Introduction
Toll-like receptors (TLRs) are a family of pattern
recognition receptors. To date, 11 human TLRs and 13 mouse TLRs
have been identified (1). Mammalian
TLRs recognize microbial products and initiate innate immune
responses (2).
Although a limited number of studies have analyzed
the correlation between TLR expression and human malignancy,
several studies concerning the expression of TLRs and cancer have
been conducted (3).
The Toll-related proteins were first identified in
mammals (4) and the mammalian TLR4
was rapidly demonstrated to be responsible for the recognition of
lipopolysaccharide (LPS) (5). It
has been previously reported that TLR4 negatively regulates the
Salmonella-induced antitumor activity (6). Notably, TLR4 has been reported to be
important in promoting the immune escape of human lung cancer cells
by inducing immunosuppressive cytokines and apoptosis resistance
(7).
In ovarian cancer patients, chemotherapy resistance
is the principal factor restricting long-term treatment (8). Previously, paclitaxel (Pac) has been
reported to be a ligand to TLR4 (9). The current study investigated the
effects of TLR4 in ovarian cancer, particularly in Pac
chemotherapy. Myeloid differentiation factor 88 (MyD88) was
originally isolated as a gene that is induced rapidly during the
interleukin (IL)-6-stimulated differentiation of M1 myeloleukemic
cells into macrophages (10). IL-6
is considered to be involved in host immune responses to types of
ovarian cancer (11,12). IL-6 has also been demonstrated to
provide paracrine growth stimulation when monocytes are attracted
to types of ovarian cancer that produce macrophage
colony-stimulating factor (13).
IL-6 signaling in ovarian cancer cells regulates tumor cell
proliferation, invasion and angiogenesis (14,15)
and IL-8 has also been reported to promote ovarian tumor growth
in vivo (16). Previously,
it has been reported that TLR4 signaling is divided into the
following two pathways: MyD88-dependent and MyD88-independent
(17,18). A correlation between MyD88
expression and patients’ progression-free survival has shown that
patients whose tumors do not express MyD88 exhibit a significantly
improved progression-free interval compared with patients whose
tumors express high levels of MyD88 (19).
The present study investigated the role of TLR4 in
ovarian cancer cells and the effect of TLR4 ligand by Pac in
MyD88+ and MyD88− human ovarian carcinoma
in vitro.
Materials and methods
Reagents
Pac was purchased from Sigma-Aldrich (St. Louis, MO,
USA) and the rabbit polyclonal antibodies against TLR4 and MyD88
were purchased from Santa Cruz Biotechnology, Inc., (Santa Cruz,
CA, USA).
Cell culture and samples
The in vitro experiments were performed with
human ovarian cancer cell lines, SKOV3, OVCAR3, A2780 and 3AO. All
the cell lines were obtained from the Basic Research Center,
Shandong Cancer Hospital and Institute (Jinan, China). Cells were
cultured in RPMI-1640 medium (Gibco-BRL, Carlsbad, CA, USA)
supplemented with 10% heat-inactivated fetal bovine serum (FBS;
Gibco-BRL) and incubated under standardized conditions (37°C; 5%
CO2 atmosphere).
Samples of normal ovarian tissue adjacent to tumor
(n=12) and borderline (n=8) and malignant (n=24) tumors were
collected with the approval of the Ethics Committee of the Shandong
Cancer Hospital and Institute.
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using the TRIzol reagent kit
(Invitrogen Life Technologies, Carlsbad, CA, USA) according to the
manufacturer’s instructions. Reverse transcription was performed
using SYBR ExScript RT-PCR kit (Takara Bio, Inc., Shiga, Japan).
The following set of primers were used for amplification: i) human
TLR4 sense, 5′-AATGGATCAAGGACCAGAGG-3′ and antisense,
5′-CAGCCAGCAAGAAGCATCAG-3′; and ii) human MyD88 sense,
5′-CGCCGGATGGTGGTGGTTGT-3′ and antisense,
5′-TGTAGTCGCAGACAGTGATGAACC-3′. The primers were used to amplify an
197-bp fragment of TLR4 cDNA and an 186-bp fragment of MyD88 cDNA.
The following primers were used for β-actin: forward,
5′-TTGTATCGTGGAAGGACTCA-3′ and reverse,
5′-TGTCATCATATTTGGCAGGTTT-3′. TLR4 was used to amplify a 197-bp
fragment. Forty cycles of PCR were performed at 95°C for 30 sec,
63°C for 30 sec and 72°C for 45 sec. The primers for human MyD88
were used to amplify a 186-bp fragment of MyD88 cDNA. Thirty cycles
of PCR were performed at 95°C for 30 sec, 61°C for 30 sec and 72°C
for 45 sec.
SDS-PAGE and western blot analysis
Protein was denatured in sample buffer [2.5% SDS,
10% glycerol, 5% β-mercaptoethanol, 0.15 mol/l Tris (pH 6.8) and
0.01% bromophenol blue] and subjected to 10–12% SDS-PAGE as
previously described (6). The
following antibodies were used: Rabbit anti-TLR4 (1:1,000), -MyD88
(1:1,000) and -actin (1:10,000) (Santa Cruz Biotechnology, Inc.).
Signals were detected using ECL western blotting detection reagents
(Pierce Biotechnology, Inc., Rockford, IL, USA) according to the
manufacturer’s instructions.
Immunohistochemistry
Paraffin sections of tumor tissues were
deparaffinized and microwaved while immersed in 0.01 M citrate
buffer (pH 6.0) for 20 min. Sections were washed with PBS and
incubated overnight at 4°C with polyclonal rabbit anti-human TLR4
antibody (1:50) or with polyclonal rabbit anti-human MyD88 (1:50;
Santa Cruz Biotechnology, Inc.). Following washing, tissues were
incubated with horseradish peroxidase-labeled anti-rabbit antibody
for 1 h, followed by 3,3′-diaminobenzidine (Dako, Carpinteria, CA,
USA). The results for TLR4 and MyD88 expression in tissues were
scored by two independent investigators based on the following
levels of staining intensity: None (−), weak (+), moderate (++), or
strong (+++).
Incubation of tumor cells with TLR4
ligands
In all experiments testing the effects of LPS or Pac
on tumor cells, LPS was used at a concentration of 10 μg/ml and Pac
at 2 μM.
Cytokine and chemokine production
Cytokine and chemokine production by the human
ovarian cancer cells was determined using a Luminex-100 System
(Luminex, Austin, TX, USA). Supernatants of tumor cells seeded in
12-well plates at 5×105 cells/well in 1 ml of LPS or Pac
medium were collected following 36 h of incubation. The levels of
IL-6 and IL-8 were measured using panels of capture antibody-coated
beads and the labeled detection antibodies, which were pretested
and qualified by the manufacturer to ensure the absence of
cross-reactivity. The assay sensitivity varied between 5 and 15
pg/ml.
Caspase-Glo 3/7 assay
In total, 10 μg of protein in a 50 μl total volume
was mixed with 50 μl equilibrated Caspase-Glo 3/7 reagents (Promega
Corporation, Madison, WI, USA). Following incubation at room
temperature for 1 h, luminescence was measured using TD 20/20
luminometer (Turner Designs, Inc., Sunnyvale, CA, USA). Blank
values were subtracted and fold increase in activity was calculated
based on the activity measured from untreated cells. Each sample
was measured in triplicate.
Construction of RNA interference
targeting the TLR4 gene in the vector
Small interfering RNA (siRNA) oligonucleotides
specifically targeting TLR4 oligonucleotides were confirmed to be
valid by the authors and were designed with the following
sequences: Sense, 5′-GGTAAGGAATGAGCTAGTA-3′ and antisense,
5′-TACTAGCTCATTCCTTACC-3′. The pGenesil-1 negative control vector
(Wuhan Genesil Biotechnology Co., Ltd., Wuhan, China) was used as
the negative control plasmid in all experiments as previously
described (20).
Transcription and production of stable
clones
The day prior to transfection, cells were
trypsinized and plated at a density of 5×105 cells/well
in six-well tissue culture plates (Corning Inc., Corning, NY, USA).
Cells were rinsed twice with serum-free RPMI-1640 when the density
reached >90% confluence. Next, pGenesil-shTLR4 (RNA interference
expression vectors) or pGenesil-shControl (control vector) and
Lipofectamine 2000 mixtures, prepared in OptiMEM (Invitrogen Life
Technologies), were added. Following 5 h of incubation, the
plasmid-Lipofectamine 2000 mixture was removed and RMPI-1640 plus
10% FBS and 800 μg/ml geneticin (Invitrogen Life Technologies) for
SKOV3 and OVCAR3 cells, 500 μg/ml G418 for 3AO cells and 600 μg/ml
geneticin for A2780 cells were added. All the non-transfected cells
died within 7 days and a number of surviving transfected cells were
harvested 21 days later.
MTT assay
Stable clone cells were seeded into 96-well culture
plates (5,000/well) and treated with 2 μmol/l Pac (2 μM) for 24 h.
As the control for normal cell proliferation, 0.1% ethanol was
used. At the end of each treatment, cells were incubated as
previously described (21).
Statistical analysis
Data are presented as the mean ± standard deviation
for continuous variables, and the frequency and percentage for
categorical variables. Results were statistically evaluated by
analysis of variance. SPSS 17.0 software for Windows was used for
statistical treatment (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of TLR4 and MyD88 in ovarian
cancer tissues
Expression of TLR4 and MyD88 were assessed in
situ on paraffin sections of normal ovarian tissue adjacent to
tumor (n=12) and borderline (n=8) and malignant (n=24) tumors. TLR4
was found to exhibit moderate (++) or strong (+++) expression in
malignant (20/24) and borderline (5/8) tumors and normal ovarian
epithelium (6/12). However, the expression of MyD88 in malignant
tumors was considerably greater (18/24) than that in normal ovarian
tissue (1/8) or in borderline tumors (1/12) (Table I).
 | Table IResults for TLR4 and MyD88 expression
in tissues. |
Table I
Results for TLR4 and MyD88 expression
in tissues.
| Definite
histology | TLR4 | MyD88 |
|---|
| Malignant tumor
cases |
| 1 | ++ | − |
| 2 | +++ | ++ |
| 3 | +++ | ++ |
| 4 | ++ | − |
| 5 | +++ | +++ |
| 6 | ++ | +++ |
| 7 | +++ | ++ |
| 8 | + | +++ |
| 9 | +++ | ++ |
| 10 | ++ | − |
| 11 | ++ | ++ |
| 12 | +++ | ++ |
| 13 | +++ | +++ |
| 14 | +++ | ++ |
| 15 | ++ | + |
| 16 | ++ | ++ |
| 17 | ++ | + |
| 18 | + | ++ |
| 19 | ++ | ++ |
| 20 | ++ | +++ |
| 21 | + | + |
| 22 | ++ | ++ |
| 23 | + | ++ |
| 24 | ++ | +++ |
| Borderline
cases |
| 1 | ++ | − |
| 2 | ++ | + |
| 3 | + | − |
| 4 | +++ | ++ |
| 5 | ++ | + |
| 6 | + | − |
| 7 | ++ | + |
| 8 | + | + |
| Normal ovarian
epithelium cases |
| 1 | ++ | + |
| 2 | +++ | ++ |
| 3 | ++ | + |
| 4 | ++ | + |
| 5 | + | + |
| 6 | + | − |
| 7 | ++ | + |
| 8 | + | + |
| 9 | + | − |
| 10 | + | + |
| 11 | ++ | + |
| 12 | + | + |
| P-value | 0.022a | 0.001b |
Expression of TLR4 and MyD88 signaling
adapter protein in epithelial ovarian cancer (EOC) cell lines
Furthermore, the expression of TLR4 and MyD88 in EOC
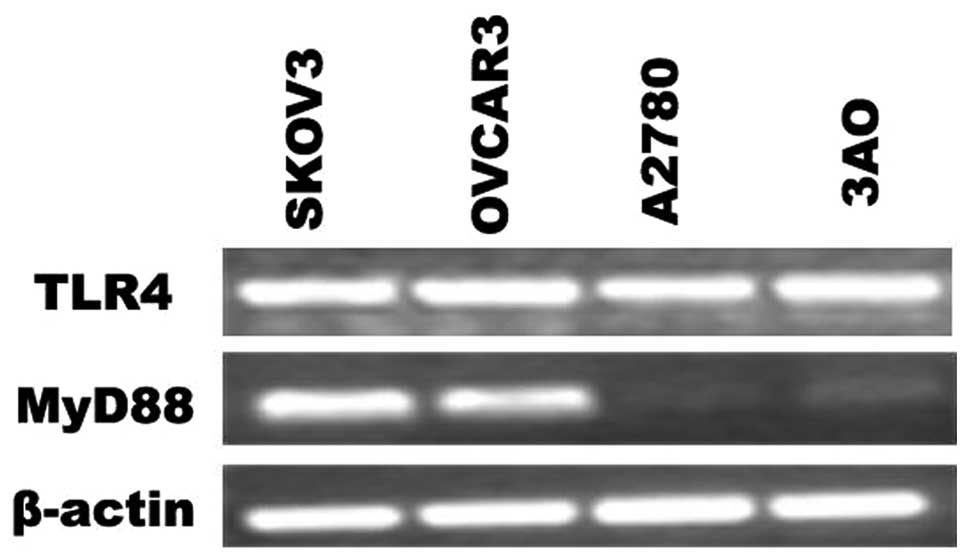
cells was evaluated by RT-PCR. As shown in Fig. 1, the mRNA of TLR4 were expressed in
EOC cell lines. However, the expression of MyD88 was found to
differ; SKOV3 and OVCAR3 cells were MyD88-positive, while A2780 and
3AO cells were MyD88-negative.
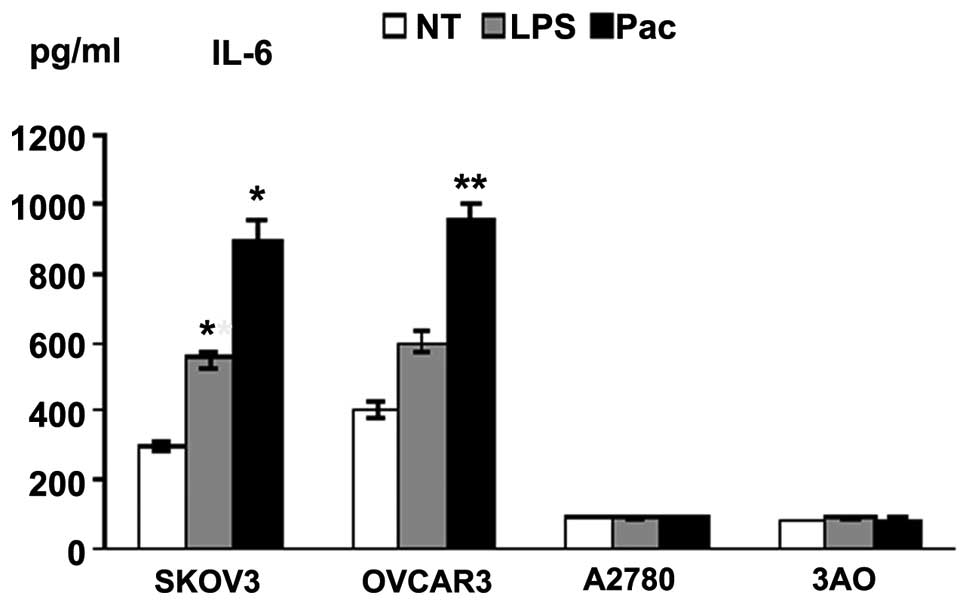
LPS- and Pac-induced cytokine production
in MyD88+ cells
Supernatants of the four cell lines exposed to LPS
or Pac for 36 h were analyzed for levels of inflammatory cytokines
and growth factors. SKOV3 and OVCAR3 cells constitutively secreted
a wide range of cytokines and chemokines, including IL-6 and IL-8.
By contrast, A2780 and 3AO cells produced low levels of these
cytokines. LPS and Pac significantly increased the secretion of
IL-6 and IL-8 in SKOV3 cells. Similarly, Pac resulted in a
significant upregulation of IL-6 and IL-8 in OVCAR3 cells, but not
in A2780 and 3AO cells (Figs. 2 and
3).
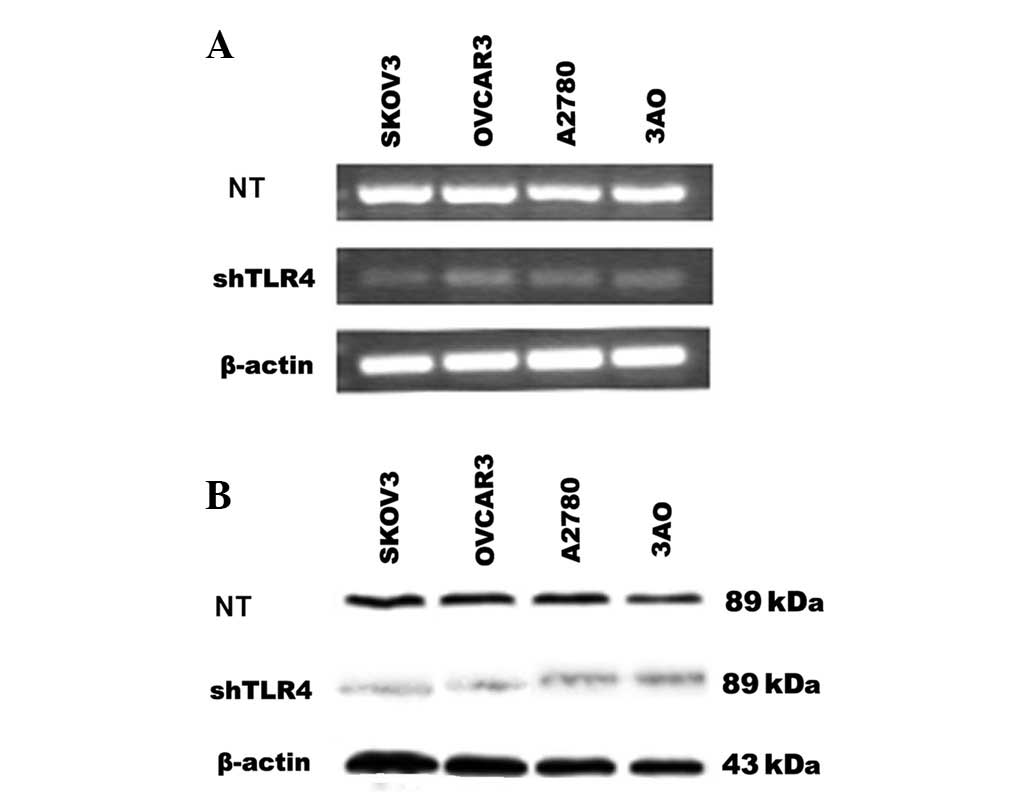
Effect of shRNA on TLR4 gene
expression
To investigate the potential role of TLR4 in EOC
cell lines, pGenesil-shTLR4 directed at nucleotides 2,202–2,220 of
TLR4 was used to selectively reduce TLR4 gene expression in EOC
cell lines, SKOV3, OVCAR3, A2780 and 3AO. As shown in Fig. 4, the shTLR4 significantly reduced
the expression of the TLR4 mRNA and protein in these cells
(SKOV3/shTLR4, OVCAR3/shTLR4, A2780/shTLR4 and 3AO/shTLR4). The
control-scrambled sequence, shControl, exhibited no effect on TLR4
gene expression in cells (SKOV3/shControl, OVCAR3/shControl,
A2780/shControl and 3AO/shControl). Thus, the application of
TLR4-directed shTLR4 was an effective and selective method of
long-term suppression of endogenous TLR4 levels, making it possible
to determine in experiments the role of endogeneous TLR4 on EOC
cells.
Knockdown of TLR4 depressed cytokine
production in MyD88+ cells
Supernatants of the four cell lines exposed to LPS
or Pac for 36 h were analyzed for levels of inflammatory cytokines
and growth factors. SKOV3/shTLR4 and OVCAR3/shTLR4 cells were found
to secrete low levels of cytokines IL-6 and IL-8. However, these
changes were not found in A2780/shTLR4 and 3AO/shTLR4. These
results suggested that LPS/Pac results in a significant
downregulation of IL-6 and IL-8 in MyD88+, but not in
MyD88− cells, in which TLR4 had been knocked down
(Figs. 5 and 6).
Effect of TLR4 on the Pac sensitivity of
MyD88+ EOC cells
Of note, a positive increase in caspase-3/7 activity
was identified between the MyD88+ and MyD88−
cell lines (Fig. 7). In order to
identify whether the MyD88+ cells respond to cell
apoptosis through TLR4-MyD88 signaling, RNA interference was used
to knock down the expression of TLR4 and the results are shown in
Fig. 7. A significant increase was
identified in caspase-3/7 activity following Pac treatment in
SKOV3/shTLR4 cells compared with SKOV3/shControl cells
(P<0.001), as was the case with OVCAR3 cells (P=0.000). No
significant difference was observed in changes of caspase-3/7
activity between A2780/shTLR4 and A2780/shControl cells, as well as
with 3AO cells. The results suggested that the TLR4-MyD88 signaling
negatively regulates ovarian cancer cell sensitivity to Pac.
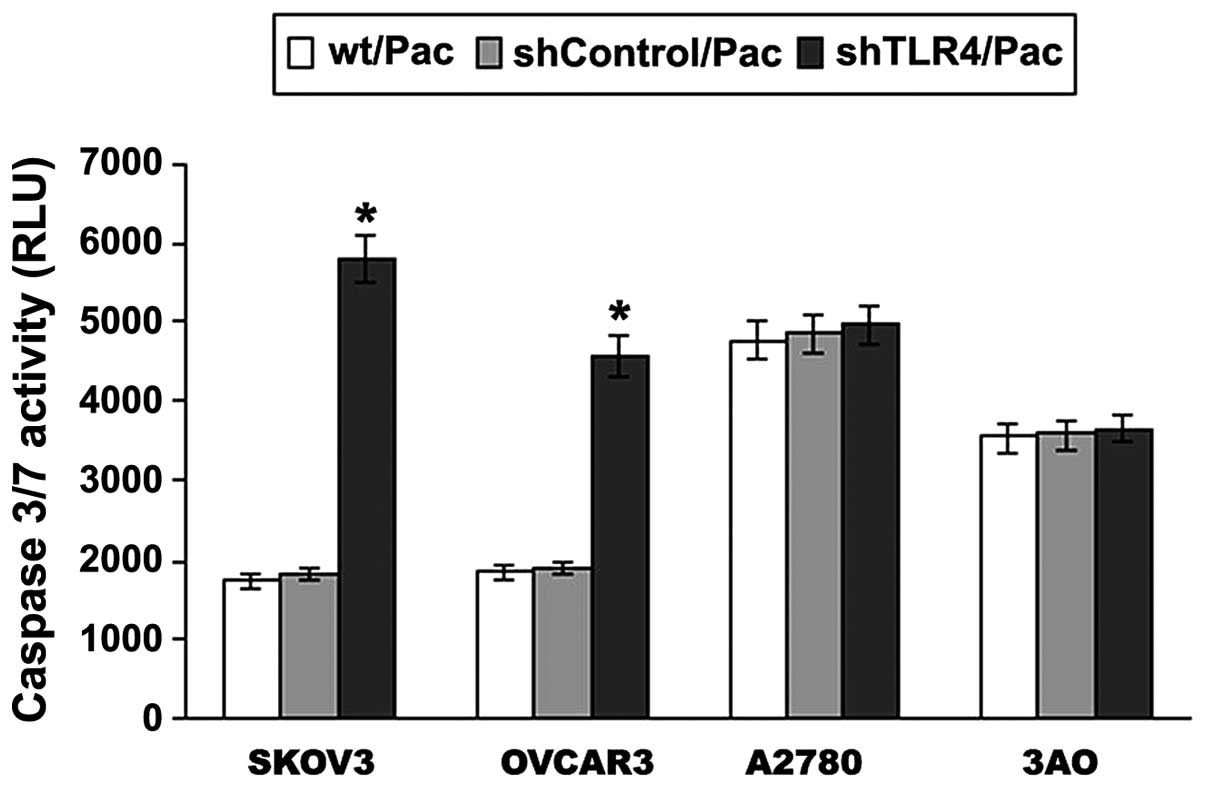 | Figure 7Following TLR4 knockdown, the
apoptotic response of Pac (2 μM) treatment in MyD88+ and
MyD88− EOC cell lines was investigated. EOC cell lines
were treated with 2 μmol/l Pac for 24 h and the levels of
caspase-3/7 were measured using the Caspase-Glo 3/7 assay. Data are
presented as the mean ± SD from at least three independent
experiments. A significant increase was identified in caspase-3/7
activity following Pac treatment in SKOV3/shTLR4 cells compared
with SKOV3 and SKOV3/shControl cells, as was the case with the
OVCAR3 cells. *P<0.001, vs. wt/Pac and shControl/Pac.
TLR4, Toll-like receptor 4; MyD88, myeloid differentiation factor
88; EOC, epithelial ovarian cancer; Pac, paclitaxel; wt/Pac,
SKOV3/OVCAR3/A2780/3AO parental cells treated with Pac;
shControl/Pac, SKOV3/OVCAR3/A2780/3AO shControl cells treated with
Pac; shTLR4/Pac, SKOV3/OVCAR3/A2780/3AO shTLR4 cells treated with
Pac. |
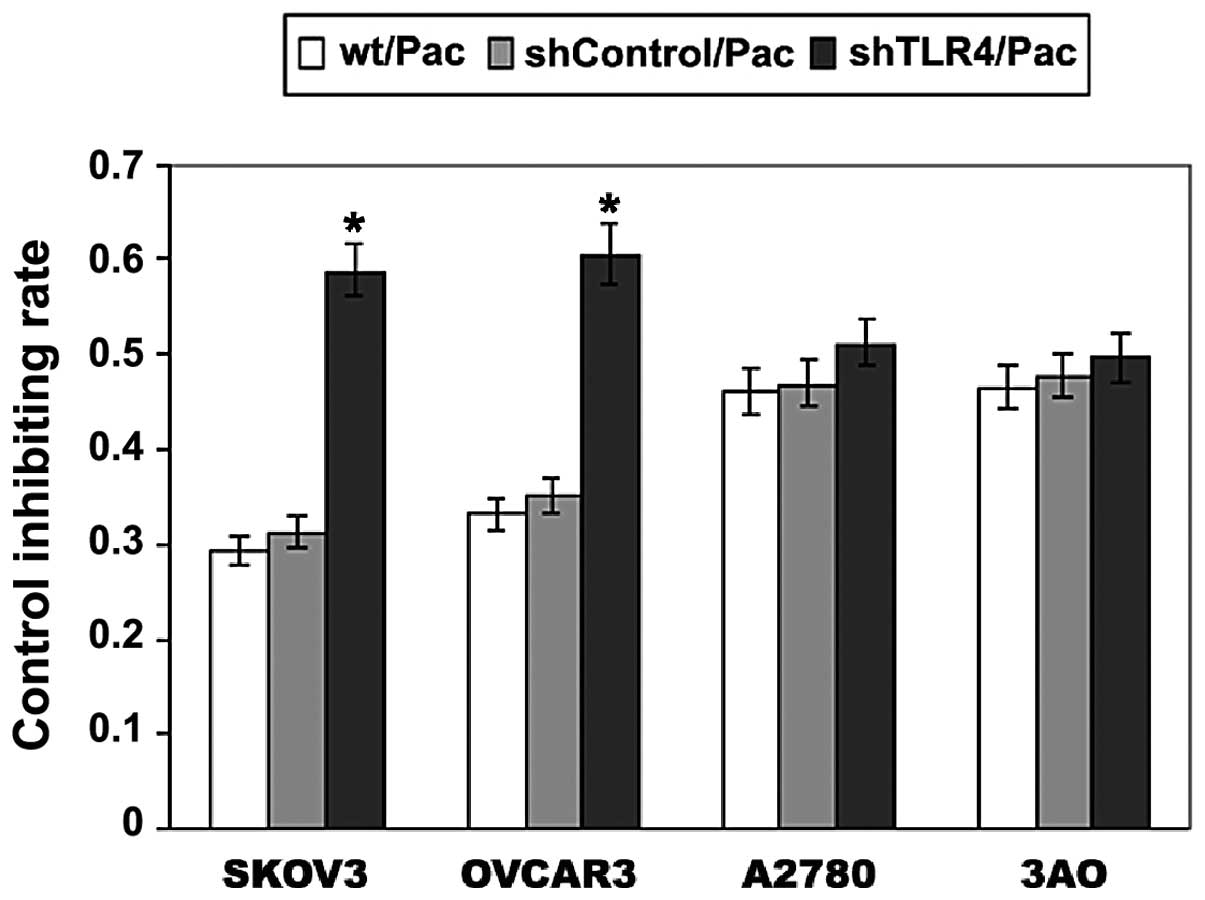
Knockdown of TLR4 depressed cell
proliferation in MyD88+ EOC cells
The effect of Pac on cell proliferation in SKOV3,
OVCAR3, A2780 and 3AO cell lines was investigated. In Fig. 8, the growth inhibiting rate (GIR) of
SKOV3/shTLR4 cells was ~60% following 24 h treatment of 2 μM Pac,
which is significantly higher than those of the parental SKOV3
(29%) and SKOV3/shControl (31%) cells (P<0.001). In the OVCAR3
cell line, the same treatments were used as with the parental
OVCAR3, OVCAR3/shControl and OVCAR3/shTLR4. The GIR was ~61% in
OVCAR3/shTLR4, which was higher than that of the parental OVCAR3
(33%) and OVCAR3/shControl (35%) (P<0.001). However, no
difference was observed in the proliferation among the three types
of A2780 cells (parental A2780, A2780/shControl and A2780/shTLR4),
as well as in the 3AO cells (parental A2780, A2780/shControl and
A2780/shTLR4). These results demonstrated that knockdown of TLR4
significantly restores the sensitivity of Pac in MyD88+
ovarian cancer cells.
Discussion
The successful treatment of ovarian cancer remains a
major challenge. Overall, >85% patients presenting with advanced
disease are likely to relapse. Recurrence defines incurable disease
in the majority of cases. The main obstacle to effective treatment
is the failure of initial therapy to eradicate a sufficient number
of tumor cells to prevent disease recurrence. Pac is a product of
the Pacific yew and its antimitotic actions are due to its ability
to bind and stabilize microtubules, which prevent accurate cell
division during mitosis (22,23).
Pac induces the secretion of inflammatory cytokines in murine
macrophages in a TLR4-dependent manner in addition to its antitumor
effects. Although, the effect of Pac on human macrophages is
controversial (24). Few previous
studies have analyzed the contribution of innate immunity pathways
to the mechanism of action of Pac (25). Pac is a first-line chemotherapeutic
agent used in the treatment of EOC as well as recurrent EOC
(26), and is known to be a TLR4
ligand (27,28). In the present study, it was
identified that Pac activates TLR4 signaling, which increases
ovarian cancer cell proliferation.
Previously, MyD88 has been reported to be a negative
regulator of TLR signaling (29,30,31).
It has been identified that MyD88 is an essential downstream
component of the TLR4 signaling cascade mediating Pac resistance
(19). In addition, it has been
reported that LPS-stimulated tumor cell supernatants inhibit T cell
proliferation and natural killer cell activity. Blockade of the
TLR4 pathway reverses the functions of these cells in vitro
and in vivo, delays tumor growth and, thus, prolongs the
survival of tumor-bearing mice (32). In the present study, TLR4 was found
to exhibit moderate (++) or strong (+++) expression in malignant
(20/24) and borderline (5/8) tumors and normal ovarian epithelium
(6/12). The expression of MyD88 in malignant tumors was
considerably greater (18/24) than that in normal ovarian tissue
(1/8) or borderline tumors (1/12). The results of the present study
support that MyD88 acts as a downstream factor and combines with
TLR4 to increase the proliferation of ovarian cancer cells.
If TLR4 signaling highlights a survival benefit to
tumor cells and alters their sensitivity to Pac, then its silencing
via siRNA must aid in identifying the molecular mechanisms
responsible for LPS- and Pac-mediated effects. We hypothesized that
in TLR4-MyD88 signaling, TLR4 is activated by Pac.
MyD88+ human ovarian carcinoma cells (SKOV3 and OVCAR3)
and MyD88− ovarian carcinoma cell lines (A2780 and 3AO)
were selected to investigate the TLR4 effects on apoptosis with Pac
chemotherapy. The molecular mechanisms of chemotherapy resistance
are considered to be associated with apoptosis inhibition (33,34).
Acquired resistance to chemotherapy is a significant impediment to
effective cancer therapy (35).
Notably, silencing of TLR4 expression in MyD88+ EOC
cells results in sensitization of the cells to Pac-induced
apoptosis and this sensitization is accompanied by the inhibition
of cytokine IL-6 and IL-8 production in response to Pac and LPS. In
the current study, MyD88+ cells (SKOV3 and OVCAR3)
constitutively secreted a wide range of cytokines including, IL-6
and IL-8. By contrast, MyD88− cells (A2780 and 3AO)
produced low levels of these cytokines. LPS and Pac significantly
increased the secretion of IL-6 and IL-8 in SKOV3 and OVCAR3 cells,
but not in A2780 and 3AO cells (Figs.
2 and 3).
The present study used RNA interference to knock
down the expression of TLR4 in SKOV3, OVCAR3, A2780 and 3AO cell
lines. Cytokine production in response to LPS and Pac stimulation
was significantly inhibited in SKOV3/shTLR4 and OVCAR3/shTLR4
cells. No changes in cytokine production were observed in
A2780/shTLR4 and 3AO/shTLR4 cells (Figs. 5 and 6).
Caspase-Glo 3/7 assay was used to investigate the
apoptosis of ovarian cancer cells. When TLR4 was knocked down, a
positive increase in caspase-3/7 activity was identified following
Pac treatment between MyD88+ and MyD88− cell
lines. The mechanism responsible for Pac resistance in ovarian
cancer is not completely understood. The present study confirmed
that there is a negative correlation between MyD88 expression and
Pac-induced apoptosis in TLR4 signaling, consistent with the
results of a previous study (36).
In addition, RNA interference was used to knockdown
the expression of TLR4 in SKOV3, OVCAR3, A2780 and 3AO cell lines.
In the caspase-Glo 3/7 assay, a significant increase of caspase-3/7
activity was identified in SKOV3/shTLR4 and in OVCAR3/shTLR4 cells
(Fig. 7).
The results of the current study indicated that
TLR4-MyD88 signaling negatively regulates Pac treatment. Knockdown
of TLR4 may increase Pac chemosensitivity in MyD88+
cells. In addition to the caspase-Gol 3/7 assay, cell proliferation
was evaluated and a significant increase of GIR was identified in
SKOV3/shTLR4 and OVCAR3/shTLR4 cell lines. However, in A2780/shTLR4
and 3AO/shTLR4 cells, no significant changes were observed compared
with their control cells (Fig. 8).
The present study revealed that the proliferation and survival of
the cancer cells is regulated by a specific defense mechanism in
TLR4/MyD88 signaling.
In conclusion, the observations of the current study
imply that Pac activates TLR4-MyD88 signaling, which increases
ovarian cancer cell proliferation. Although the results suggest
that the knockdown of TLR4 inhibits cell growth and that IL-6 and
IL-8 levels are associated with MyD88 (+) EOC cells, the precise
underlying molecular mechanisms responsible for these observations
remain to be established. As TLR4 is functionally associated with
tumor progression, TLR4 is likely to be a promising target for
tumor therapy in the future.
Acknowledgements
The authors would like to thank Professor Pei-Shu
Liu (Department of Obstetrics and Gynecology, Qilu Hospital,
Shandong University, Jinan, China) for support.
Abbreviations:
|
TLR4
|
Toll-like receptor 4
|
|
Pac
|
paclitaxel
|
|
MyD88
|
myeloid differentiation factor 88
|
References
|
1
|
Akira S, Uematsu S and Takeuchi O:
Pathogen recognition and innate immunity. Cell. 124:783–801. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takeda K, Kaisho T and Akira S: Toll-like
receptors. Annu Rev Immunol. 21:335–376. 2003. View Article : Google Scholar
|
|
3
|
Yu L and Chen S: Toll-like receptors
expressed in tumor cells: targets for therapy. Cancer Immunol
Immunother. 57:1271–1278. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Medzhitov R, Preston-Hurlburt P and
Janeway CA Jr: A human homologue of the Drosophila Toll protein
signals activation of adaptive immunity. Nature. 388:394–397. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Poltorak A, He X, Smirnova I, et al:
Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations
in Tlr4 gene. Science. 282:2085–2088. 1998. View Article : Google Scholar
|
|
6
|
Pathak SK, Basu S, Bhattacharyya A, Pathak
S, Kundu M and Basu J: Mycobacterium tuberculosis
lipoarabinomannan-mediated IRAK-M induction negatively regulates
Toll-like receptor-dependent interleukin-12 p40 production in
macrophages. J Biol Chem. 280:42794–42800. 2005. View Article : Google Scholar
|
|
7
|
He W, Liu Q, Wang L, Chen W, Li N and Cao
X: TLR4 signaling promotes immune escape of human lung cancer cells
by inducing immunosuppressive cytokines and apoptosis resistance.
Mol Immunol. 44:2850–2859. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fallows S, Price J, Atkinson RJ, Johnston
PG, Hickey I and Russell SE: P53 mutation does not affect prognosis
in ovarian epithelial malignancies. J Pathol. 194:68–75. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Byrd-Leifer CA, Block EF, Takeda K, Akira
S and Ding A: The role of MyD88 and TLR4 in the LPS-mimetic
activity of Taxol. Eur J Immunol. 31:2448–2457. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lord KA, Hoffman-Liebermann B and
Liebermann DA: Nucleotide sequence and expression of a cDNA
encoding MyD88, a novel myeloid differentiation primary response
gene induced by IL6. Oncogene. 5:1095–1097. 1990.
|
|
11
|
Asschert JG, Vellenga E, Ruiters MH and de
Vries EG: Regulation of spontaneous and TNF/IFN-induced IL-6
expression in two human ovarian-carcinoma cell lines. Int J Cancer.
82:244–249. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Watson JM, Sensintaffar JL, Berek JS and
Martinez-Maza O: Constitutive production of interleukin 6 by
ovarian cancer cell lines and by primary ovarian tumor cultures.
Cancer Res. 50:6959–6965. 1990.
|
|
13
|
Wu S, Rodabaugh K, Martinez-Maza O, et al:
Stimulation of ovarian tumor cell proliferation with monocyte
products inducing interleukin 1, interleukin 6 and tumor necrosis
factor-alpha. Am J Obstet Gynecol. 166:977–1007. 1992.PubMed/NCBI
|
|
14
|
Obata NH, Tamakoshi K, Shibata K, Kikkawa
F and Tomoda Y: Effects of interleukin-6 on in vitro cell
attachment, migration and invasion of human ovarian carcinoma.
Anticancer Res. 17:337–342. 1997.PubMed/NCBI
|
|
15
|
Nilsson MB, Langley RR and Fidler IJ:
Interleukin-6, secreted by human ovarian carcinoma cells, is a
potent proangiogenic cytokine. Cancer Res. 65:10794–10800. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shahzad MM, Arevalo JM, Armaiz-Pena GN, et
al: Stress effects on FosB- and interleukin-8 (IL8)-driven ovarian
cancer growth and metastasis. J Biol Chem. 285:35462–35470. 2010.
View Article : Google Scholar
|
|
17
|
Kawai T, Adachi O, Ogawa T, Takeda K and
Akira S: Unresponsiveness of MyD88-deficient mice to endotoxin.
Immunity. 11:115–122. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Björkbacka H, Fitzgerald KA, Huet F, et
al: The induction of macrophage gene expression by LPS
predominantly utilizes Myd88-independent signaling cascades.
Physiol Genomics. 19:319–330. 2004.PubMed/NCBI
|
|
19
|
Kelly MG, Alvero AB, Chen R, et al: TLR-4
signaling promotes tumor growth and Pac chemoresistance in ovarian
cancer. Cancer Res. 66:3859–3868. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Wang AC, Su QB, Wu FX, Zhang XL and Liu
PS: Role of TLR4 for Pac chemotherapy in human epithelial ovarian
cancer cells. Eur J Clin Invest. 39:157–164. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mao HL, Liu PS, Zheng JF, et al:
Transfection of Smac/DIABLO sensitizes drug-resistant tumor cells
to TRAIL or Pac-induced apoptosis in vitro. Pharmacol Res.
56:483–492. 2007. View Article : Google Scholar
|
|
22
|
Wani MC, Taylor HL, Wall ME, Coggon P and
McPhail AT: Plant antitumor agents. VI The isolation and structure
of taxol, a novel antileukemic and antitumor agent from Taxus
brevifolia. J Am Chem Soc. 93:2325–2327. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Manfredi JJ, Parness J and Horwitz SB:
Taxol binds to cellular microtubules. J Cell Biol. 94:688–696.
1982. View Article : Google Scholar
|
|
24
|
Wang J, Kobayashi M, Han M, et al: MyD88
is involved in thesignalling pathway for Taxol-induced apoptosis
and TNF-alpha expression in human myelomonocytic cells. Br J
Haematol. 118:638–645. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zimmer SM, Liu J, Clayton JL, Stephens DS
and Snyder JP: Paclitaxelbinding to human and murine MD-2. J Biol
Chem. 283:27916–27926. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ozols RF, Bundy BN, Greer BE, et al: Phase
III trial of carboplatin and Paclitaxel compared with cisplatin and
Pac in patients with optimally resected stage III ovarian cancer: a
Gynecologic Oncology Group study. J Clin Oncol. 21:3194–3200. 2003.
View Article : Google Scholar
|
|
27
|
Song G, Ouyang G and Bao S: The activation
of Akt/PKB signaling pathway and cell survival. J Cell Mol Med.
9:59–71. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ding AH, Porteu F, Sanchez E and Nathan
CF: Shared actions of endotoxin and taxol on TNF receptors and TNF
release. Science. 248:370–372. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Brint EK, Xu D, Liu H, et al: ST2 is an
inhibitor of interleukin 1 receptor and Toll-like receptor 4
signaling and maintains endotoxin tolerance. Nat Immunol.
5:373–379. 2004. View
Article : Google Scholar : PubMed/NCBI
|
|
30
|
Burns K, Janssens S, Brissoni B, Olivos N,
Beyaert R and Tschopp J: Inhibition of interleukin 1
receptor/Toll-like receptor signaling through the alternatively
spliced, short form of MyD88 is due to its failure to recruit
IRAK-4. J Exp Med. 197:263–268. 2003. View Article : Google Scholar
|
|
31
|
Schmitz I, Kirchhoff S and Krammer PH:
Regulation of death receptor-mediated apoptosis pathways. Int J
Biochem Cell Biol. 32:1123–1136. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Huang B, Zhao J, Li H, et al: Toll-like
receptors on tumor cells facilitate evasion of immune surveillance.
Cancer Res. 65:5009–5014. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Pérez-Tomás R: Multidrug resistance:
retrospect and prospects in anti-cancer drug treatment. Curr Med
Chem. 13:1859–1876. 2006.PubMed/NCBI
|
|
34
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Nakanishi C and Toi M: Nuclear
factor-kappaB inhibitors as sensitizers to anticancer drugs. Nat
Rev Cancer. 5:297–309. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Silasi DA, Alvero AB, Illuzzi J, et al:
MyD88 predicts chemoresistance to Pac in epithelial ovarian cancer.
Yale J Biol Med. 79:153–163. 2006.PubMed/NCBI
|