Introduction
Lymphatic hygromas are rare lymphatic vessel
malformations, which are localized in areas of abnormal development
of the lymphatic system. Commonly, the disease is diagnosed during
childhood, is located in the head and neck region and occurs in one
out of every 2,000–4,000 live births (1,2). Only
5% are found in the abdominal cavity, including the small
intestine, colon and mesentery (2–4).
Lymphatic hygromas predominantly present in childhood, with >50%
diagnosed at birth and 90% by 2 years of age (5,6).
However, a large number of abdominal lymphatic hygromas do not
manifest until adulthood (7).
Proper diagnosis remains difficult for abdominal lymphatic hygroma
during routine examinations, particularly in adults. Further
progression in standard therapy is necessary for improving
treatment of all lymphatic hygromas. The current study presents the
case of a male adult with mesenteric lymphatic hygroma and a
literature review of lymphatic hygroma.
Case report
A 23-year-old male was admitted to Huashan Hospital
Affiliated to Fudan University (Shanghai, China) with the chief
complaint of progressive dull pain in the upper abdominal region
that had been present for seven years. This symptom worsened when
the patient was lying down, particularly in a lateral position, and
was slightly relieved by a horizontal position. In addition, the
patient suffered from a change in bowel habits with an irregular
alternation between diarrhea and constipation. The patient did not
receive any related medical examinations until seven days prior to
admission. A computed tomography (CT) scan indicated a huge cystic
mass in the midabdomen, with an estimated maximum diameter of
>15 cm (Fig. 1). The top three
suspected radiological diagnoses were teratoma, lipoblastoma and
lymphatic hygroma. A physical examination revealed a huge mass with
soft feedback and a vague boundary upon palpation. No other
significant features were found.
A laparotomy was performed with a median abdominal
incision. Intraoperative exploration revealed a huge mass within
the small bowel mesentery extending into the mesentery of the left
colon. The size of the mass was measured to be ~30×20×15 cm. The
appearance of the mass was found to be yellowish, cystic and
lobulated. The capsule was almost complete, with the exception of
an unclear section of the Toldt’s fascia, which was confused with
the Gerota’s fascia of the left kidney. Tight adhesion was found
near to the descending section of the duodenum. Following entrance
to the potential space of the mesentery by peritoneal incision
lateral to the descending colon, the mass was easily separated by
blunt dissection. The trunk of the superior mesenteric vessels was
carefully protected, and sharp and blunt dissection were practiced
to control the tight adhesion in the duodenal region. Visible
tumor-related lymph vessels were most concentrated in the
superficial layer covering the head of the pancreas and drained
towards the retroperitoneum. These lymph vessels were ligated
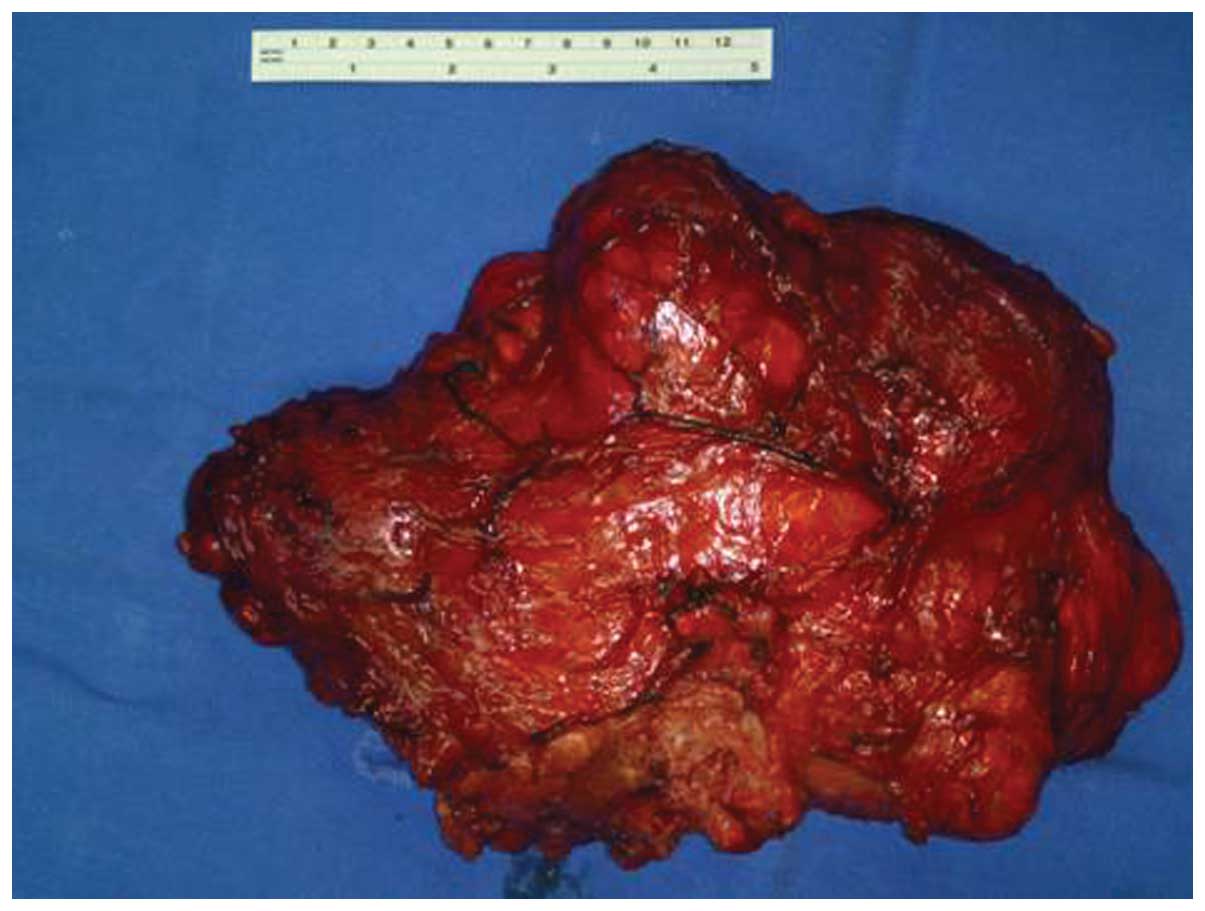
carefully. The mass was removed completely without tumor
perforation and intra-abdominal pollution (Fig. 2). The duodenum, small intestine and
capsule of the pancreas were checked to confirm that there was no
damage. Two drainage tubes were used; tube 1 in the left paracolic
gutter and tube 2 near to the descending section of the duodenum
above the head of the pancreas.
Investigation of the resected tumor identified that
each lobule was filled with a milky white fluidic content.
Following fixation with formalin, the size of the tumor was reduced
to 29.5×16.5×3.0 cm. Multicystic structures were found with
morphological observation, and the diameter of the cysts ranged
between 0.3 and 2 cm. Caseous material remained inside the cysts
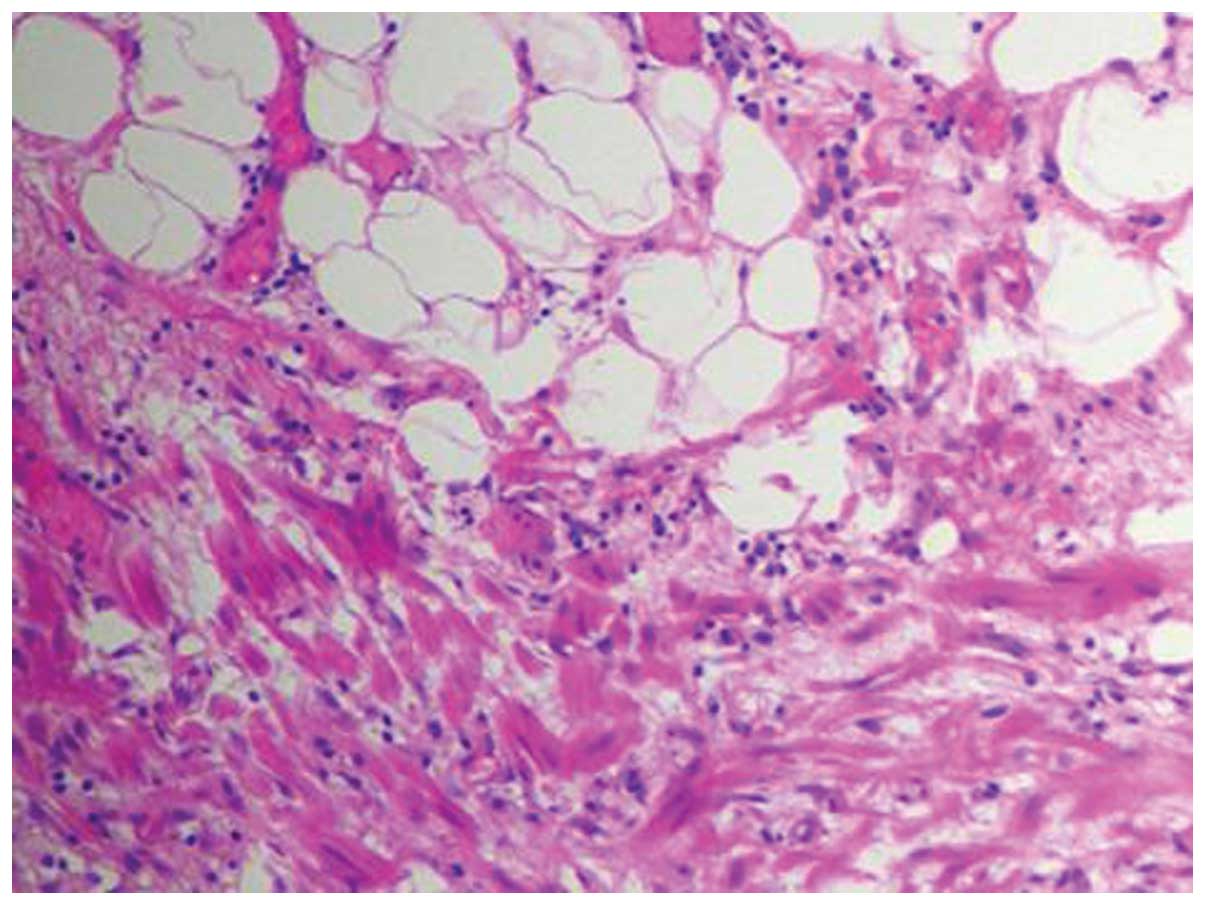
following drainage of the milky white fluid. Microscopically, the
cystic walls of the tumor were comprised of flat endothelial and
smooth muscle cells (Fig. 3). Lipid
deposition occupied the intracystic space lined by flat endothelial
cells and lymphocyte infiltration (Fig.
4). The pathological diagnosis supported lymphatic hygroma.
The patient’s first bowel movement occurred three
days after surgery. Drainage tube 1 (in the left paracolic gutter)
was removed without any adverse events on day 6. A milky chylous
fluid appeared in tube 2 (placed at the descending section of the
duodenum), since a fluid diet had been administered on day 4. A
chyle test of the drainage indicated a positive result that
confirmed the diagnosis of chylous leakage. Since the volume of the
drainage was 30–50 ml each day, which is clinically acceptable, no
special treatment was provided and a semi-fluid diet was initiated
on day 7. The patient recovered well post-operatively and the
drainage was removed on day 14 when the drainage volume had
decreased to 2–3 ml per day. The patient provided written informed
consent.
Discussion
Lymphangiomas are divided into the following three
major histological types: Simple or capillary hemangioma, cavernous
hemangioma and lymphatic hygroma (5,6). The
cavernous type is most frequently found in the mesenteric region
and colonic wall (8). Lymphatic
hygromas are defined as malformations of the lymphatic system
appearing as single or multiloculated cavities (9). Pathologically, lymphatic hygromas are
composed of dilated cysts containing proteinaceous eosinophilic
fluid, separated by endothelial cells (10,11).
Symptoms occur only after the lymphatic hygroma compresses the
adjacent structures (12). The
symptoms of abdominal lymphatic hygroma are not specific, including
transient pain, nausea, vomiting, abdominal distention, ascites and
fever. Complications include inflammation, intestinal or ureter
obstructions, cystic ruptures and bleeding (13–15).
The most common location of mesenteric lymphangioma is the small
bowel volvulus, usually complicated by acute intestinal obstruction
(16). In the present case, the
mesenteric lymphangioma was located at the small bowel volvulus,
but did not cause the complication of intestinal obstruction.
The etiology and pathogenesis of lymphatic hygroma
are poorly understood. It has been indicated that its possible
etiologies are attributed to chronic inflammation and fibrosis,
trauma, lymph node atrophy and lymphatic endothelial disturbances
(5). Genetic factors are also
important for the formation of lymphatic hygroma, which may lead to
the loss of connection between the lymphatic and venous systems,
abnormal budding of lymphatic structures from the cardinal vein and
abnormal sequestration of lymphatic tissue in early embryogenesis
(17). Previously, Chen et
al (18) showed that the
congenital malformation causes sequestration of lymphatic vessels
during the embryonic period.
A pre-operative diagnosis of lymphatic hygroma is
often difficult, since it is difficult to distinguish lymphatic
hygroma from other cystic masses due to its rarity. The
radiographic characterizations are profitable for arranging
treatments and predicting lower-stage lesion treatment outcomes.
The sonographic characteristic of a multilocular lesion filled with
clear fluid matches the diagnosis of lymphatic hygroma (19). Lymphatic hygroma typically appears
as a large, thin-walled, multiseptate cystic mass on CT scan
(20,21). Enhanced CT scans show enhancement of
the cyst walls and septa of the lymphatic hygroma (22). In addition, Choi et al
(23) previously described the use
of magnetic resonance in the diagnosis of lymphatic hygroma. A
correct diagnosis for lymphatic hygroma is only possible based on
these examinations, which complement each other and whose use may
not be beneficial alone (15,24).
Multiple staging systems have been developed for
lymphatic hygromas. According to histological results, lymphatic
hygromas are divided into three categories (5,6).
However, such categories do not correspond with the clinical
behavior or therapeutic response. Previous studies by de Serres
et al (25)and Hamoir et
al (26) demonstrated an
additional staging system for lymphatic hygroma, according to the
predicted prognosis and surgical results. In addition, Smith et
al (27) categorized lymphatic
hygromas as macrocystic, microcystic or mixed type based on the
sclerotherapy outcome. Currently, no standard classification system
exists. Further clinical study is required for establishing a
suitable classification procedure.
The goal of lymphatic hygroma treatment is the
restoration or preservation of functional integrity and the
patients’ quality of life. Lymphatic hygromas do not only compress
and infiltrate adjacent structures, but occur with complications,
including intracystic hemorrhage, cyst rupture, volvulus or
infection (28). As a result,
surgery is a beneficial therapy for such cases. If no significant
functional deficit is identified, treatment may be delayed and
consist of surgery, sclerotherapy or observation (29). The current report set the following
indications for abdominal lymphatic hygroma: i) Acute abdominal
symptoms; ii) large mass affecting patients’ quality of life; iii)
intracystic hemorrhage, cyst rupture, volvulus or infection; iv)
obstruction of the intestine, colon or urinary system; and v)
compression of the main vein or artery. All these factors also
contribute to the timing of intervention. In the current case, the
lymphatic hygroma was extremely large in size, compressing the
adjacent structures and therefore, matched the indication for
surgery.
The standard treatment of lymphatic hygroma is
surgical excision (1). A complete
resection of the lymphatic hygroma and a margin of the healthy
tissue must be performed in order to prevent recurrence (8). The surgical approach must take into
account that the process warrants preservation of vital structures.
Subtotal or partial lymphatic hygroma excision is also common,
dictated by special organ infiltration or proximity to
neurovascular structures (30). A
study by Raveh et al (31)
showed that incomplete resection may not cause recurrence,
requiring additional therapeutic intervention.
The current study presents the case of a 23-year-old
male patient diagnosed with mesenteric lymphatic hygroma, with the
chief complaint of progressive dull pain in the upper abdominal
region that had been present for seven years. A correct diagnosis
was possible following combined analysis of a CT imaging
examination and routine abdominal ultrasound. Treatment by complete
resection was successful and no severe complications occurred.
Large mesenteric lymphatic hygromas continue to pose a therapeutic
challenge. In the majority of cases, the treatment planning for
adults is primarily determined by the presence of functional
compromise or associated symptoms. The mainstay of lymphatic
hygroma treatment is surgical resection (32,33).
Incomplete excision is the only reason for disease recurrence
(34). However, whether to use
complete or staged surgical excision, sclerosing agents or other
therapeutic modalities must be determined by the size, location and
characteristics of the lymphatic hygroma. Progress has been made in
understanding the etiology, diagnosis and treatment of lymphatic
hygroma, however, future studies remain necessary to improve the
management of mesenteric lymphatic hygroma.
References
|
1
|
Kennedy TL, Whitaker M, Pellitteri P and
Wood WE: Cystic hygroma/lymphangioma: a rational approach to
management. Laryngoscope. 111:1929–1937. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Takiff H, Clabria R, Yin L and Strabile
BE: Mesenteric cysts and intraabdominal cystic lymphangiomas. Arch
Surg. 120:1266–1269. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Chung JH, Suh YL, Park IA, et al: A
pathologic study of abdominal lymphangiomas. J Korean Med Sci.
14:257–262. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kullendorff CM and Malmgren N: Cystic
abdominal lymphangioma in children. Case report. Eur J Surg.
159:499–501. 1993.PubMed/NCBI
|
|
5
|
Gallagher PG, Mahoney MJ and Gosche JR:
Cystic hygroma in the fetus and newborn. Semin Perinatol.
23:341–356. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Giguère CM, Bauman NM, Sato Y, et al:
Treatment of lymphangiomas with OK-432 (Picibanil) sclerotherapy: a
prospective multi-institutional trial. Arch Otolaryngol Head Neck
Surg. 128:1137–1144. 2002.PubMed/NCBI
|
|
7
|
Enzinger FM and Weiss SW: Tumors of lymph
vessels. Soft Tissue Tumors. Mosby; St. Louis, MO: pp. 679–699.
1995
|
|
8
|
Roisman I, Manny J, Fields S and Shiloni
E: Intra-abdominal lymphangioma. Br J Surg. 76:485–489. 1989.
View Article : Google Scholar
|
|
9
|
Chervenak FA, Isaacson G, Blakemore KJ,
Breg WR, Hobbins JC, Berkowitz RL, Tortora M, Mayden K and Mahoney
MJ: Fetal cystic hygroma: cause and natural history. N Engl J Med.
309:822–825. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gray G, Fried K and Iraci J: Cystic
lymphangioma of the pancreas: CT and pathologic findings. Abdom
Imaging. 23:78–80. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Colovic Radoje B, Grubor Nikica M, Micev
Marjan T, et al: Cystic lymphangioma of the pancreas. World J
Gastroenterol. 14:6873–6875. 2008.
|
|
12
|
Wilson SR, Bohrer S, Losada R and Price
AP: Retroperitoneal lymphangioma: an unusual location and
presentation. J Pediatric Surg. 41:603–605. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hauser H, Mischinger HJ, Beham A, et al:
Cystic retroperitoneal lymphangiomas in adults. Eur J Surg Oncol.
23:322–326. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Allen JG, Riall TS, Cameron JL, et al:
Abdominal lymphangiomas in adults. J Gastrointest Surg. 10:746–751.
2006. View Article : Google Scholar
|
|
15
|
Ohba K, Sagauchi F, Orito E, et al: Cystic
lymphangioma of the gall-bladder: a case report. J Gastroenterol
Hepatol. 10:693–696. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Campbell WJ, Irwin ST and Biggart JD:
Benign lymphangioma of the jejunal mesentery: an unusual cause of
small bowel obstruction. Gut. 32:15681991. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Giguère CM, Bauman NM and Smith RJ: New
treatment options for lymphangioma in infants and children. Ann
Otol Rhinol Laryngol. 111:1066–1075. 2002.PubMed/NCBI
|
|
18
|
Chen CW, Hsu SD, Lin CH, Cheng MF and Yu
JC: Cystic lymphangioma of the jejunal mesentery in an adult: a
case report. World J Gastroenterol. 11:5084–5086. 2005.PubMed/NCBI
|
|
19
|
Shilo L, Hirsch D, Ellis M and Shenkman L:
Pseudoascites - still a diagnostic pitfall. Isr Med Assoc J.
3:770–771. 2001.PubMed/NCBI
|
|
20
|
Yang DM, Jung DH, Kim H, et al:
Retroperitoneal cystic masses: CT, clinical, and pathological
findings and literature review. Radiographics. 24:1353–1365. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Konen O, Rathaus V, Dlugy E, et al:
Childhood abdominal cystic lymphangioma. Pediatr Radiol. 32:88–94.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Levy AD, Cantisani V and Miettinen M:
Abdominal lymphangiomas: imaging features with pathologic
correlation. AJR Am J Roentgenol. 182:1485–1491. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Choi JY, Kim MJ, Chung JJ, et al:
Gall-bladder lymphangioma: MR findings. Abdom Imaging. 27:54–57.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Davidson AJ and Hartman DS: Lymphangioma
of the retroperitoneum: CT and sonographic charateristics.
Radiology. 175:507–510. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Serres LM, Sie KC and Richardson MA:
Lymphatic malformations of the head and neck. A proposal for
staging. Arch Otolaryngol Head Neck Surg. 121:577–582.
1995.PubMed/NCBI
|
|
26
|
Hamoir M, Plouin-Gaudon I, Rombaux P, et
al: Lymphatic malformations of the head and neck: a retrospective
review and a support for staging. Head Neck. 23:326–337. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Smith RJ, Burke DK, Sato Y, et al: OK-432
therapy for lymphangiomas. Arch Otolaryngol Head Neck Surg.
122:1195–1199. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rani DV, Srilakshmi R, Malathi S,
Raghupathy V and Bagdi RK: Unusual presentation of a
retroperitoneal lymphangioma. Indian J Pediatr. 73:617–618. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Perkins JA, Manning SC, Tempero RM, et al:
Lymphatic malformations: review of current treatment. Otolaryngol
Head Neck Surg. 142:795–803. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Riechelmann H, Muehlfay G, Keck T, et al:
Total, subtotal, and partial surgical removal of cervicofacial
lymphangiomas. Arch Otolaryngol Head Neck Surg. 126:1378–1382.
2000.
|
|
31
|
Raveh E, de Jong AL, Taylor GP, et al:
Prognostic factors in the treatment of lymphatic malformations.
Arch Otolaryngol Head Neck Surg. 123:706–710. 1997.
|
|
32
|
Paal E, Thompson LD and Heffess CS: A
clinicopathologic and immunohistochemical study of ten pancreatic
lymphangiomas and a review of the literature. Cancer. 82:2150–2158.
1998. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Igarashi A, Maruo Y, Ito T, Ohsawa K, et
al: Huge cystic lymphangioma of the pancreas: report of a case.
Surg Today. 31:743–746. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Daltrey IR and Johnson CD: Cystic
lymphangioma of the pancreas. Postgrad Med J. 72:564–566. 1996.
View Article : Google Scholar : PubMed/NCBI
|