Introduction
The frequency of physical examinations and the
introduction of computed tomography (CT) of the chest have
increased the detection of peripheral nodular lesions in the lung
(PNLL). These lesions used to be followed up without intervention
or occasionally diagnosed by transbronchial aspiration
biopsy/cytology (TBAB/C) and brush cytology. However, when the
nodules are small and located in a peripheral region of the lung,
tumor cells may not be collected due to the difficulty of direct
aspiration. As a result, diagnostic accuracy has been far from
satisfactory. An increasing number of institutes have carried out a
CT-guided percutaneous lung biopsy (CTGLB) for PNLL (1,2).
Furthermore, aspiration biopsy cytology (ABC) or cytology using
lavage fluid from the aspiration needle have been used in these
examinations. Using these methods, tumor cells can be collected
from the samples for cytology, even in cases when a diagnosis is
not reached by biopsy. However, as there are only a small number of
cells in the aspiration needle and lavage fluid, it is imperative
to succeed in collecting tumor cells for an accurate diagnosis.
Conventionally, various methods have been used to smear the sample
attached to the aspiration needle directly onto a glass slide, and
to collect material by centrifugation of the lavage fluid (3). However, tumor cells are often
desquamated from the glass slide during sample preparation. In
addition, various contaminants, such as necrotic debris and
inflammatory cells, are included in the sample and may interfere
with the analysis. Several studies have attempted to solve these
problems. The present study investigated liquid-based cytology
(LBC), which has recently become a focus of attention.
LBC was first introduced in the area of
gynecological cytology, and has since been developed. LBC enables
not only reliable cell collection, but also the evaluation of
uniform samples. In addition, certain useful features have been
reported, including a reduction in problems in screening
observations. Cytology using LBC has also been used in the
pathological evaluation of other organs (4,5).
Reports indicate that LBC is an excellent method for reliably
collecting cells on glass slides from samples with a small number
of cells (6–10). LBC has also been used recently for
purposes other than cytological examination, for example, in
molecular biological tests and in the genotyping of the human
papilloma virus in patients with uterine cervical lesions (11,12).
The fixation solution used in LBC is alcohol-based, so the
destruction of the DNA and RNA is limited and the structure is
stable for a relatively long period. Therefore, it has been used
for various analyses in addition to cytology (13,14).
The present study investigated whether LBC, with
these various advantages, is useful for the cytology of lavage
fluid from the aspiration needle in CTGLB for PNLL.
Materials and methods
Case selection and specimen
collection
Of the cases that underwent CTGLB at the Ibaraki
Prefectural Central Hospital (Kasama, Japan) between 2004 and 2010,
130 were enrolled in this study. Biopsy samples were collected from
these cases, and LBC samples were prepared from the lavage fluid of
the aspiration needle. The cases were divided into two groups. One
group was comprised of 73 cases in which tumor lesions were
diagnosed or suspected from at least one of the following: Biopsy,
LBC and the conventional method (CM); and in which partial lung
resection was performed after thoracotomy or under thoracoscopy. CM
is an ordinary sample processing process without the LBC method.
These cases were diagnosed histologically from the resected
specimens. The other group was comprised of 57 cases in which a
definite diagnosis was not made clinically or by any other method.
These cases were followed up radiologically and considered to have
non-tumorous lesions, as the nodules showed no aggravation (nodule
size increase), disappeared or remained unchanged. The study
protocol was approved by the ethics committee of Ibaraki
Prefectural Central Hospital. Written informed consent was obtained
from the patients or patient’s family.
Sample preparation
The biopsy tissue that was obtained was fixed
immediately in 10% buffered formaldehyde solution, embedded in
paraffin, sliced according to the CM and stained with
hematoxylin-eosin for observation. The lavage fluid obtained by
washing the aspiration needle following biopsy with physiological
saline was centrifuged (1,700 × g for 10 min). The sediment was
smeared on silane-coated slides using the wedge method and fixed
immediately in 95% ethanol using the CM. LBC samples were prepared
with ThinPrep2000™ (Cytyc Corporation, Boxbough, MA, USA),
according to the manufacturer’s instructions. The centrifuged
sediment was resuspended and fixed in cytopreservative solution,
and smear samples were automatically prepared with ThinPrep, for
which special filters and glass slides were set up. Samples
constructed by the CM, and the LBC samples, were subjected to
Papanicolaou staining for observation.
Evaluation and classification
For diagnostic evaluation of the cytological samples
prepared by LBC and the CM, two observers first observed and
diagnosed them independently. When the diagnosis was inconsistent,
the two observers reexamined the case under a double-headed
microscope and reached a joint conclusion. A cytological diagnosis
was made according to three grades: i) Benign, no malignant cells
present; ii) suspicion of malignancy, presence of atypical cells
suspicious of malignancy; and iii) malignant, presence of malignant
cells. When malignancy was diagnosed, the histological types were
estimated. The cytological, biopsy-based and radiological diagnoses
were made in an independent and blinded manner, so that information
from one method could not affect the diagnosis by other
methods.
Statistical analysis
The sensitivity, specificity and accuracy of these
diagnostic methods were calculated. The χ2 test was
employed for the comparison between two groups, and P<0.05 was
considered to indicate a statistically significant difference.
Results
Sample preparation and evaluation
Samples were prepared and evaluated appropriately by
LBC in 94/130 cases (72%) and by CM in 47/130 cases (36%).
Inappropriate sample preparation, such as no cell collection on the
glass slide, occurred by LBC in 36/130 cases (28%) and by CM in
83/130 cases (64%) (Table I).
Table II shows the cases that
could be evaluated according to the three-grade cytological
criteria.
 | Table ISuitability of the specimens for LBC
and CM. |
Table I
Suitability of the specimens for LBC
and CM.
| Suitability | LBC, n (%) | CM, n (%) |
|---|
| Adequate | 94 (72) | 47 (36) |
| Inadequate | 36 (28) | 83 (64) |
 | Table IIResults of cytological evaluation by
LBC and CM. |
Table II
Results of cytological evaluation by
LBC and CM.
| Evaluation | LBC, n (%) | CM, n (%) |
|---|
| Benign | 47 (50) | 32 (68) |
| Suspicious | 24 (26) | 9 (19) |
| Malignant | 23 (24) | 6 (13) |
Cytological findings
The cellular findings by LBC and the CM were as
follows. In the squamous cell carcinoma cases, the cellular
findings were comparable between the two methods. Contamination
with necrotic debris and inflammatory cells in the background was
limited, and the majority of tumor cells were scattered and present
in an isolated or solitary manner. The cytoplasm of the tumor cells
was dense and either eosinophilic or amphophilic. The nuclei were
hyperchromatic with an uneven distribution of coarse granular
chromatin, while the nuclear membrane was irregular and nucleoli
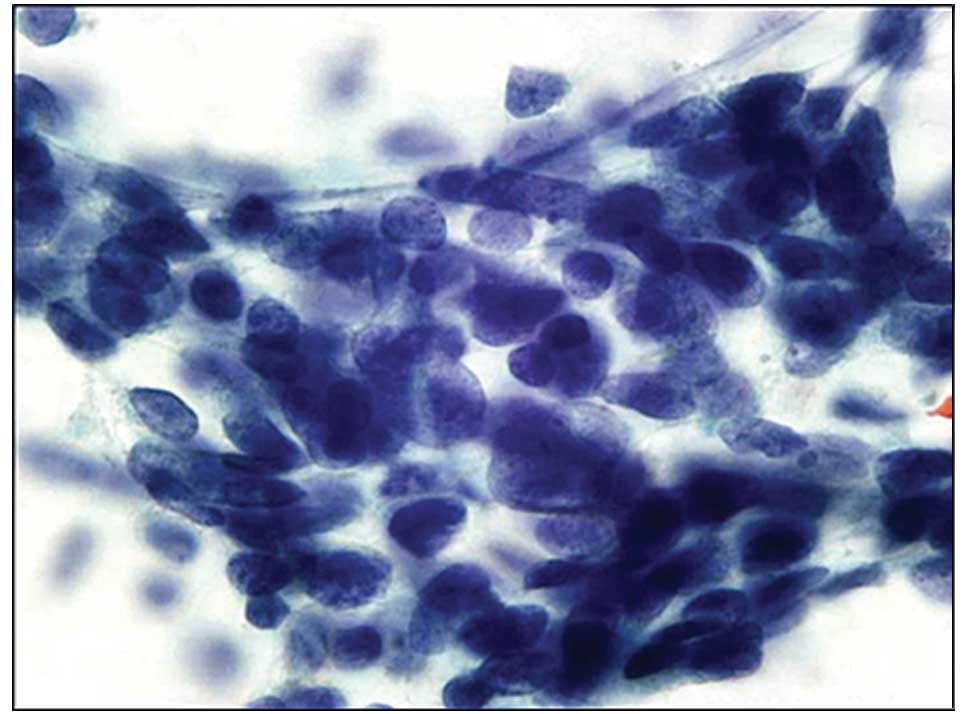
were prominent. In the adenocarcinoma cases, cell clusters were
three-dimensional or overlapping and maintained a sheet-like or
papillary cluster. Small glandular lumina were also observed within
the cluster. However, the tumor cells showed a slight difference in
nuclear findings. Compared with LBC, swollen nuclei were prominent
in the CM (Fig. 1). Conversely, in
LBC, the nuclei were smaller than in the CM, and fine granular
chromatin and prominent nucleoli were observed (Fig. 2). Small cell carcinoma cells
exhibited a pavement-like arrangement with stripped and
hyperchromatic nuclei (Fig. 3). In
the metastatic tumor cases, tumor cells showed similar cellular
findings to the primary lesion. In the cases of metastatic tumor
derived from colon cancer, the clusters were composed of columnar
tumor cells containing mucinous fluid or necrotic debris in the
background.
Histopathological correlation
The final histological diagnosis of the resected
material, biopsy-based diagnosis and estimated histological
diagnosis by LBC and the CM are shown in Table III. In the final diagnosis, there
were 65 cases with primary tumors (64 malignant and 1 benign), 6
with metastatic tumors and 2 with non-tumorous lesions. Malignant
tumors were diagnosed by biopsy in 64 cases. A total of 47 cases
were diagnosed as malignant by LBC and 31 cases were diagnosed as
malignant by CM. Malignancy was finally confirmed in the surgical
specimens. Although the definite diagnosis was not obtained by
biopsy-based examination, 10 cases were finally diagnosed as
malignant in the resected specimens. Six of them were diagnosed as
malignant or having suspected malignancy by LBC. In the remaining
four cases, the samples showed no sign of malignancy or were
inappropriate for evaluation by LBC. Furthermore, of the cases that
obtained a definite diagnosis by biopsy, four had tuberculosis or
inflammatory disease. Either their evaluation by LBC was benign or
the samples were inappropriate.
 | Table IIIDetails of histological subtype and
the number of cases in the specimen from resection, biopsy, LBC and
CM. |
Table III
Details of histological subtype and
the number of cases in the specimen from resection, biopsy, LBC and
CM.
| Histological
subtype | Resection | Biopsy | LBC | CM |
|---|
| Primary tumor |
| SCC | 8 | 8 | 6 | 3 |
| AC | 46 | 37 | 33 | 24 |
| SM | 2 | 4 | 3 | 1 |
| Other | 8 | 0 | 2 | 1 |
| Non-small | 0 | 6 | 1 | 1 |
| Malignant cells | 0 | 0 | 2 | 1 |
| AAH | 0 | 1 | 0 | 0 |
| Atypical cells | 0 | 1 | 10 | 2 |
| Benign tumor | 1 | 2 | 0 | 0 |
| Metastatic tumor | 6 | 5 | 2 | 0 |
| Non tumorous
lesion | 2 | 7 | 6 | 1 |
| Inadequate | 0 | 2 | 8 | 39 |
| Total | 73 | 73 | 73 | 73 |
Statistical findings
LBC on its own provided a sensitivity of 68%, a
specificity of 61% and an accuracy of 65%. A combination of biopsy
and LBC provided a sensitivity of 94%, a specificity of 81% and an
accuracy of 84% (Table IV).
 | Table IVReliability of biopsy, LBC and the
two combined. |
Table IV
Reliability of biopsy, LBC and the
two combined.
| Reliability | Biopsy | LBC | Biopsy + LBC |
|---|
| Sensitivity | 86 | 68 | 94 |
| Specificity | 89 | 61 | 81 |
| Accuracy | 87 | 65 | 84 |
Discussion
With regard to the utility of ABC for PNLL, previous
studies have shown that CTGLB cytology provides an accuracy of
67.6%. In the present study, inappropriate samples were eliminated
by staining and the collected samples were evaluated immediately on
site (3). This contributed to the
high accuracy of this approach, which was superior to the
conventional smear method, but inferior to LBC in the present
study. The skill of the examiner and the diameter of the aspiration
needle may have had a certain level of impact on the degree of
accuracy. Furthermore, the procedure in which the aspiration needle
touches the glass slide directly in the conventional smear method
may have had a certain effect on the results. It is easy to obtain
a large number of cells with this method, but it is not possible to
examine the samples other than on a glass slide. In certain cases,
this method is likely to cause mechanical damage to the biopsy
material, which may occasionally affect the diagnosis.
LBC, initially introduced in the field of
gynecology, has begun to be used frequently in other fields
(15). Urine cytology has been
employed most frequently at various institutes. Liquid samples are
the subject of urine cytological examination, so there is the
disadvantage that the cells may desquamate easily from the glass
slide. Several methods have been devised to prevent this. Following
reports that a large number of cells may be collected most
efficiently by LBC, the diagnostic accuracy of urine cytology has
improved (16,17). With regard to the application of
fluid samples from the body cavity, diagnostic accuracy is not
improved compared to the conventional CytoSpin method (18). In addition, LBC has been applied for
lavage fluid of the brush in biliary disease (19), and the diagnostic accuracy was
improved by combination with the CM. Moreover, LBC has been used
for ABC for various diseases and organs other than those providing
liquid samples. This application is aimed at avoiding the loss of
cells and preparing samples efficiently, as ABC collects only a
small number of cells (7–10).
To date, a few studies have used LBC for PNLL,
similar to the present study. One study showed that, compared with
the CM, the overall accuracy improved and the number of
inappropriate samples decreased with LBC (20). In the present study, there was a
large difference between LBC and the CM in terms of the sample
number that could be evaluated. A large number of cells on the LBC
slides improved the accuracy of the diagnosis. Conversely, more
than half of the cases in the CM could not be evaluated, as
insufficient numbers of cells were collected. The fact that there
was a large difference in the percentage of samples that could be
evaluated was attributable to several factors. Few cells were
collected reliably and smeared on glass slides, and the cells were
difficult to desquamate from the LBC samples. Although the
diagnostic specificity of LBC was inferior to biopsy, LBC exceeded
the CM in terms of sensitivity and accuracy. However, data from a
study by Konofaos et al (20) differed greatly from the present
study results, as it reported that the specificity was 100% for LBC
and the CM. In the study, the tumors were resected by thoracotomy
and all 80 were histologically diagnosed as benign or malignant and
then analyzed. However, non-tumor cases were not included. In such
groups, the sensitivities are calculated with the number of cases
evaluated as positive by cytological diagnosis. However, it is
difficult to understand how the specificities were calculated, as
the non-tumor cases were not included. Moreover, a negative
predictive value was also calculated (20). Wallace et al (21) constructed cell blocks from LBC
samples and made a diagnosis. It was reported that the LBC samples
were high in quality and that the construction of cell blocks
enabled immunohistochemistry to be applied. Furthermore, although
the analysis was made only for the case of small cell carcinoma of
the lung, Kim and Owens (22)
concluded that LBC should replace the CM in constructing
pathological samples.
Endoscopic ultrasound-guided transbronchial or
transesophageal lymph node aspiration (23) and aspiration biopsy under
thoracoscopy (24) have been
carried out in a number of institutes for lung hilar lymph nodes to
determine the staging of tumor cases. Construction of LBC samples
from these aspiration biopsy materials may be useful for various
reasons.
In the present study, the number of cases diagnosed
as malignant by LBC was comparable to that by biopsy. However, six
cases were not diagnosed by biopsy, but were determined to be
either malignant or suspected of malignancy by LBC. These results
indicate that a combination of biopsy and LBC would increase
diagnostic accuracy. In fact, sensitivity, specificity and accuracy
all improved with this combination compared with either method on
its own. Four cases that were diagnosed as being malignant in the
resected specimens failed to be diagnosed as malignant by biopsy or
LBC. In these cases, it was suspected that the tumors were not
properly aspirated by biopsy or LBC.
There was no significant difference in the
appearance pattern of tumor cells and the shapes of clusters
between the two methods in the cases with adenocarcinoma that could
be evaluated. While the nuclei of the tumor cells showed marginal
swelling with the CM, this finding was either not observed or the
nuclei tended to be smaller in size with LBC. These varying results
may be attributable to the nuclei being swollen in physiological
saline, but then reduced in size by alcoholic fixation in the LBC
preparation solution.
LBC enables the evaluation of a large number of
uniform samples and has several advantages in being able to collect
material from samples containing only a few cells. Furthermore,
liquid samples can be stored for a certain period of time prior to
smearing and can be used for other analyses, including
immunocytochemical analysis. In LBC samples, the cell membranes and
the cytoplasmic and intranuclear antigenicity are maintained, and
the samples are suitable for immunocytochemical analysis (25–27).
In certain cases, immunocytochemical analysis using LBC samples
would be useful for the determination of benign or malignant tumors
in the lung (28). In addition, LBC
has also been used for molecular diagnosis, such as in fluorescent
in situ hybridization (29,30).
Molecular targeting therapy has been carried out in inoperable
cases with non-small cell lung carcinoma and in pre-operative
neoadjuvant therapy. The indication for gefitinib therapy is
currently evaluated based on the presence or absence of a mutation
in the epidermal growth factor receptor gene. Biopsy samples and
surgically-resected specimens have been used mostly for
exploration, and cytology specimens may also be increasingly
employed in the future (31,32).
LBC is likely to become one of the applicable methods in this
field.
In conclusion, CTGLB is likely to be employed more
frequently in the pre-operative diagnosis of PNLL. Diagnostic
accuracy is likely to improve as CT instrument and aspiration
devices are developed. Furthermore, LBC is the most appropriate
method to enable the collection of cells from samples containing
only a few cells. Aspiration biopsy and LBC may be used in
combination more frequently in the future, as this can improve the
sensitivity, specificity and accuracy of a diagnosis.
References
|
1
|
Fassina A, Corradin M, Zardo D,
Cappellesso R, Corbetti F and Fassan M: Role and accuracy of rapid
on-site evaluation of CT-guided fine needle aspiration cytology of
lung nodules. Cytopathology. 22:306–312. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Khouri NF, Stitik FP, Erozan YS, et al:
Transthoracic needle aspiration biopsy of benign and malignant lung
lesions. AJR Am J Roentgenol. 144:281–288. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kothary N, Lock L, Sze DY and Hofmann LV:
Computed tomography-guided percutaneous needle biopsy of pulmonary
nodules: impact of nodule size on diagnostic accuracy. Clin Lung
Cancer. 10:360–363. 2009. View Article : Google Scholar
|
|
4
|
Kirschner B, Simonsen K and Junge J:
Comparison of conventional Papanicolaou smear and SurePath
liquid-based cytology in the Copenhagen population screening
programme for cervical cancer. Cytopathology. 17:187–194. 2006.
View Article : Google Scholar
|
|
5
|
Rinas AC, Mittman BW Jr, Le LV, Hartmann
K, Cayless J and Singh HK: Split-sample analysis of discarded cells
from liquid-based Pap smear sampling devices. Acta Cytol. 50:55–62.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fetsch PA, Simsir A, Brosky K and Abati A:
Comparison of three commonly used cytologic preparations in
effusion immunocytochemistry. Diagn Cytopathol. 26:61–66. 2002.
View Article : Google Scholar
|
|
7
|
Joseph L, Edwards JM, Nicholson CM, Pitt
MA and Howat AJ: An audit of the accuracy of fine needle aspiration
using a liquid-based cytology system in the setting of a rapid
access breast clinic. Cytopathology. 13:343–349. 2002. View Article : Google Scholar
|
|
8
|
Kontzoglou K, Moulakakis KG, Konofaos P,
Kyriazi M, Kyroudes A and Karakitsos P: The role of liquid-based
cytology in the investigation of breast lesions using fine-needle
aspiration: a cytohistopathological evaluation. J Surg Oncol.
89:75–78. 2005. View Article : Google Scholar
|
|
9
|
Nasuti JF, Tam D and Gupta PK: Diagnostic
value of liquid-based (Thinprep) preparations in nongynecologic
cases. Diagn Cytopathol. 24:137–141. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Parfitt JR, McLachlin CM and Weir MM:
Comparison of ThinPrep and conventional smears in salivary gland
fine-needle aspiration biopsies. Cancer. 111:123–129. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Prétet JL, Vidal C, Le Bail Carval K, et
al: Novaprep(®) Vial Test is a suitable liquid-based cytology
medium for high risk human papillomavirus testing by Hybrid Capture
2. J Clin Virol. 49:286–289. 2010.
|
|
12
|
Valasoulis G, Koliopoulos G, Founta C, et
al: Alterations in human papillomavirus-related biomarkers after
treatment of cervical intraepithelial neoplasia. Gynecol Oncol.
121:43–48. 2011. View Article : Google Scholar
|
|
13
|
Apostolidou S, Hadwin R, Burnell M, et al:
DNA methylation analysis in liquid-based cytology for cervical
cancer screening. Int J Cancer. 125:2995–3002. 2009. View Article : Google Scholar
|
|
14
|
Komatsu K, Nakanishi Y, Seki T, et al:
Application of liquid-based preparation to fine needle aspiration
cytology in breast cancer. Acta Cytol. 52:591–596. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Imura J, Uchida Y, Tomita S, Ichikawa K
and Fujimori T: Current and future of liquid based cytology. Pathol
Clin Med. 27:1144–1151. 2009.(In Japanese).
|
|
16
|
Hwang EC, Park SH, Jung SI, et al:
Usefulness of liquid-based preparation in urine cytology. Int J
Urol. 14:626–629. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lu DY, Nassar A and Siddiqui MT:
High-grade urothelial carcinoma: comparison of SurePath
liquid-based processing with cytospin processing. Diagn Cytopathol.
37:16–20. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Ylagan LR and Zhai J: The value of
ThinPrep and cytospin preparation in pleural effusion cytological
diagnosis of mesothelioma and adenocarcinoma. Diagn Cytopathol.
32:137–144. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Volmar KE, Vollmer RT, Routbort MJ and
Creager AJ: Pancreatic and bile duct brushing cytology in 1000
cases: review of findings and comparison of preparation methods.
Cancer. 108:231–238. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Konofaos P, Tomos P, Malagari K, et al:
The role of ThinPrep cytology in the investigation of lung tumors.
Surg Oncol. 15:173–178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Wallace WA, Monaghan HM, Salter DM,
Gibbons MA and Skwarski KM: Endobronchial ultrasound-guided
fine-needle aspiration and liquid-based thin-layer cytology. J Clin
Pathol. 60:388–391. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim S and Owens CL: Analysis of ThinPrep
cytology in establishing the diagnosis of small cell carcinoma of
lung. Cancer. 117:51–56. 2009.PubMed/NCBI
|
|
23
|
Wallace WA and Rassl DM: Accuracy of cell
typing in nonsmall cell lung cancer by EBUS/EUS-FNA cytological
samples. Eur Respir J. 38:911–917. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Iwasaki A, Kamihara Y, Yoneda S, Kawahara
K and Shirakusa T: Video-assisted thoracic needle aspiration
cytology for malignancy of the peripheral lung. Thorac Cardiovasc
Surg. 51:89–92. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Harton AM, Wang HH, Schnitt SJ and Jacobs
TW: p63 Immunocytochemistry improves accuracy of diagnosis with
fine-needle aspiration of the breast. Am J Clin Pathol. 128:80–85.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Piaton E, Faÿnel J, Ruffion A, Lopez JG,
Perrin P and Devonec M: p53 immunodetection of liquid-based
processed urinary samples helps to identify bladder tumours with a
higher risk of progression. Br J Cancer. 93:242–247. 2005.
View Article : Google Scholar
|
|
27
|
Sartelet H, Lagonotte E, Lorenzato M, et
al: Comparison of liquid based cytology and histology for the
evaluation of HER-2 status using immunostaining and CISH in breast
carcinoma. J Clin Pathol. 58:864–871. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Simsir A, Wei XJ, Yee H, Moreira A and
Cangiarella J: Differential expression of cytokeratins 7 and 20 and
thyroid transcription factor-1 in bronchioloalveolar carcinoma: an
immunohistochemical study in fine-needle aspiration biopsy
specimens. Am J Clin Pathol. 121:350–357. 2004. View Article : Google Scholar
|
|
29
|
Mian C, Lodde M, Comploj E, et al:
Liquid-based cytology as a tool for the performance of uCyt+ and
Urovysion Multicolour-FISH in the detection of urothelial
carcinoma. Cytopathology. 14:338–342. 2003.PubMed/NCBI
|
|
30
|
Sui W, Ou M, Chen J, et al: Human
telomerase RNA gene (TERC) gain and polysomy of chromosome 3 in
cervicovaginal liquid-based pap preparations: a fluorescence in
situ hybridization study. Eur J Gynaecol Oncol. 31:375–379.
2010.PubMed/NCBI
|
|
31
|
Billah S, Stewart J, Staerkel G, Chen S,
Gong Y and Guo M: EGFR and KRAS mutations in lung carcinoma:
molecular testing by using cytology specimens. Cancer Cytopathol.
119:111–117. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
van Eijk R, Licht J, Schrumpf M, et al:
Rapid KRAS, EGFR, BRAF and PIK3CA mutation analysis of fine needle
aspirates from non-small-cell lung cancer using allele-specific
qPCR. PLoS One. 6:e177912011.
|