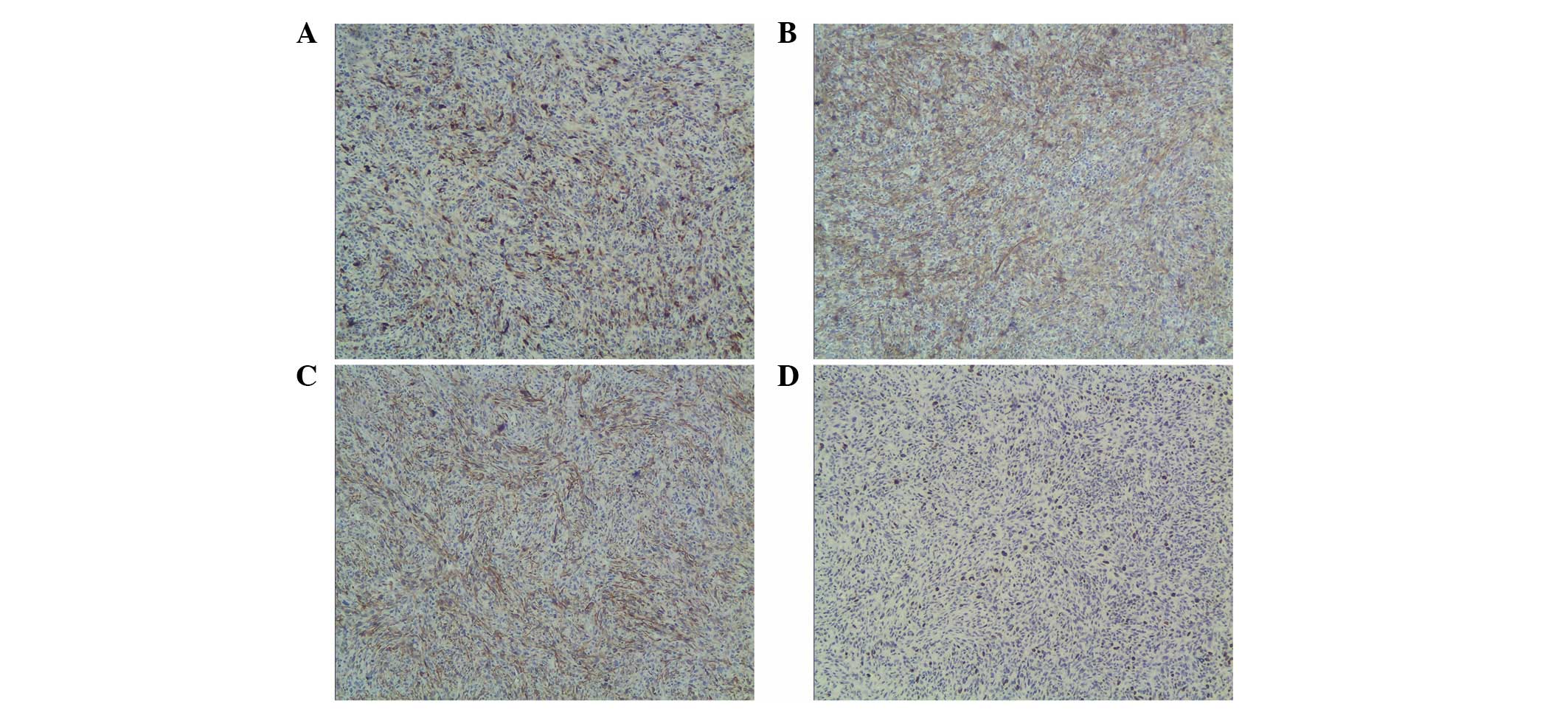
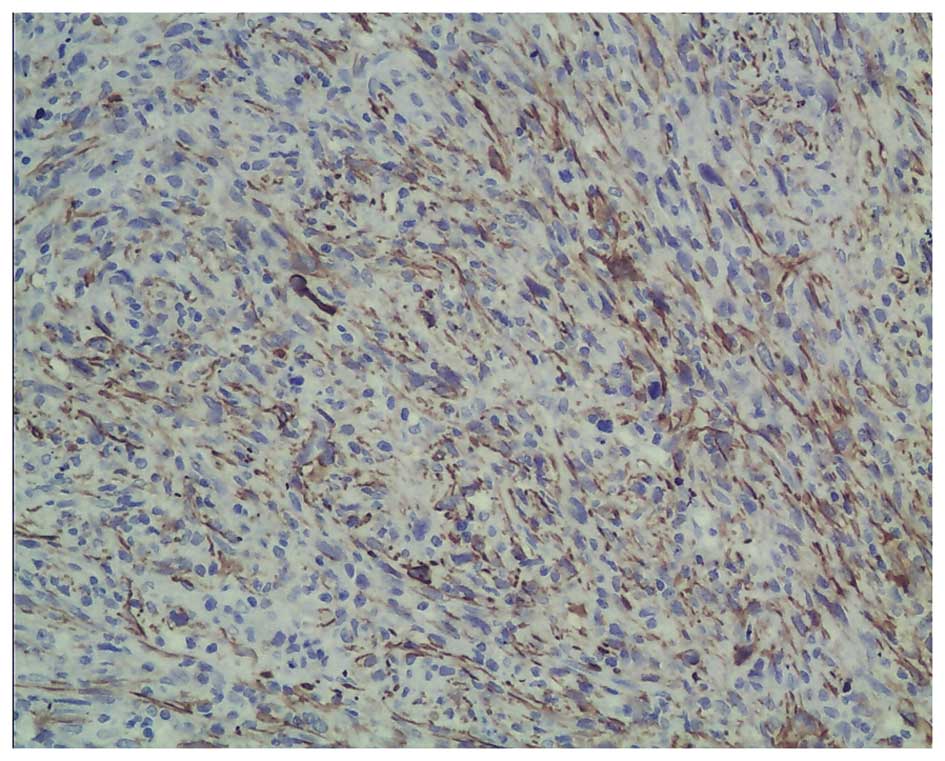
tumor had been resected completely. Immunohistochemistry showed
positive staining for S-100, Vim and SMA, and negative staining for
CKpan, CK7, ALK, desmin, actin, CD21, CD23, CD1a, CD34, HMB45,
MelanA, RCC and CD10 (Fig. 2A–C).
In total, ~10% of the tumor cells showed immunoreactivity for Ki67
(Fig. 2D). Based on the
pathological results, the diagnosis of IDCS was considered.
Following surgery, the patient did not receive adjuvant therapy,
but received active surveillance. In April 2012, the patient
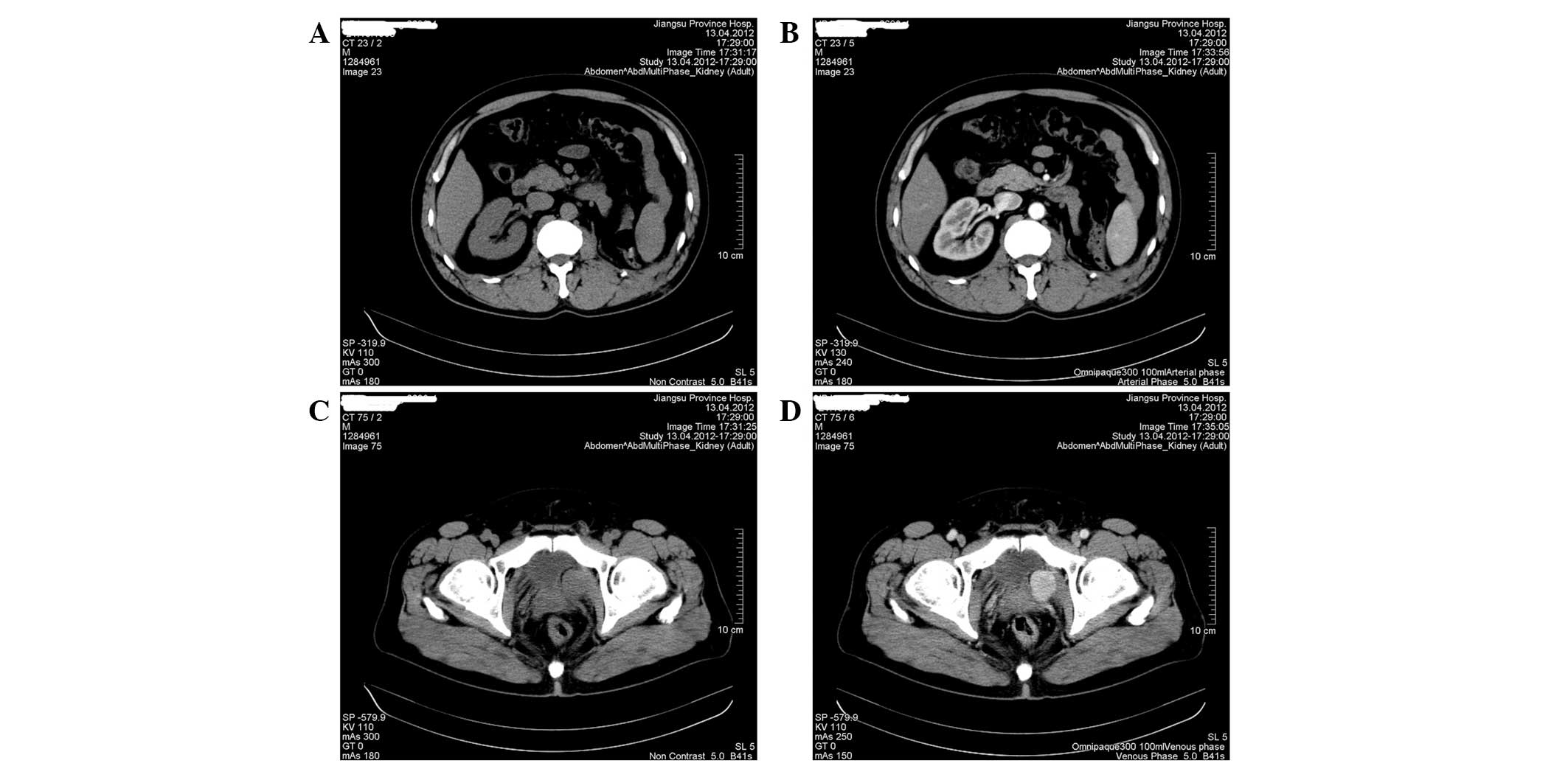
underwent CT scan, which showed the absence of the left kidney
without tumor recurrence or metastasis in the abdomen (Fig. 3A and B). However, the volume of the
periprostatic mass had evidently increased (3.5×2.8 cm2
in maximum diameter), which exhibited apparent enhancement effect
(Fig. 3C and D). The patient was
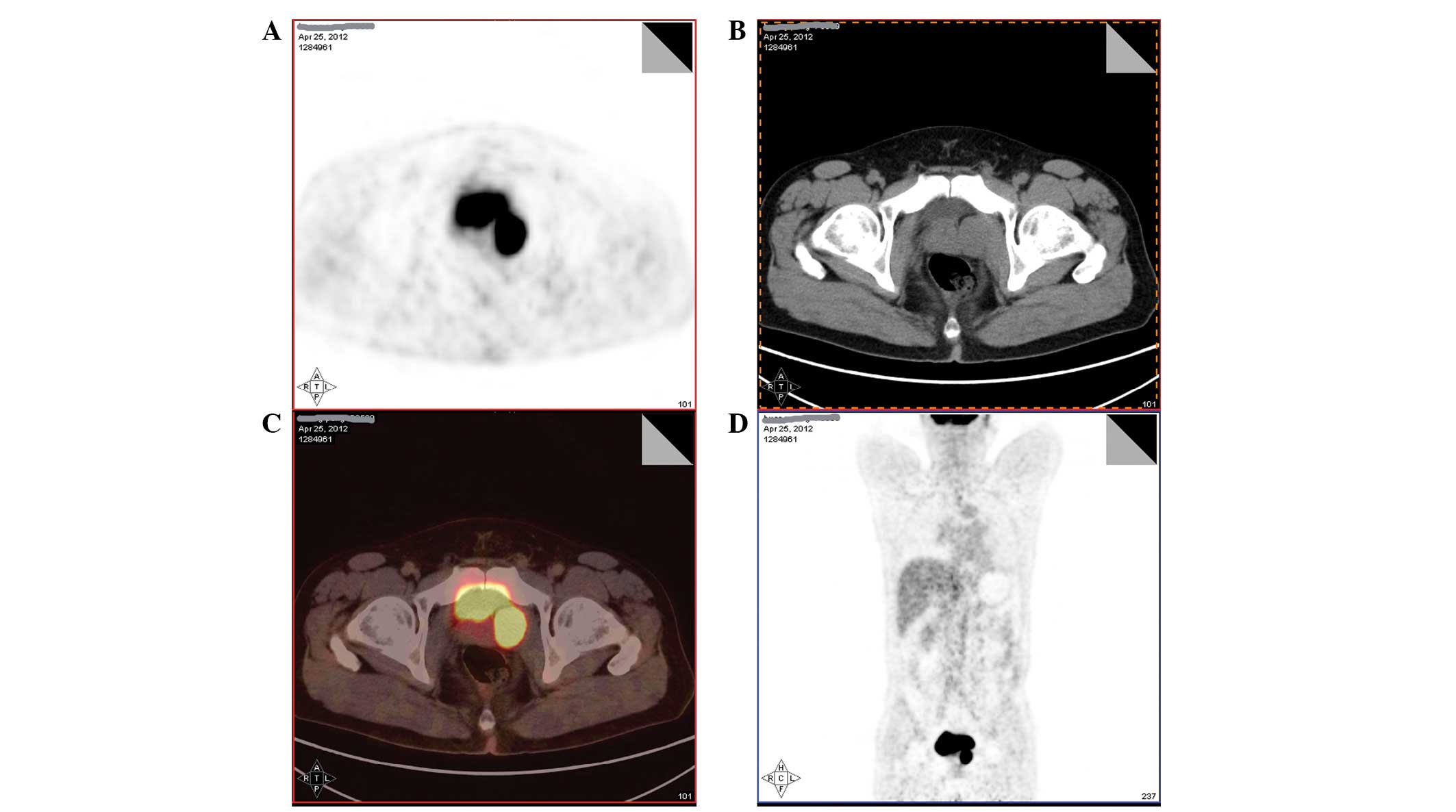
readmitted to the Department of Urology and underwent positron
emission tomography (PET)-CT to evaluate the possible metastasis.
PET-CT showed a periprostatic soft tissue nodule with a 36-Hu CT
value and fluorodeoxyglucose hypermetabolism, which suggested the
high possibility of metastasis (Fig.
4). Therefore, the patient underwent laparoscopic pelvic tumor
resection in May 2012. During surgery, the periprostatic mass was
found to be located at the retroperitoneum, which had not invaded
the prostate. Postoperative immunohistochemistry showed positive
staining for SMA and negative staining for actin, desmin, S-100,
CD21, CD23, CD117 and CD34, which demonstrated the diagnosis of
retroperitoneal leiomyosarcoma (Fig.
5). Following communication with the oncologists, the patient
did not receive adjuvant treatment, but received close follow-up.
The patient recovered without evidence of recurrence or metastasis
and the follow-up examination (abdominal CT scan only, as chest CT
scan was omitted; Fig. 6) revealed
no evident abnormality.
Discussion
IDCS belongs to the dendritic cell family, which is
a type of professional antigen-presenting cell, involved in innate
and adaptive immune responses (1).
Dendritic cell neoplasm is a rare tumor and the World Health
Organization has classified dendritic cell neoplasms into the
following five groups: Langerhans cell histiocytosis, Langerhans
cell sarcoma, interdigitating dendritic cell sarcoma/tumor,
follicular dendritic cell sarcoma/tumor and dendritic cell sarcoma
(7). IDCS is exceedingly rare and,
to date, <90 cases of IDCS have been reported worldwide. IDCS
affects individuals of any age, with a slight male predominance,
although, the majority of the cases are in middle-aged individuals
(4,8). The etiology of IDCS remains obscure. A
previous study have suggested that BCL2 chromosomal translocation
is associated with IDCS (9), but
another study contradicts this (10). The most common lesion site of IDCS
is in the lymph node. In total, approximately one-third of the
cases have extranodal sites, such as the liver, spleen, lung,
intestine, bone marrow, nasopharynx, breast, bladder, testis and
skin (2–5). To the best of our knowledge, the
current case is the first reported case of extranodal IDCS in the
kidney. Patients with IDCS usually present with painless lymph node
enlargement or extranodal mass. Systemic symptoms, including fever,
fatigue or weight loss, are extremely rare. The atypical symptoms
of IDCS and its histological similarity to other soft tissue
sarcomas increases the diagnostic difficulties. A correct diagnosis
usually depends on postoperative pathological features
(immunoreactivity for specific markers). In general, the tumor
cells of IDCS are positive for S-100 and Vim and negative for CD21,
CD35 and CD1a (7), which are of
benefit to differentiate IDCS from other dendritic cell neoplasms.
There is no standard therapeutic method for IDCS. Approximately
half of the localized IDCS may be curative by successful surgery
without adjuvant therapy (2,7).
Systematic radiotherapy or chemotherapy is used for extensive IDCS.
Chemotherapy has achieved good effects only in a few cases
(11,12). However, the optimal regimen and
exact role of adjuvant therapy remains unclear due to the absence
of previous cases and clinical data (2,7). The
reliable prognostic factors remain unknown. In general, extensive
IDCS exhibits a significantly poorer prognosis than localized
disease (2).
Notably, ~14.6% of patients with IDCS exhibit
previous concurrent or subsequent malignancy, particularly
non-Hodgkin's lymphoma (2). The
current patient exhibited an additional tumor, retroperitoneal
leiomyosarcoma. To date, the present report is a unique case of
IDCS combined with retroperitoneal leiomyosarcoma. Primary
retroperitoneal neoplasm is a rare tumor originating from
retroperitoneal smooth muscle tissue, such as vascular smooth
muscle, smooth muscle in the retroperitoneal potential gap and
residual embryonic smooth muscle (6). The etiology of retroperitoneal
leiomyosarcoma remains unknown. Rapidly increasing retroperitoneal
or pelvic mass and corresponding compression symptoms are often the
most common clinical manifestations. Immunohistochemistry of
retroperitoneal leiomyosarcoma shows positive for SMA and desmin
and negative for CD117, S-100, HMB45 and CD34, which are of benefit
to differentiate leiomyosarcoma from other soft tissue tumors
(13). Radical surgical resection
is the main treatment (14,15). Radiotherapy or chemotherapy may be
of benefit for reducing the recurrence and metastasis (14,16).
The current report presents a unique and noteworthy
case. Firstly, we considered the lesion in the kidney as primary
renal carcinoma and the periprostatic mass as the metastasis. The
imaging study was similar to that of renal cell carcinoma. However,
the final pathological examinations demonstrated IDCS and
retroperitoneal leiomyosarcoma, the two distinct tumors. As far as
we know, the current case of IDCS presenting in the kidney is the
first to be reported. In addition, it is the first to report the
combined appearance of IDCS and retroperitoneal leiomyosarcoma in
the same patient. The CT and PET-CT suggested that the two tumors
were localized diseases. Complete surgical resection for the masses
was performed, without adjuvant therapy. Close follow-up identified
no recurrence or metastasis until two-months following the final
surgery. IDCS and retroperitoneal leiomyosarcoma are extremely rare
tumors and optimal diagnosis, treatment and prognosis remain
unknown. The study suggests that successful surgery is curative to
localized IDCS and leiomyosarcoma.
Acknowledgements
The present study was supported by a project funded
by the Priority Academic Program Development of Jiangsu Higher
Education Institutions (Nanjing, China; JX10231801).
References
|
1
|
Kadowaki N: The divergence and interplay
between pDC and mDC in humans. Front Biosci (Landmark Ed).
14:808–817. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
2
|
Zhou J, Zhou W, Bai C, Zhou Y and Wang Y:
Interdigitating dendritic cell sarcoma: case report with review of
the literature. Onkologie. 34:634–637. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Han HS, Lee OJ, Lim SN, An JY, Lee KM,
Choe KH, Lee KH and Kim ST: Extranodal interdigitating dendritic
cell sarcoma presenting in the pleura: a case report. J Korean Med
Sci. 26:304–307. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ye Z, Liu F, Cao Q and Lin H:
Interdigitating dendritic cell sarcoma of lymph node mimicking
granuloma: a case report and review of the literature. Pol J
Pathol. 62:274–277. 2011.PubMed/NCBI
|
|
5
|
Parada D, Peña KB, Gil I, Queralt R,
Garcia A and Alos L: Interdigitating dendritic cell sarcoma
presenting in the nasal region. Pathol Res Pract. 208:368–371.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shvarts O, Han KR, Lam JS and Belldegrun
AS: Primary leiomyosarcoma of the inferior vena cava presenting as
a renal mass. Rev Urol. 6:39–42. 2004.PubMed/NCBI
|
|
7
|
De Pas T, Spitaleri G, Pruneri G,
Curigliano G, Noberasco C, Luini A, Andreoni B, Testori A and de
Braud F: Dendritic cell sarcoma: an analytic overview of the
literature and presentation of original five cases. Crit Rev Oncol
Hematol. 65:1–7. 2008.PubMed/NCBI
|
|
8
|
Perkins SM and Shinohara ET:
Interdigitating and follicular dendritic cell sarcomas: a SEER
analysis. Am J Clin Oncol. 36:395–398. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nayer H, Murphy KM, Hawkins AL, Long PP,
Gillison M, Borowitz M and Griffin CA: Clonal cytogenetic
abnormalities and BCL2 rearrangement in interdigitating dendritic
cell sarcoma. Leuk Lymphoma. 47:2651–2654. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang HY, Li S, Woodford RL, Mills SE and
Cousar JB: BCL2 chromosomal translocation is not a general feature
of the interdigitating dendritic cell sarcoma. Diagn Mol Pathol.
19:169–171. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Olnes MJ, Nicol T, Duncan M, Bohlman M and
Erlich R: Interdigitating dendritic cell sarcoma: a rare malignancy
responsive to ABVD chemotherapy. Leuk Lymphoma. 43:817–821. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lee SY, Lee SR, Chang WJ, Kim HS, Kim BS
and Kim IS: Successful treatment of disseminated interdigitating
dendritic cell sarcoma with adriamycin, bleomycin, vinblastine, and
dacarbazine chemotherapy. Korean J Hematol. 47:150–153. 2012.
View Article : Google Scholar
|
|
13
|
Paal E and Miettinen M: Retroperitoneal
leiomyomas: a clinicopathologic and immunohistochemical study of 56
cases with a comparison to retroperitoneal leiomyosarcomas. Am J
Surg Pathol. 25:1355–1363. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kyriazi MA, Stafyla VK, Chatzinikolaou I,
Koureas A, Chatziioannou A, Kondi-Paphiti A, Arkadopoulos N and
Smyrniotis V: Surgical challenges in the treatment of
leiomyosarcoma of the inferior vena cava: analysis of two cases and
brief review of the literature. Ann Vasc Surg. 24:826.e13–17. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Theodosopoulos T, Psychogiou V, Yiallourou
AI, Polymeneas G, Kondi-Pafiti A, Papaconstantinou I and Voros D:
Management of retroperitoneal sarcomas: main prognostic factors for
local recurrence and survival. J BUON. 17:138–142. 2012.PubMed/NCBI
|
|
16
|
Yamashita R, Muraoka K, Matsuzaki M,
Matsui T, Yamaguchi R, Niwakawa M, Tobisu K and Ito I:
Clinicopathological study of retroperitoneal sarcoma. Nihon
Hinyokika Gakkai Zasshi. 102:628–632. 2011.(In Japanese).
|