Introduction
Breast cancer is the most common malignancy and the
second leading cause of cancer-related mortality among females
worldwide (1). Chemotherapy is one
of the most important therapeutic approaches for breast cancer
patients; however, the efficacies of drug treatments on breast
cancers are often limited due to the resistance of tumor cells
(2).
The pregnane X receptor (PXR) belongs to the nuclear
hormone receptor (NR) superfamily of ligand-activated transcription
factors (3), and is alternatively
referred to as the steroid and xenobiotic receptor (SXR) or the
pregnane-activated receptor (PAR), also termed as PXR or hPXR in
humans. PXR regulates the expression of a number of downstream
targeted genes, which are mostly related to the metabolism and
transport of xenobiotics and associated with drug resistance in a
number of cancers (4), such as
cytochrome P450 (CYP450), multidrug resistance 1 (MDR1), breast
cancer resistance protein (BCRP) and multidrug
resistance-associated protein 2 (5–7).
In this study, we used SR12813, a potent and
selective agonist of hPXR, to upregulate and activate the PXR
protein in breast cancer cells, and analyzed the correlation
between PXR and drug resistance in breast cancer, this study was
designed to explore the formation mechanism of drug resistance of
breast cancer cells and provide theoretical basis for clinical
chemotherapy.
Materials and methods
Materials
Thirty-three breast carcinoma tissues and
corresponding normal tissues were obtained from Jiangsu Cancer
Hospital Affiliated to Nanjing Medical University (Nanjing, China).
Informed consent was provided in compliance with the Declaration of
Helsinki. Breast cancer cell lines, MCF-7 and MDA-MB-231, were
purchased from Cell Bank of the Chinese Academy of Sciences,
Shanghai Life Science Institute (Shanghai, China). Cells were
cultured in Dulbecco’s modified Eagle’s medium (DMEM)-high glucose
(Invitrogen, Carlsbad, CA, USA) supplemented with 10% fetal bovine
serum (Invitrogen) and 100 U/ml penicillin-streptomycin
(Invitrogen).
Reagents and instruments
hPXR (H-11) sc-48340 mouse monoclonal antibody and
relevant horseradish peroxidase (HRP)-labeled secondary antibodies
were purchased from Santa Cruz Biotechnology Inc., (Santa Cruz, CA,
USA). β-actin AP0060 rabbit antibody was from Bioworld Technology,
Inc. (Minneapolis, MN, USA). SR12813, dissolved in dimethyl
sulfoxide (DMSO), and 4-hydroxytamoxifen were purchased from
Sigma-Aldrich (St. Louis, MO, USA). Docetaxel was purchased from
Qilu Pharmaceutical Co., Ltd (Jinan, China). The Cell Counting
Kit-8 (CCK-8) cell proliferation-toxicity test kits were from
Dojindo (Kumamoto, Japan). An RNA extraction kit, reverse
transcription system and semi-quantitative PCR reagents were
purchased from Takara Bio Inc. (Shiga, Japan). The total protein
extraction kit was purchased from Beyotime Institute of
Biotechnology (Shanghai, China). The enhanced chemiluminescence
(ECL) detection system was purchased from Millipore (Billerica, MA,
USA). The 7300 Real Time PCR system was from ABI (Warrington, UK).
The Microplate Reader system was from Promega (Madison, WI, USA)
and the BD FACSCalibur Flow Cytometer was purchased from
Becton-Dickinson (New York, NY, USA).
Western blotting
Cells were directly lysed with 2X sodium dodecyl
sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) sample buffer
(Beyotime Institute of Biotechnology), then boiled and sonicated.
The total protein was obtained following centrifugation for 10 min
at 15,407 × g, then total protein was isolated, separated on a 10%
SDS-PAGE gel and transferred to polyvinylidene difluoride (PVDF)
membranes at 100 V for 1 h. Then, the PVDF membranes were incubated
with either anti-hPXR monoclonal antibody H-11 (diluted to 1:800 in
blocking buffer) or anti-β-actin antibody AP0060 (diluted to 1:4000
in blocking buffer) overnight at 4°C. For staining, a goat
anti-mouse HRP-labeled secondary antibody (diluted to 1:10,000 in
blocking buffer) or goat anti-rabbit HRP-labeled secondary antibody
(diluted to 1:2,000 in blocking buffer) were used for 1.5 h at room
temperature. The protein bands were detected by an ECL detection
system. Following normalization by the corresponding expression of
β-actin, protein expression levels of PXR were determined by
densitometry scans.
Semi-quantitative RT-PCR
Total RNA was extracted from cell cultures and from
breast cancer and corresponding normal tissues with
TRIzol® reagent (Invitrogen). Reverse transcription was
performed by a Superscript T3000 Thermocycler system
(Mettler-Toledo, Biometra Goettingen, Giessen, Germany). SYBR
Green-based semi-quantitative PCR was used to measure relative gene
expression with the following primer pairs: hPXR: 5′-CGA GCT CCG
CAG CAT CA-3′ and 5′-TGT ATG TCC TGG ATG CGC A-3′; MDR1: 5′-GTT GCT
GCT TAC ATT CAG GTT TC-3′ and 5′-ACC AGC CTA TCT CCT GTC GC-3′;
BCRP: 5′-TCC ACT GCT GTG GCA TTA AA-3′ and 5′-TGC TGA AAC ACT GGT
TGG TC-3′; β-actin: 5′-TCA CCC ACA CTG TGC CCA TCT ACG A-3′ and
5′-CAG CGG AAC CGC TCA TTG CCA ATG G-3′. PCR conditions were as
follows: One cycle at 95°C for 30 sec, followed by 40 cycles of PCR
amplification, each consisting of 95°C for 5 sec and 60°C for 31
sec. The concentration of mRNA was calculated according to the
standard curve and then normalized to that of β-actin. The data
processing methods were according to a previous study (8).
CCK-8 assay
The cells were seeded in 96-well plates at an
initial density of 8,000 cells per well. After incubation for 12 h,
cells were treated with 4-hydroxytamoxifen or docetaxel at
different concentration gradients (4-hydroxytamoxifen, 0.5, 1.0,
5.0, 10.0, 15.0 and 20.0 μM; docetaxcel, 0.05, 0.1, 0.2, 0.4, 0.8
and 1.6 μg/ml) for 24, 48 or 72 h directly or following a 24 h
treatment of 0.3 μM SR12813 or 0.1% DMSO. For the evaluation of
SR12813 cell cytotoxicity, cells were treated with SR12813 (0.3 μM)
or 0.1% DMSO for 24, 48 or 72 h. The cell viability was measured by
CCK-8 assay according to the manufacturer’s instructions. In brief,
90 μl fresh serum-free medium and 10 μl CCK-8 reagent were added
into each well after decanting the old medium and culture was
continued at 37°C for 1 h. The optical density (OD) at 450 nm was
measured by a microplate reader (Promega). The above steps were
repeated three times and the average was calculated. The cell
viability fraction (%) was calculated as follows: [OD450 nm in
test cells − OD450 nm in blank
control]/[OD450 nm in control cells − OD450
nm in blank control]. The relative drug resistance folds were
analyzed by comparison with IC50.
Flow cytometry assay of cell
apoptosis
The MCF-7 and MDA-MB-231 cells were inoculated in
six-well plates at a density of 2×105 cells/well. They
were respectively divided into three groups, including a control
group, single drug group and SR12813 (0.3 μM) pretreatment group,
and each group was set into three repeated wells. After 24 h, the
cells were treated respectively with complete medium containing 5
μM 4-hydroxytamoxifen (MCF-7) or 0.1 μg/ml docetaxel (MDA-MB-231)
for 24 h. The control group cells were treated with complete medium
containing the same volume of phosphate-buffered saline (PBS). Cell
apoptosis was detected by BD FACSCalibur flow cytometry. The cells
were detached with 0.25% trypsin and resuspended in PBS.
Fluorescein isothiocyanate (FITC)-Annexin V and propidium iodide
(PI) were added to 105 cells, after which the cells were
placed in the dark at room temperature for 15 min according to the
manufacturer’s instructions (Apoptosis Detection kit; BD
Biosciences, Franklin Lakes, NJ, USA). Early apoptotic cells were
stained with FITC-Annexin V (20 μg/ml) alone, and late apoptotic
cells and necrotic cells were stained with FITC-Annexin V and PI
(50 μg/ml).
Statistical analysis
Statistical analysis was performed using SPSS 16.0
software (SPSS Inc., Chicago, IL, USA). Data are expressed as the
mean ± standard deviation. Student’s t-test (two-tailed) was used
to analyze the difference between two groups and one-way analysis
of variance was used to analyze the difference among three groups.
Data were considered to be statistically significant when
P≤0.05.
Results
Comparison of PXR gene expressions
between in breast carcinoma tissues and corresponding normal
tissues
Comparison of PXR gene expression levels between
breast carcinoma tissues and corresponding normal tissues is shown
in Table I. The data showed that
there is higher expression of PXR in breast cancer tissues.
Compared with the normal breast tissues, the expression levels of
PXR gene were significantly higher in breast carcinoma tissues
(t=17.979, P<0.001), ~6.92±1.86 times according to the
statistical results.
 | Table IComparison of PXR gene expression
between in breast carcinoma tissues and corresponding normal
tissues. |
Table I
Comparison of PXR gene expression
between in breast carcinoma tissues and corresponding normal
tissues.
| Group | n | ΔCT |
2−ΔΔCT | T-value | P-value |
|---|
| T | 33 | 3.34±0.51 | | | |
| N | 33 | 6.10±0.72 | 6.92±1.86 | 17.979 | <0.001 |
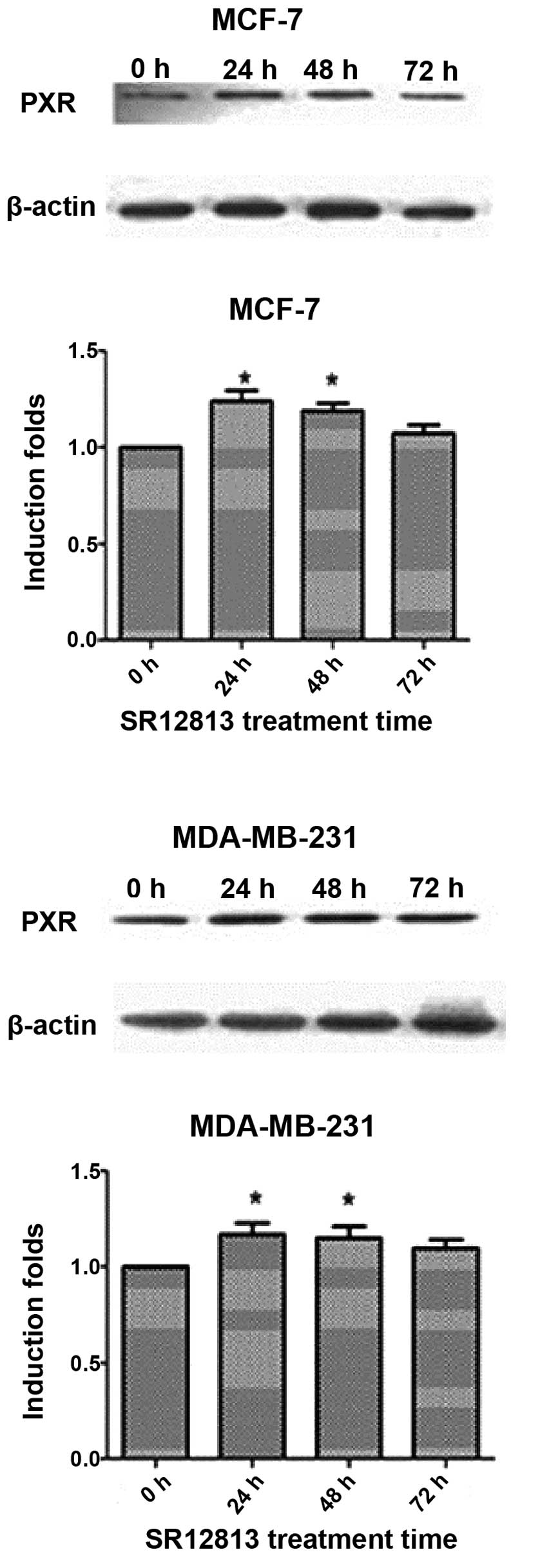
Increased PXR protein expression levels
in MCF-7 and MDA-MB-231 cells following SR12813 treatment
MDA-MB-231 cells had a higher PXR expression
compared with MCF-7. After treatment with 0.3 μM SR12813 for 24, 48
or 72 h, the levels of PXR protein were increased (particularly at
24 and 48 h) (P<0.05) (Fig.
1).
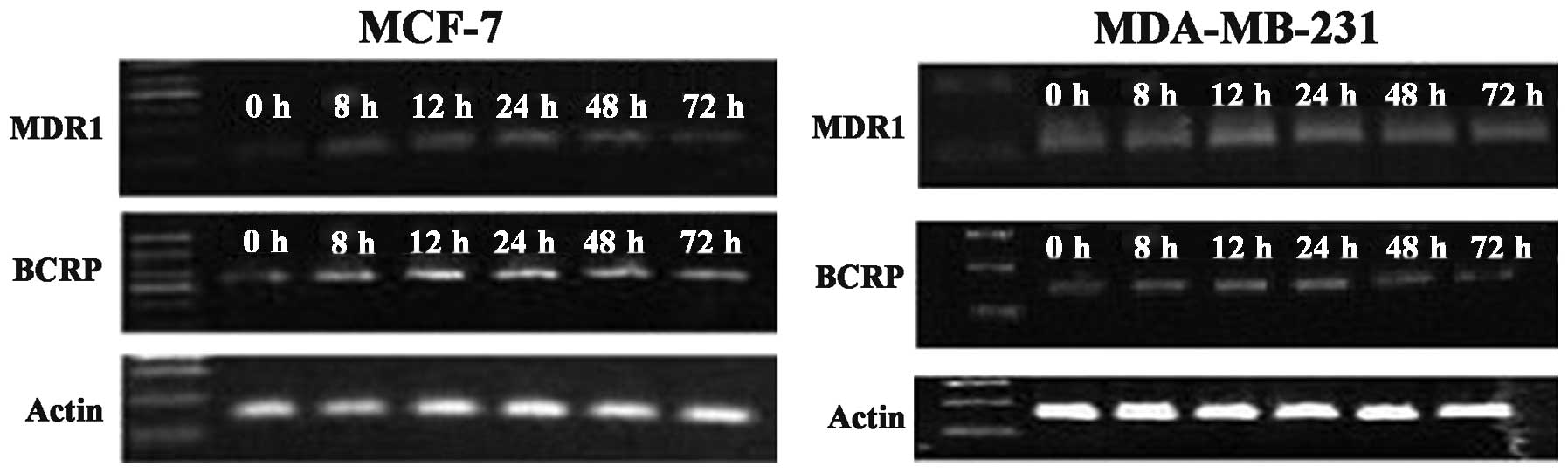
Changes in MDR1 and BCRP gene expression
levels in breast cancer cells before and after SR12813
treatment
Compared with the control group, after treatment
with 0.3 μM SR12813 for 8, 12, 24, 48 and 72 h, the levels of MDR1
and BCRP mRNA in MCF-7 or MDA-MB-231 cells were significantly
increased by 1.5–4 times. The increased levels of MDR1 and BCRP
mRNA in MCF-7 treated by SR12813 at 24 h were highest, 3.61±0.33
and 3.24±0.17 times higher, respectively. The levels of MDR1 and
BCRP mRNA in breast cancer cell lines MCF-7 or MDA-MB-231 before to
and following SR12813 treatment were significantly different
(P<0.001). In MDA-MB-231 cells treated with 0.3 μM SR12813, the
levels of MDR1 mRNA were highest at 12 h (up to 3.61±0.25 times;
P<0.001). BCRP mRNA levels were highest at 12 and 24 h
(3.28±0.24 and 3.23±0.16 times, respectively; P<0.001) (Fig. 2). Combined with the western blot
assay, the results indicate that activated PXR enhances MDR1 and
BCRP gene levels in breast cancer cells.
Increased resistance of MCF-7 and
MDA-MB-231 to therapeutic agents after upregulated PXR expression
by SR12813 treatment
The IC50 values of 4-hydroxytamoxifen to
MCF-7 cells at 48 h were 9.81±0.49 μM in the control group,
9.40±0.69 μM in the DMSO treatment group and 11.57±0.83 μM in the
SR12813 pretreatment group. At 72 h, these values were 8.35±0.64,
8.22±0.59 and 9.78±0.68 μM, respectively. The differences between
the SR12813 pretreatment group and the other groups were
significant (P<0.05) (Table
II). Similarly, the IC50 values of docetaxel to
MDA-MB-231 cells at 24 h were 0.45±0.025 μg/ml in the control
group, 0.44±0.021 μg/ml in the DMSO treatment group and 0.67±0.091
μg/ml in the SR12813 pretreatment group. At 48 h, these values were
0.40±0.042, 0.39±0.025 and 0.53±0.056 μg/ml, and at 72 h, these
values were 0.35±0.021, 0.36±0.036 and 0.46±0.040 μg/ml,
respectively. These values in the SR12813 pretreatment group were
significantly different compared with the other groups at 24 h
(P=0.003), 48 h (P=0.015) and 72 h (P=0.025) (Table III).
 | Table IIEffect of PXR on MCF-7 cell
sensitivity to 4-hydroxytamoxifen. |
Table II
Effect of PXR on MCF-7 cell
sensitivity to 4-hydroxytamoxifen.
| Group | IC50 at 48
h (μM) | F-value | P-value | IC50 at 72
h (μM) | F-value | P-value |
|---|
| Control | 9.81±0.49 | | | 8.35±0.64 | | |
| DMSO treatment | 9.40±0.69 | | | 8.22±0.59 | | |
| SR12813
pretreatment | 11.57±0.83a | 8.529 | 0.018 | 9.78±0.68a | 16.295 | 0.045 |
 | Table IIIEffect of PXR on MDA-MB-231 cell
sensitivity to docetaxel. |
Table III
Effect of PXR on MDA-MB-231 cell
sensitivity to docetaxel.
| Group | IC50 at 24
h (μg/ml) | F-value | P-value | IC50 at 48
h (μg/ml) | F-value | P-value | IC50 at 72
h (μg/ml) | T-value | P-value |
|---|
| Control | 0.45±0.025 | | | 0.40±0.042 | | | 0.35±0.021 | | |
| DMSO treatment | 0.44±0.021 | | | 0.39±0.025 | | | 0.36±0.036 | | |
| SR12813
pretreatment | 0.67±0.091b | 16.885 | 0.003 | 0.53±0.056a | 9.079 | 0.015 | 0.46±0.040a | 7.248 | 0.025 |
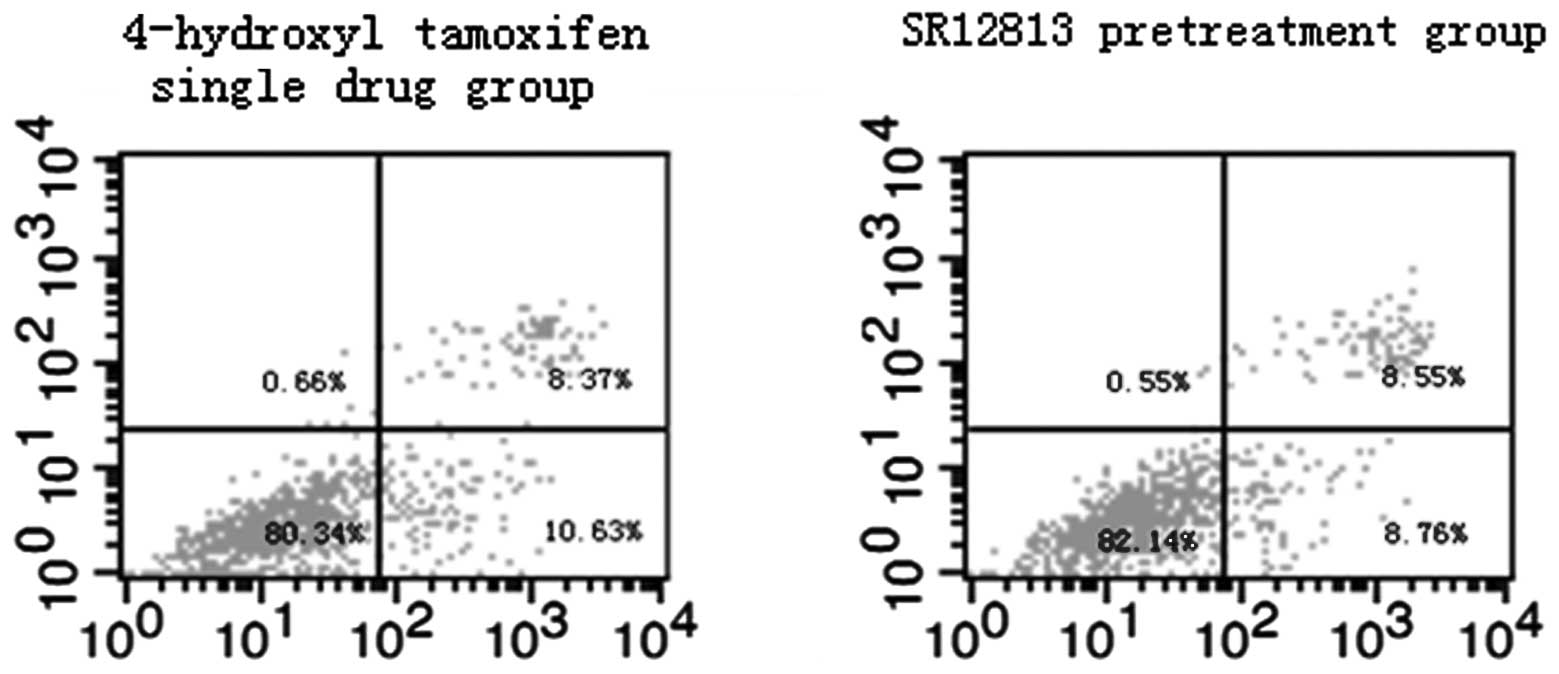
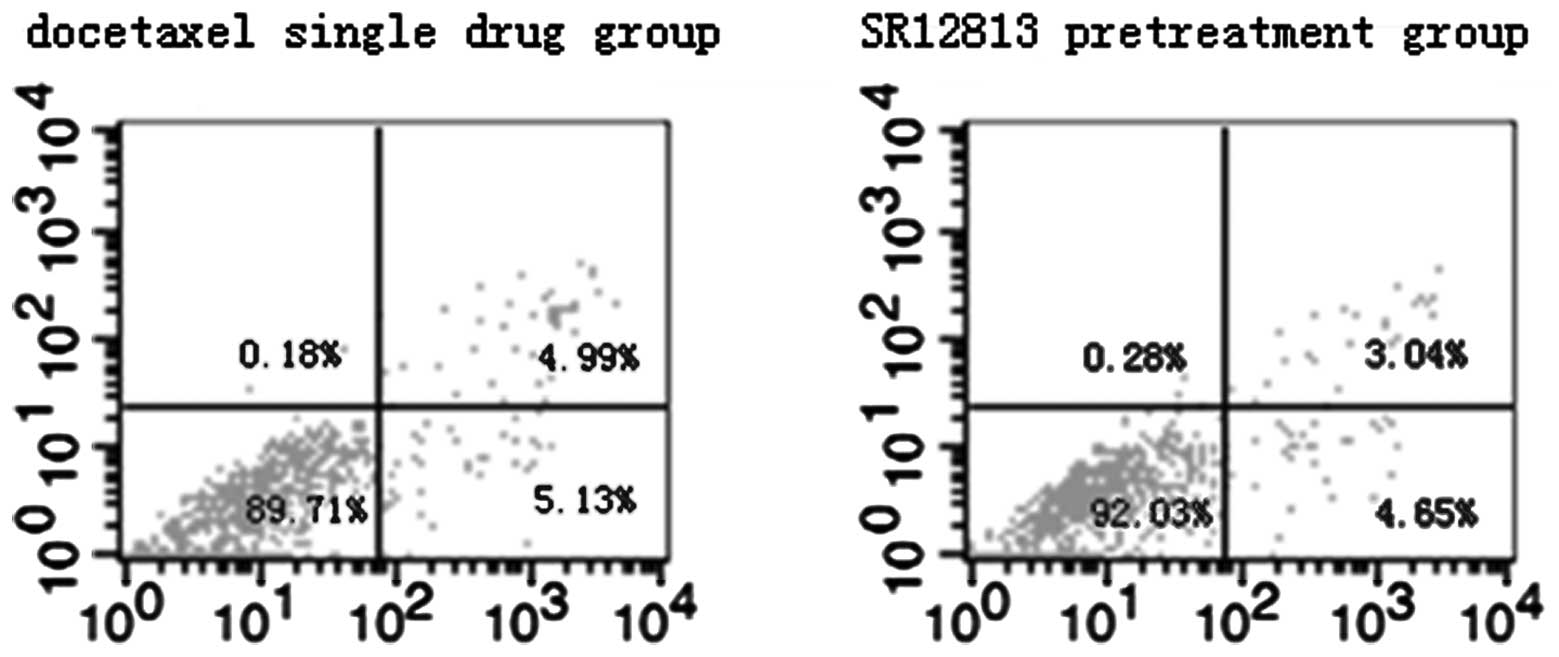
Effect of PXR on MCF-7 and MDA-MB-231
cell apoptosis induced by therapeutic agents
Results of flow cytometric assays from control and
experimental treatment groups are shown in Figs. 3 and 4. The total rates of apoptosis of cells in
the control group were 5.81±0.62% (MCF-7) and 5.17±0.46%
(MDA-MB-231), which were significantly increased after treatment
with therapeutic agents for 24 h (P<0.001). Compared with the
single drug groups (19.43±0.97 and 10.27±0.80%, in MCF-7 and
MDA-MB-231 cells, respectively), the total rates of apoptosis of
cells in the SR12813 pretreatment groups were significantly
decreased (17.26±0.40%, t=3.571, P=0.023 and 7.69±0.54%, t=4.647,
P=0.01 in MCF-7 and MDA-MB-231 cells, respectively).
Discussion
Drug resistance of tumor cells is a complicated
process with multiple factors, genes and steps. PXR is a member of
the ligand-activated transcription factor superfamily called
nuclear receptor subfamily 1 group I member 2 (NR1I2) whose
downstream target genes are involved in the production of phase I
metabolic enzymes, including CYP450 enzymes, phase II metabolic
enzymes, including glucuronyltransferase (UGT) and phase III drug
transporters, including P-glycoprotein (P-gp) and BCRP (6,7). Since
these target genes are mostly involved in the formation of tumor
MDR, PXR may be a novel master regulator of drug resistance.
P-gp and BCRP are the main members of the ATP
binding cassette (ABC) superfamily of transporters, and are
important PXR target genes (6).
Their normal physiologic function is to protect the body from
cytotoxicity caused by drugs or other xenobiotics. P-gp
overexpression is a major cause of MDR. The protein is able to
increase the outflow of a number of intracellular structurally and
functionally unrelated chemotherapy drugs, including anthracyclines
and taxanes, thereby reducing the efficacy (9). Navarro et al (10) reported that the combination of
adriamycin and P-gp silencing formulations led to a double increase
of adriamycin uptake and a significant improvement of the
therapeutic effect of adriamycin in MCF-7 cells. Yuan et al
(11) demonstrated there was a
significant negative correlation between BCRP expression and 5-FU
resistance in breast cancer cells, which may be used to optimize
the chemotherapy scheme in BCRP-positive breast cancer patients.
Liu et al (12) also found
that P-gp and BCRP proteins are implicated in the formation of MDR
in breast cancer and P-gp expression can be used to evaluate the
efficacy of anthracycline chemotherapy in breast cancer. It is thus
clear that the two proteins play an important role in breast cancer
resistance mechanisms. Our study results demonstrated that
treatment with SR12813 resulted in an upregulation of MDR1 and BCRP
gene levels. Correspondingly, there was a significant increase in
breast cancer cell resistance, which was consistent with the above
findings.
A number of studies have demonstrated that PXR is
involved in MDR of tumor cells. Chen et al (13) demonstrated that PXR is expressed in
normal and cancerous prostate tissues and in the prostate cancer
cell line PC-3. Treatment with SR12813 activated PXR and improved
the level of MDR1, and increased the resistance of PC-3 cells to
paclitaxel or vinblastine. The targeted knockdown of PXR reduced
the resistance of PC-3 cells to paclitaxel or vinblastine.
Similarly, Raynal et al (14) reported that activation of PXR
increased the resistance of colorectal cancer cells to irinotecan.
The increase in chemoresistance was reversed by the PXR antagonist
sulforaphane. For breast cancer, Dotzlaw et al (15) first detected the expression of PXR
mRNA in human breast cancer tissues by PCR amplification. Conde
et al (16) further
demonstrated the overexpression of PXR protein in breast cancer
cells by immunohistochemistry and western blot analysis. The
organic anion transporter polypeptide 1A2 (OATP1A2) is capable of
mediating the cellular uptake of estrogen metabolites. Meyer zu
Schwabedissen et al (17)
identified that the PXR-OATP1A2 promoter interaction induced the
expression of OATP1A2 in breast cancer cells, increased the uptake
of the estrogen metabolite estrone 3-sulfate, and improved the
estrogen effect of breast cancer. This process was also reversed by
a specific antagonist of PXR, A-792611. Chen et al (18) confirmed hPXR expression in normal
and cancerous human breast specimens and in breast cancer cell
lines MCF-7 and MDA-MB-231. Activation of hPXR by SR12813 led to an
increased expression of CYP3A4 and MDR1 in the two cell strains and
improved resistance to Taxol or tamoxifen. Furthermore, knockdown
of hPXR via shRNA sensitized the two cell strains to Taxol,
vinblastine or tamoxifen. Jiang et al (19) demonstrated that PXR mRNA and protein
were expressed in human colon cancer cell lines. The expression of
PXR was increased following treatment with the PXR agonist
rifampicin. PXR being activated or knocked down, accordingly
increased or inhibited the cell proliferation, and enhanced or
reduced the resistance of cells to the chemotherapeutic agents.
However, the specific mechanism is unknown. This is one of the
limited studies on PXR and tumor resistance. Our results
demonstrated that treatment with SR12813 increased hPXR expression
and significantly increased the expression of MDR1 and BCRP in
MDA-MB-231 and MCF-7 cells, and distinctly reduced sensitivity to
4-hydroxytamoxifen or docetaxel, which was mostly consistent with
previous studies. However, there were also some opposing study
results. Honorat et al (20)
found that PXR was also able to downregulate ABCG2 expression in
PXR- and glucocorticoid receptor-positive MCF-7 cells and improved
the sensitivity of MCF-7 to mitoxantrone.
Studies have demonstrated that PXR is implicated in
apoptosis of tumor cells, which may be an important cause of MDR.
Masuyama et al (21)
confirmed that PXR overexpression led to a significant decrease in
endometrial cancer cell growth inhibition and inhibited apoptosis
induced by cisplatin or paclitaxel. Wang et al (22) demonstrated that in colon cancer
cells, activated PXR was able to induce fibroblast growth factor 19
(FGF19)-dependent cancer cell proliferation and inhibit cell
apoptosis. Moreover, the authors observed that PXR caused FGF19
activation only in the cancer cells. The present study also
demonstrated that following activation and upregulated PXR
expression by SR12813 treatment in MCF-7 and MDA-MB-231 cells, cell
apoptosis induced by 4-hydroxytamoxifen or docetaxel was
significantly inhibited. The data were consistent with the above
previous findings; however, the specific mechanism requires further
exploration. Constrastingly, Liu et al (23) demonstrated that Tanshinone IIA (Tan
IIA) had marked growth inhibition effects on U-937 cells through
the induction of cell apoptosis. The Tan IIA-induced apoptosis may
be due to the activation of PXR, which inhibited the activity of
NF-κB and led to the downregulation of monocyte chemoattractant
protein (MCP)-1 (MCP-1/CCL2) expression. The aforementioned results
indicate that PXR plays a dual role in tumor cell apoptosis.
To date, literature that discusses the correlation
between PXR and breast cancer are relatively limited and the
majority are related to colon cancer. In our study, we detected the
expression of PXR in normal and cancerous breast tissues and in
breast cancer cell lines. PXR played a significant role in MDR of
breast cancer cells, and treatment with the PXR agonist SR12813
activated and increased PXR protein expression, increased the
resistance of cancer cells to chemotherapy drugs and decreased cell
apoptosis. The specific mechanisms and the correlation between PXR
and the development of breast cancer are yet to be explored. There
are a number of opposing results among the studies discussed;
therefore, further investigation is required.
Acknowledgements
This study was supported by grants from the Natural
Science Foundation of China (no. 30840093), Natural Science
Foundation of Zhejiang Province (no. LY12H16030), Foundation of
Science Technology Department of Zhejiang Province (no. 2011C23043)
and Traditional Chinese Medical Research Foundation of Zhejiang
Province (no. 2011ZA016).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar
|
|
2
|
Leonessa F and Clarke R: ATP binding
cassette transporters and drug resistance in breast cancer. Endocr
Relat Cancer. 10:43–73. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kliewer SA, Moore JT, Wade L, et al: An
orphan nuclear receptor activated by pregnanes defines a novel
steroid signaling pathway. Cell. 92:73–82. 1998. View Article : Google Scholar
|
|
4
|
Minemura M, Tanimura H and Tabor E:
Overexpression of multidrug resistance genes MDR1 and cMOAT in
human hepatocellular carcinoma and hepatoblastoma cell lines. Int J
Oncol. 15:559–563. 1999.PubMed/NCBI
|
|
5
|
Tolson AH and Wang H: Regulation of
drug-metabolizing enzymes by xenobiotic receptors: PXR and CAR. Adv
Drug Deliv Rev. 62:1238–1249. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Chen T: Overcoming drug resistance by
regulating nuclear receptors. Adv Drug Deliv Rev. 62:1257–1264.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Campa D, Butterbach K, Nieters A, et al: A
comprehensive study of polymorphisms in the ABCB1, ABCC2, ABCG2,
NR1I2 genes and lymphoma risk. Int J Cancer. 131:803–812. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Heid CA, Stevens J, Livak KJ and Williams
PM: Real time quantitative PCR. Genome Res. 6:986–994. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mellor HR and Callaghan R: Resistance to
chemotherapy in cancer: a complex and integrated cellular response.
Pharmacology. 81:275–300. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Navarro G, Sawant RR, Biswas S, Essex S,
Tros de Ilarduya C and Torchilin VP: P-glycoprotein silencing with
siRNA delivered by DOPE-modified PEI overcomes doxorubicin
resistance in breast cancer cells. Nanomedicine (Lond). 7:65–78.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yuan JH, Cheng JQ, Zhuang ZX, et al: Study
on the relationship between breast cancer resistance protein
expression and 5-fluorouracil resistance. Zhonghua Yu Fang Yi Xue
Za Zhi. 42:506–510. 2008.(In Chinese).
|
|
12
|
Liu X, Chen P, Cui J and Zhang G:
Expression of drug resistance related proteins in breast cancer
tissues and its relationship with chemotherapy therapeutic effect.
Chin J Cancer Prev Treatment. 18:1354–1357. 2011.
|
|
13
|
Chen Y, Nie D, Wang MT, et al: Human
pregnane X receptor and resistance to chemotherapy in prostate
cancer. Cancer Res. 67:10361–10367. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Raynal C, Evrard A, Kantar J, et al:
Pregnane X receptor (PXR) expression in colorectal cancer cells
restricts irinotecan chemosensitivity through enhanced SN-38
glucuronidation. Mol Cancer. 9:462010. View Article : Google Scholar
|
|
15
|
Dotzlaw H, Leygue E, Watson P and Murphy
LC: The human orphan receptor PXR messenger RNA is expressed in
both normal and neoplastic breast tissue. Clin Cancer Res.
5:2103–2107. 1999.
|
|
16
|
Conde I, Lobo MV, Zamora J, et al: Human
pregnane X receptor is expressed in breast carcinomas, potential
heterodimers formation between hPXR and RXR-alpha. BMC Cancer.
8:1742008. View Article : Google Scholar
|
|
17
|
Meyer zu Schwabedissen HE, Tirona RG, Yip
CS, et al: Interplay between the nuclear receptor pregnane X
receptor and the uptake transporter organic anion transporter
polypeptide 1A2 selectively enhances estrogen effects in breast
cancer. Cancer Res. 68:9338–9347. 2008.
|
|
18
|
Chen Y, Tang Y, Chen S and Nie D:
Regulation of drug resistance by human pregnane X receptor in
breast cancer. Cancer Biol Ther. 8:1265–1272. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Jiang H, Chen W, Chen K, He J and Liang H:
Roles of pregnane X receptor for proliferation and drug resistance
in human colon cancer cells. Prog Mod Biomed. 9:2012–2015.
2009.
|
|
20
|
Honorat M, Mesnier A, Di Pietro A, et al:
Dexamethasone down-regulates ABCG2 expression levels in breast
cancer cells. Biochem Biophys Res Commun. 375:308–314. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Masuyama H, Nakatsukasa H, Takamoto N and
Hiramatsu Y: Down-regulation of pregnane X receptor contributes to
cell growth inhibition and apoptosis by anticancer agents in
endometrial cancer cells. Mol Pharmacol. 72:1045–1053. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang H, Venkatesh M, Li H, et al: Pregnane
X receptor activation induces FGF19-dependent tumor aggressiveness
in humans and mice. J Clin Invest. 121:3220–3232. 2011. View Article : Google Scholar
|
|
23
|
Liu C, Li J, Wang L, et al: Analysis of
tanshinone IIA induced cellular apoptosis in leukemia cells by
genome-wide expression profiling. BMC Complement Altern Med.
12:52012. View Article : Google Scholar : PubMed/NCBI
|