Introduction
The most common type of salivary gland tumor is a
slow-growing benign tumor of the parotid gland. The most commonly
observed clinical presentation is the appearance of a symptomless
mass or lump over the affected area. Less often, signs include
fluid draining from the ear, pain, numbness, weakness and trouble
swallowing. However, lump formation or swelling of the salivary
glands can also originate from non-neoplastic causes, including
infection, sialolithiasis, sarcoidosis, amyloidosis and Sjogren
syndrome. Salivary gland cancers are rare, comprising only 2% of
head and neck tumors. According to the latest data released by the
Taiwan Bureau of Health Promotion, the incidence of salivary gland
neoplasia is ~1.18 cases per 100,000 individuals in Taiwan. In
2009, 55 mortalities (0.32 per 100,000 males and 0.16 per 100,000
females) associated with salivary gland tumors occurred (1).
As with the majority of benign tumors, benign
salivary gland tumors largely appear to be symptomless. However,
the most common symptom of major salivary gland cancer is also a
painless lump in the affected gland. Although occasionally
accompanied by paralysis of the facial nerve, malignancy is hard to
determine on the basis of this particular symptom, which only
appears in one-third of patients with parotid gland malignancies
(2,3). Various attempts have been made to
improve the diagnostic accuracy of salivary gland tumors before
further measures, including surgery, are necessary. Fine needle
aspiration (FNA) cytology appears to be a useful tool, with overall
accuracy reported at ~90% (4–12). The
majority of previous studies have focused on the comparison of
cytological diagnosis and histopathological diagnosis. However, in
clinical practice, decisions concerning salivary gland tumor
management are not usually based on single examinations but also
incorporate information gathered from patient histories, clinical
symptoms and signs, physical examinations and imaging studies.
In this study, general data from patients with
salivary gland tumors diagnosed and managed at Taipei Medical
University Hospital (Taipei, Taiwan) between 1992 and 2011 are
presented. The overall impressions of the clinicians prior to
surgery were compared with the final pathology results. The main
goal of this study was to evaluate, when analyzing patients with
salivary gland tumors, the accuracy of pre-operative clinical
diagnosis based on various available clinical examinations.
Materials and methods
The study was approved by the Institutional Review
Board of Taipei Medical University Hospital. A retrospective chart
review was conducted and data from patients diagnosed with salivary
gland tumors at Taipei Medical University Hospital, between 1992
and 2011, were retrieved. Patients who did not undergo surgery or
with inconclusive pathology results were excluded. A total of 101
patients were enrolled. Special emphasis was placed on comparing
the clinical diagnostic accuracy between various locations of the
salivary gland tumor.
Clinical and pathological diagnosis
Following the exclusion of patients without a
complete pre-operative study, including FNA and image evaluation,
53 patients were enrolled in this comparison. Malignancy was
suspected when the following observations were made: i) On physical
examination the tumor appears immobile or without a clearly defined
border; ii) there are signs of facial or other cranial nerve
involvement; and iii) there is lymphadenopathy associated with the
tumor. Final clinical diagnosis is based on patient history,
symptoms/signs, tumor texture under physical examination, imaging
studies and FNA.
All 32 patients treated prior to 2008 received
computed tomography (CT) scans for their imaging evaluations. From
2008 onwards, 12 of the remaining 21 patients received CT scans and
9 received magnetic resonance imaging (MRI) scans for their imaging
evaluations.
Since FNA is not always correct and reliable, it was
only considered as a supplement in achieving clinical diagnosis.
Among these 53 patients, 26 received FNA. Of these 26 aspirations,
10 were of poor quality. The remaining 16 FNA samples showed
non-specific changes. None of the patients received a second
FNA.
Pathological diagnoses were purely based on
post-operative findings. Correlation between the clinical diagnosis
and the pathological diagnosis was taken to indicate an accurate
clinical diagnosis.
Clinical diagnostic accuracy
Clinical diagnostic accuracy was defined as the
number of correct clinical predictions of malignancy divided by the
total enrolled case numbers. The pathological diagnoses were
regarded as the diagnostic standard.
Results
General patient data
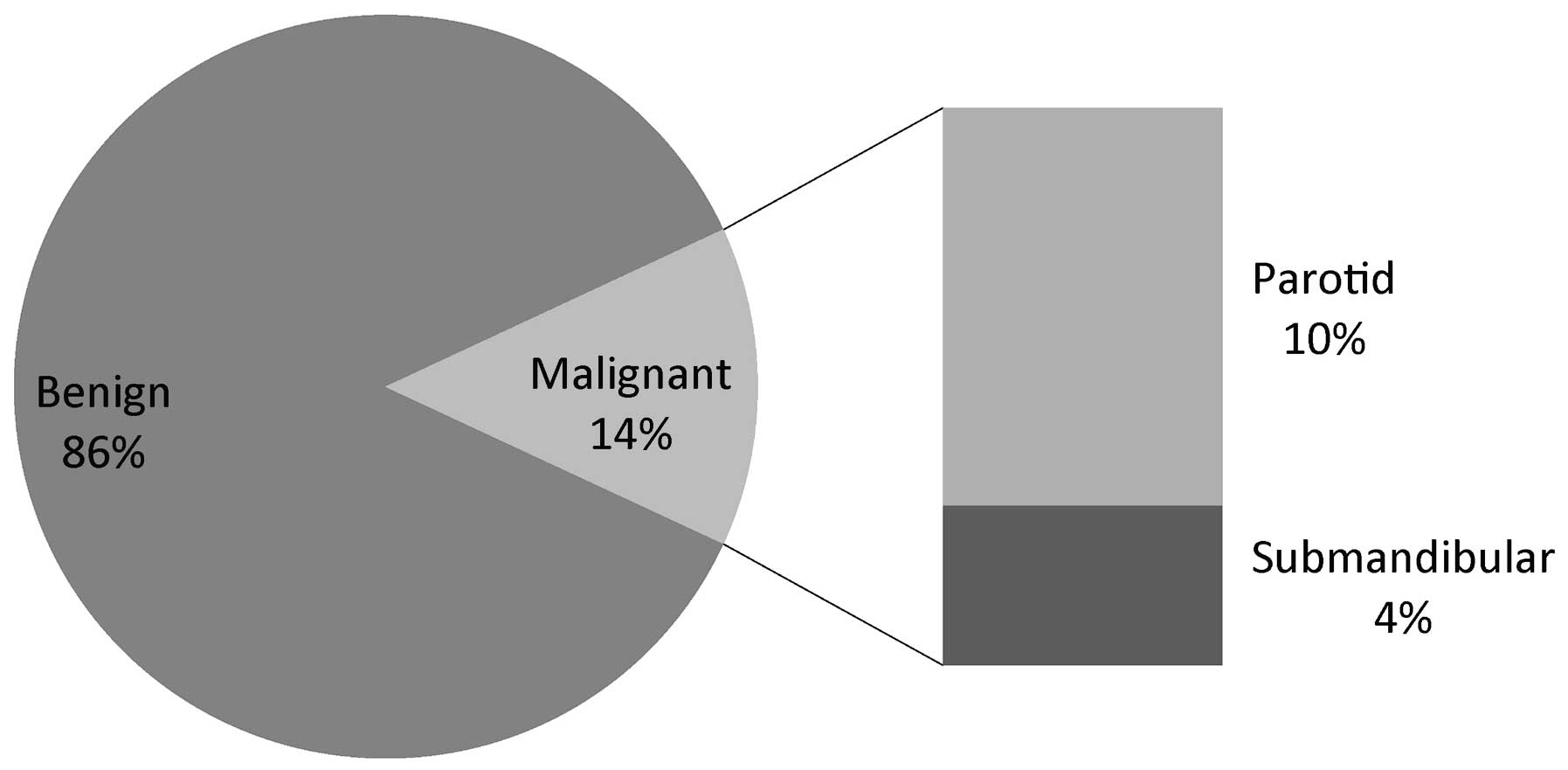
Of the 101 patients enrolled, 86% (n=87) had benign
pathology. The remaining 14% (n=14) appeared to be malignant in
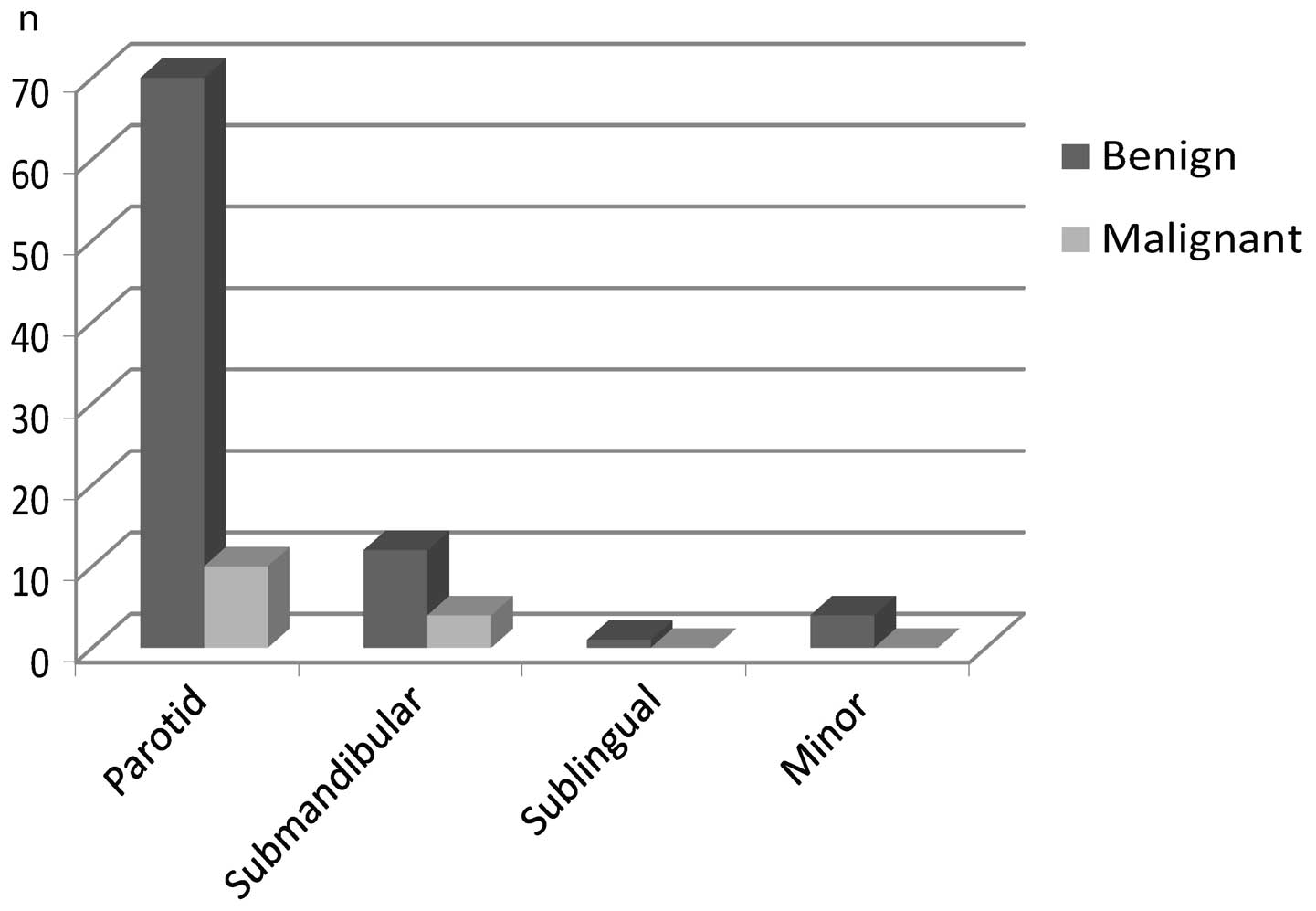
nature. When viewed according to tumor site, the malignancy rates
for parotid and submandibular glands were 14 and 33%, respectively.
All sublingual and minor salivary gland tumors appeared benign
(Figs. 1 and 2). The most common benign histology was
mixed tumor (n=63; 72%), followed by Warthin’s tumor (n=23; 26%)
and monomorphic adenoma (n=1; 1%) (Table I). Of the 14 patients with malignant
pathology, the most common malignant histology was acinic cell
carcinoma (n=4; 29%), followed by mucoepidermoid carcinoma (n=3;
21%), adenoid cystic carcinoma (n=3; 21%), carcinoma ex pleomorphic
adenoma (n=3; 21%) and epithelial-myoepithelial carcinoma (n=1; 7%)
(Table II). The male-to-female
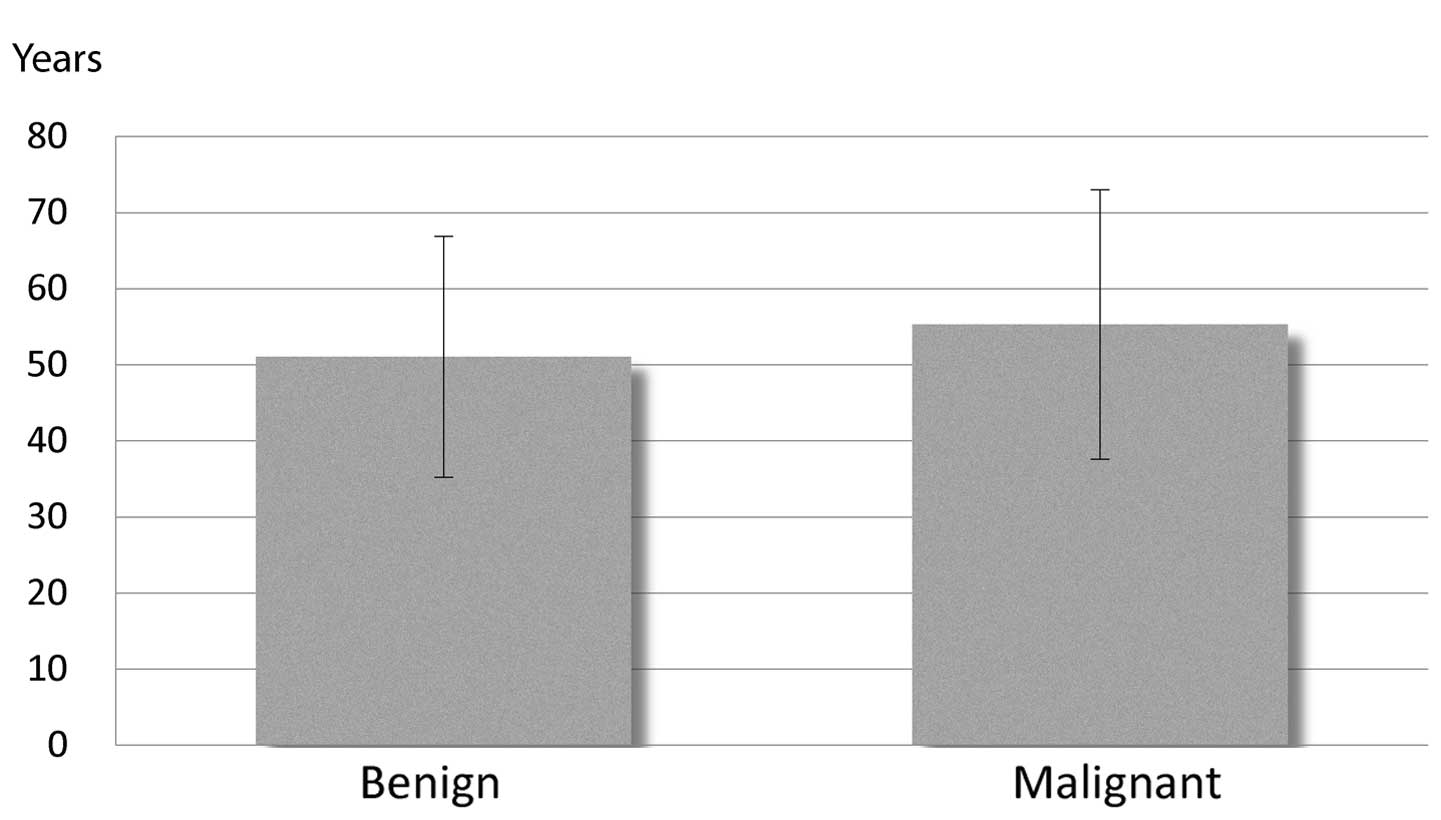
ratio for patients with malignant tumors was 6:8. Additionally, no
statistically significant difference in mean age was identified
between benign and malignant tumor patients (Fig. 3).
 | Table IHistology types for benign salivary
gland tumors. |
Table I
Histology types for benign salivary
gland tumors.
| Histology type | Patients, n | % |
|---|
| Mixed tumor | 63 | 72 |
| Warthin’s tumor | 23 | 26 |
| Monomorphic
adenoma | 1 | 1 |
| Total | 87 | |
 | Table IIHistology types for malignant salivary
gland tumors. |
Table II
Histology types for malignant salivary
gland tumors.
| Histology type | Patients, n | % |
|---|
| Mucoepidermoid
carcinoma | 3 | 21 |
| Adenoid cystic
carcinoma | 3 | 21 |
| Acinic cell
carcinoma | 4 | 29 |
| Carcinoma ex
pleomorphic adenoma | 3 | 21 |
|
Epithelial-myoepithelial carcinoma | 1 | 7 |
| Total | 14 | |
Clinical diagnostic accuracy
The clinical diagnostic accuracies for diagnosis of
parotid tumors as benign or malignant were 100 and 57%,
respectively. The clinical diagnostic accuracies for diagnosis of
submandibular tumors as benign or malignant were both 67% (Table III). There were no cases of
malignancy arising from the sublingual gland and the minor salivary
gland. Tumors arising from these regions were all correctly
interpreted as benign in nature.
 | Table IIIClinical diagnostic accuracies for
salivary gland tumors. |
Table III
Clinical diagnostic accuracies for
salivary gland tumors.
| Total | Accurate | Inaccurate | Accuracy (%) |
|---|
| Parotid |
| Benign | 28 | 28 | 0 | 100.00 |
| Malignant | 14 | 8 | 6 | 57.14 |
| Submandibular |
| Benign | 6 | 4 | 2 | 66.67 |
| Malignant | 3 | 2 | 1 | 66.67 |
| Others |
| Benign | 2 | 2 | 0 | 100.00 |
| Malignant | 0 | 0 | 0 | 0.00 |
Discussion
Pre-operative diagnosis remains a challenge for
clinical otolaryngologists and head and neck surgeons. The present
study is the first to discuss this issue from the aspect of overall
clinical judgment and diagnosis. The management of benign and
malignant tumors in the salivary gland is different, and correct
identification of the nature of the tumor can lead to completely
different management recommendations. For instance, if a parotid
tumor is considered to be at risk of malignancy, a parotidectomy is
considered for the majority of cases. Currently, surgical excision
or parotidectomy are also standard procedures for benign parotid
tumors, with the exception of Warthin’s tumor in the elderly.
However, according to a previous study by Eng et al, the
incidence of temporary facial palsy following parotidectomy is
56–57%, and the incidence of permanent facial palsy is 2–7%
(13). In addition, the risk of
complication of parotidectomy greatly depends on the experience of
the surgeon. At such considerable risk, patients may alternatively
choose to monitor the tumor if the chance of malignancy is
relatively low. The significant morbidity of facial nerve palsy
must be carefully weighed against any possible oncological benefit
(14).
Another important issue is the management of the
neck lymph nodes. Neck dissection is clearly unnecessary in benign
disease. However, if malignancy is suspected, neck dissection is
occasionally considered in pre-operative planning. A number of
factors, including advanced tumor stages and extracapsular
invasions, are considered to determine whether neck dissection is
necessary, but malignancy remains the fundamental factor when
considering neck dissection (15–18).
The two most common non-surgical strategies for
pre-operative diagnosis of salivary gland tumors are FNA cytology
and imaging studies. Various studies have concluded that the
sensitivity of cytological diagnosis of malignancy ranges between
53 and 90% with positive and negative predictive values of ~90%
(4–6,8,10,19–23).
However, in studies by Goncalves et al and Murai et
al, the sensitivity of FNA cytology for malignancy was found to
be only 42.5 and 42.9%, respectively (10,24).
Even with the incorporation of immunohistochemistry, sensitivity
did not improve significantly (9).
Numerous surgeons question the necessity of FNA cytology and state
that results from this procedure rarely define the management of
parotid masses, specifically surgical excision. Despite these
limitations, FNA cytology remains a technique considered to offer
additional information in salivary gland tumor diagnosis. However,
even if the cytological results are negative, the test does not
replace the need for clinical judgment in the management of a
suspected salivary gland neoplasm. At present, the majority of
otolaryngologists conclude that no single investigative modality is
suitable for diagnosis of specific lesions of the salivary gland
(25,26).
Another frequently used diagnostic tool is imaging
evaluation. Frequently used imaging techniques include sialography
and scintigraphy, plain radiographs, sonography, CT and MRI
(27–33). In a study by Klein et al,
exact diagnosis was only possible in 57% of cases with malignant
tumors using sonography (34). Arab
et al reported that MRI was 73–91% accurate in
differentiating between benign and malignant tumors (31). Similar results were demonstrated in
a study by Prades et al, which reported the diagnostic
accuracy of MRI at 83%. The findings of these studies imply that
the MRI seems to have reached its limitation in this application
(35). In a study by Kan et
al, positron emission tomography (PET) was evaluated for the
differential diagnosis of malignant head and neck tumors from
benign lesions. Although 65–70% of benign lesions were correctly
identified, there were no patients with salivary gland malignancies
enrolled in this study (36). A
study by Jeong et al revealed that PET/CT provides more
accurate diagnostic information for the evaluation of high-grade
salivary cancer compared with CT (37). The diagnostic accuracy reported by
Jeong et al was 97.6%. However, only high-grade salivary
cancers were included in the study.
In the present study, not all patients underwent the
same imaging procedures. It was not until 2008 that high resolution
MRI became available for diagnosis at Taipei Medical University
Hospital, and ~50% of the cases treated after 2008 received MRI.
This may potentially result in bias toward the clinical diagnosis
accuracy as the MRI was generally regarded to be more accurate than
the CT scan. However, due to the limited case numbers using MRI (9
cases), it is difficult to draw a conclusion that favors the use of
MRI.
Few studies have focused on diagnostic accuracy for
salivary gland tumors based on non-surgical or non-invasive
procedures. Ultrasound is frequently used together with FNA, and is
an effective guidance tool to facilitate the aspiration procedures
(6). The first published study
discussing the combination of multiple strategies to obtain higher
diagnostic accuracy for salivary gland lesions, was carried out by
Taylor et al in 2011 (5).
The authors concluded that sonography, sialography and FNA are
effective diagnostic tools guiding the decision for surgical
intervention, with CT, MRI and core biopsies declared as useful
adjuncts in diagnosis.
In the present study, the subjective judgment of the
clinician was also included in an attempt to combat the notion that
no single investigative modality can be utilized to diagnose a
specific lesion of the salivary gland. Clinical information,
including patient history, family history, symptoms/signs and
physical examinations, along with the findings revealed by imaging
studies and cytology, is essential in informing clinical judgment
and decision-making. Therefore, the current study attempted to
compare and analyze the results of clinical and pathological
diagnosis to assess the reliability of clinical judgments.
The final clinical judgment made by the clinician to
distinguish between benign and malignancy was unreliable in
submandibular tumors, with an accuracy of only 67%. The only
reliable judgment made by the clinician in this study was
associated with benign parotid tumors.
In the current study, all sublingual and minor
salivary gland tumors appeared to be benign. Case numbers were also
low (n=2). Thus, the clinical diagnostic accuracy for tumors in
these areas should be regarded as of no clinical significance.
One of the limitations of this retrospective study
is that patients who did not receive surgery were excluded and thus
it is possible that that the accuracy of clinical diagnosis could
be underestimated as there may be a number of patients who refused
surgery despite clinically malignant salivary masses. Another
limitation of this study is that although subjective judgment is
necessary for clinicians to evaluate the disease, this judgment is
based on a number of inconclusive and imprecise parameters and the
concept is difficult to communicate with colleagues or students. It
is also difficult to generalize from subjective results and the
value of this study may be questionable in clinical practice.
Nevertheless, based on the results of the current
study it is recommended that, in certain situations, delaying
treatment should only be advised in cases with clinically diagnosed
benign parotid tumors. Surgical intervention should be recommended,
if possible, in patients with parotid tumors clinically suspected
to be malignant, and in all submandibular, sublingual and minor
salivary gland tumors.
The results from the data presented correlate well
with the existing literature, as 86% of the parotid gland tumors
and 67% of the submandibular gland tumors appeared benign. Overall
clinical judgment distinguishing benign and malignant tumors in the
submandibular gland was unreliable. While it is difficult to draw
any conclusions for non-parotid gland tumors, surgical intervention
should be recommended in patients with parotid tumors clinically
suspected to be malignant, and all submandibular, sublingual and
minor salivary gland tumors.
References
|
1
|
Cancer Registry Annual Report. Bureau of
Health Promotion, Department of Health; Taiwan: 2009, http://www.hpa.gov.tw/Bhpnet/Web/Stat/Statistics.aspx.
Accessed August 13, 2012
|
|
2
|
Katoh T, Ishige T, Kasai H, et al:
Malignant parotid gland tumors and facial nerve paralysis. Arch
Otorhinolaryngol. 240:139–144. 1984.PubMed/NCBI
|
|
3
|
Eneroth CM: Facial nerve paralysis. A
criterion of malignancy in parotid tumors. Arch Otolaryngol.
95:300–304. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kechagias N, Ntomouchtsis A, Valeri R, et
al: Fine-needle aspiration cytology of salivary gland tumours: a
10-year retrospective analysis. Oral Maxillofac Surg. 16:35–40.
2012.PubMed/NCBI
|
|
5
|
Taylor MJ, Serpell JW and Thomson P:
Preoperative fine needle cytology and imaging facilitates the
management of submandibular salivary gland lesions. ANZ J Surg.
81:70–74. 2011. View Article : Google Scholar
|
|
6
|
Sharma G, Jung AS, Maceri DR, Rice DH,
Martin SE and Grant EG: US-guided fine-needle aspiration of major
salivary gland masses and adjacent lymph nodes: accuracy and impact
on clinical decision making. Radiology. 259:471–478. 2011.
View Article : Google Scholar
|
|
7
|
Viguer JM, Vicandi B, Jiménez-Heffernan
JA, López-Ferrer P, Gonzalez-Peramato P and Castillo C: Role of
fine needle aspiration cytology in the diagnosis and management of
Warthin’s tumour of the salivary glands. Cytopathology. 21:164–169.
2010.
|
|
8
|
Christensen RK, Bjørndal K, Godballe C and
Krogdahl A: Value of fine-needle aspiration biopsy of salivary
gland lesions. Head Neck. 32:104–108. 2010.PubMed/NCBI
|
|
9
|
Chakrabarti S, Bera M, Bhattacharya PK, et
al: Study of salivary gland lesions with fine needle aspiration
cytology and histopothology along with immunohistochemistry. J
Indian Med Assoc. 108:833–836. 2010.
|
|
10
|
Gonçalves AJ, Menezes MB, Kavabata NK,
Bertelli AA, Souza RA and Joelsons D: Fine needle aspiration in
salivary gland tumors: specificity and sensitivity. Rev Assoc Med
Bras. 53:267–271. 2007.(In Portuguese).
|
|
11
|
Mihashi H, Kawahara A, Kage M, et al:
Comparison of preoperative fine-needle aspiration cytology
diagnosis and histopathological diagnosis of salivary gland tumors.
Kurume Med J. 53:23–27. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cohen EG, Patel SG, Lin O, et al:
Fine-needle aspiration biopsy of salivary gland lesions in a
selected patient population. Arch Otolaryngol Head Neck Surg.
130:773–778. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Eng CY, Evans AS, Quraishi MS and Harkness
PA: A comparison of the incidence of facial palsy following
parotidectomy performed by ENT and non-ENT surgeons. J Laryngol
Otol. 121:40–43. 2007.
|
|
14
|
Guntinas-Lichius O, Gabriel B and
Klussmann JP: Risk of facial palsy and severe Frey’s syndrome after
conservative parotidectomy for benign disease: analysis of 610
operations. Acta Otolaryngol. 126:1104–1109. 2006.
|
|
15
|
Wang YL, Li DS, Gan HL, et al: Predictive
index for lymph node management of major salivary gland cancer.
Laryngoscope. 122:1497–1506. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ishinaga H, Kato A and Yamada H: Neck
dissection for salivary gland carcinoma. Nihon Jibiinkoka Gakkai
Kaiho. 101:895–899. 1998.(In Japanese).
|
|
17
|
Medina JE: Neck dissection in the
treatment of cancer of major salivary glands. Otolaryngol Clin
North Am. 31:815–822. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Korkmaz H, Yoo GH, Du W, et al: Predictors
of nodal metastasis in salivary gland cancer. J Surg Oncol.
80:186–189. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ivanová S, Slobodníková J, Janská E and
Jozefáková J: Fine needle aspiration biopsy in a diagnostic workup
algorithm of salivary gland tumors. Neoplasma. 50:144–147.
2003.PubMed/NCBI
|
|
20
|
Oka K, Chikamatsu K, Eura M, Katsura F,
Yumoto E and Tokunaga H: Clinical significance of fine-needle
aspiration biopsy in major salivary gland tumors. Nihon Jibiinkoka
Gakkai Kaiho. 105:1109–1115. 2002.(In Japanese).
|
|
21
|
Al-Khafaji BM and Afify AM: Salivary gland
fine needle aspiration using the ThinPrep technique: diagnostic
accuracy, cytologic artifacts and pitfalls. Acta Cytol. 45:567–574.
2001. View Article : Google Scholar
|
|
22
|
Stewart CJ, MacKenzie K, McGarry GW and
Mowat A: Fine-needle aspiration cytology of salivary gland: a
review of 341 cases. Diagn Cytopathol. 22:139–146. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Jan IS, Chung PF, Weng MH, et al: Analysis
of fine-needle aspiration cytology of the salivary gland. J Formos
Med Assoc. 107:364–370. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Murai N, Taniguchi Z, Takahashi Y,
Yasuhara Y, Kuboshima F and Tateya I: A study of salivary gland
aspiration cytology reporting: guideline validity. Nihon Jibiinkoka
Gakkai Kaiho. 114:615–619. 2011.(In Japanese).
|
|
25
|
Folia M, Kany M, Fillola G, Serrano E and
Pessey JJ: Value of of fine-needle aspiration cytology and MRI in
parotid gland masses. Rev Laryngol Otol Rhinol (Bord). 123:153–157.
2002.(In French).
|
|
26
|
Jafari A, Royer B, Lefevre M, Corlieu P,
Périé S and St Guily JL: Value of the cytological diagnosis in the
treatment of parotid tumors. Otolaryngol Head Neck Surg.
140:381–385. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Schmitt G, Lehmann G, Strötges MW, et al:
The diagnostic value of sialography and scintigraphy in salivary
gland diseases. Br J Radiol. 49:326–329. 1976. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schall GL, Smith RR and Barsocchini LM:
Radionuclide salivary imaging usefulness in a private
otolaryngology practice. Arch Otolaryngol. 107:40–44. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Isacsson G, Isberg A, Haverling M and
Lundquist PG: Salivary calculi and chronic sialoadenitis of the
submandibular gland: a radiographic and histologic study. Oral Surg
Oral Med Oral Pathol. 58:622–627. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Chisin R, Markitziu A, Hoffer S, Shani J
and Atlan H: The clinical value of quantitative dynamic
scintigraphy in salivary gland disorders. Int J Rad Appl Instrum B.
15:313–317. 1988. View Article : Google Scholar
|
|
31
|
Arbab AS, Koizumi K, Toyama K, et al:
Various imaging modalities for the detection of salivary gland
lesions: the advantages of 201Tl SPET. Nucl Med Commun. 21:277–284.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Salaffi F, Carotti M, Argalia G, Salera D,
Giuseppetti GM and Grassi W: Usefulness of ultrasonography and
color Doppler sonography in the diagnosis of major salivary gland
diseases. Reumatismo. 58:138–156. 2006.(In Italian).
|
|
33
|
Nakahara T, Suzuki T, Hashimoto J, et al:
Role of salivary gland scintigraphy with Tc-99m pertechnetate in
determining treatment of solitary parotid gland tumors: a
retrospective study. Clin Nucl Med. 32:363–366. 2007. View Article : Google Scholar
|
|
34
|
Klein K, Turk R, Gritzmann N and Traxler
M: The value of sonography in salivary gland tumors. HNO. 37:71–75.
1989.(In German).
|
|
35
|
Prades JM, Oletski A, Faye MB, et al:
Parotid gland masses: diagnostic value of MR imaging with
histopathologic correlations. Morphologie. 91:44–51. 2007.(In
French).
|
|
36
|
Khan N, Oriuchi N, Ninomiya H, Higuchi T,
Kamada H and Endo K: Positron emission tomographic imaging with
11C-choline in differential diagnosis of head and neck
tumors: comparison with 18F-FDG PET. Ann Nucl Med.
18:409–417. 2004.
|
|
37
|
Jeong HS, Chung MK, Son YI, et al: Role of
18F-FDG PET/CT in management of high-grade salivary
gland malignancies. J Nucl Med. 48:1237–1244. 2007.
|