Introduction
Acute lymphoblastic leukemia (ALL) is the most
prevalent hematological malignancy in children (1). Although remission is achieved in the
majority of children with ALL using modern therapies, disease
relapse occurs in 15–20% of patients (2). The greatest number of relapses occur
in the bone marrow (BM), in an isolated form or combined with
involvement of another site, mainly the central nervous system
(CNS) or testes. Isolated CNS or testicular relapse or, less
frequently, relapse involving other extramedullary sites, may also
occur. Isolated extramedullary relapse in childhood ALL is
associated with a wide variety of clinical symptoms and often
presents a diagnostic challenge (2). The current report presents a case of
relapsed ALL with unusual intermittent and migrating bone pain
caused by multiple bone invasions prior to clinical manifestation
of BM relapse of ALL.
Case report
An eight-year-old male was admitted to the
University of Tokyo Hospital (Tokyo, Japan) with a history of
intermittent and migrating limb pain, claudication and a 9-month
fever. Repeated laboratory examinations during that period revealed
no hematological abnormalities. Patient history included diagnosis
of ALL at the age of 5 years, and remission for 2 years prior to
admission. Physical examination revealed no pallor,
lymphadenopathy, organomegaly or petechiae. Laboratory studies
revealed normal blood cell counts. Serum calcium and alkaline
phosphatase were also within the normal range. C-reactive protein
levels were slightly elevated (2.61 mg/dl) and blood cultures were
negative. Soluble interleukin 2 receptor levels were elevated to
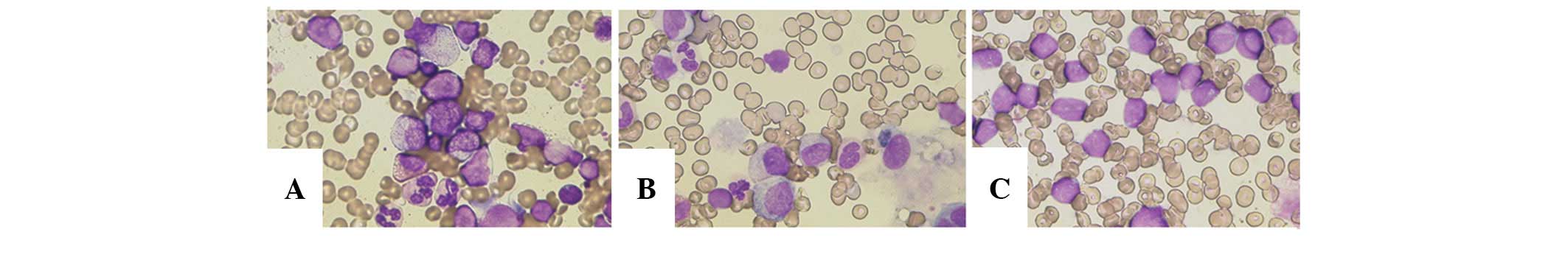
960 U/ml (127–582 U/ml: normal range). BM aspiration of the
anterior left ilium revealed lymphoid hyperplasia with 52.2%
blast-like cells (Fig. 1A).
However, flow cytometry was not performed at this time, and thus,
diagnosis of relapse could not be confirmed. Therefore, a second BM
aspiration from the posterior left ilium was performed, but no
monoclonal blasts were evident on the basis of morphological and
cell surface marker analyses (Fig.
1B).
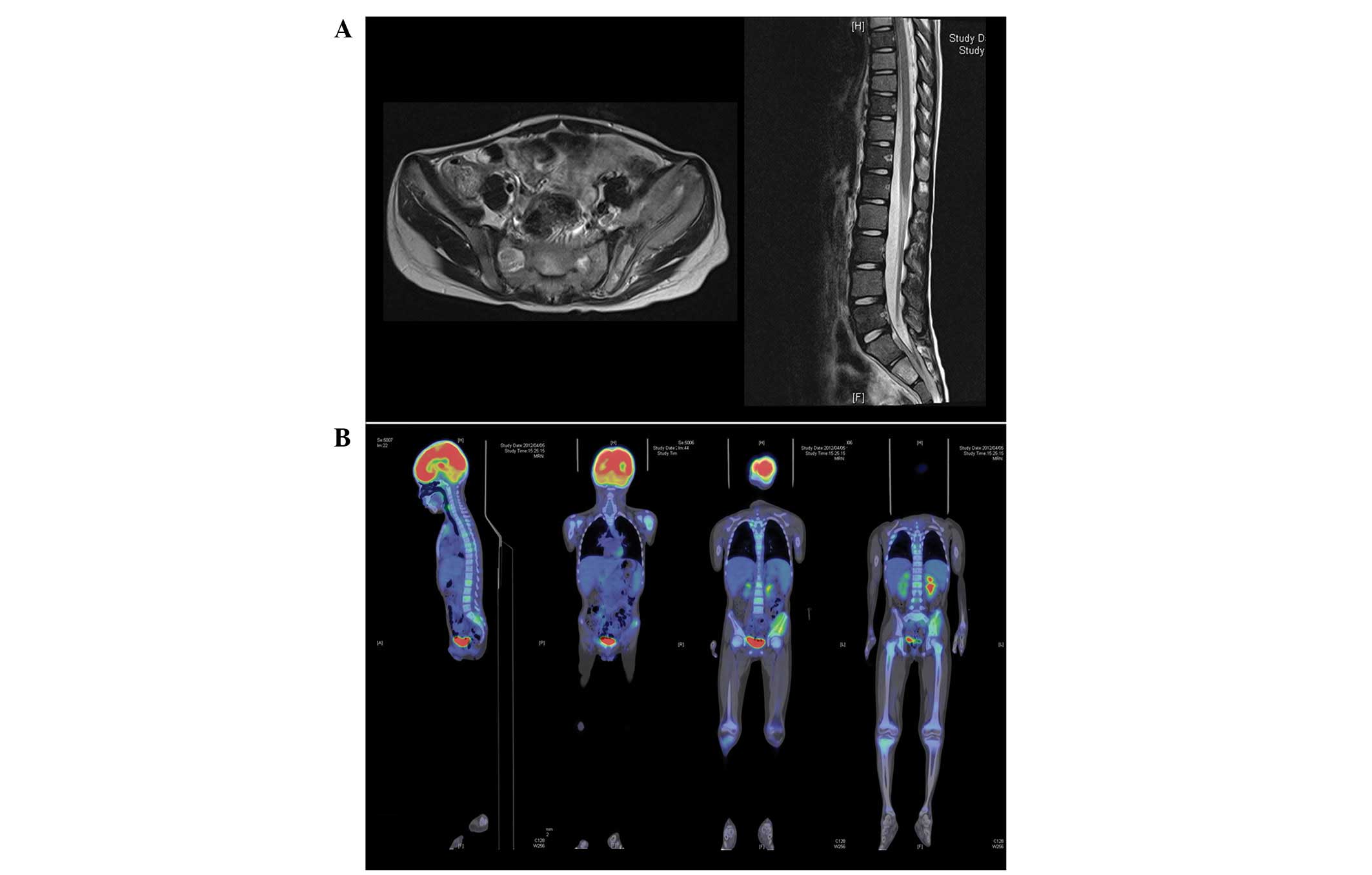
Abdominal computed tomography (CT) revealed lysis
and destruction of the left ilium. Magnetic resonance imaging (MRI)
revealed infiltrative processes in the ilium, the adjacent soft
tissue and multiple vertebral bodies (Fig. 2A). Whole-body positron emission
tomography (PET) with 18F-fluorodeoxyglucose (FDG)
revealed hypermetabolic foci in the left ilium, the epiphysis of
the left humerus, the proximal end of the right tibia and multiple
vertebral bodies corresponding to the areas of marrow infiltration
visualized on MRI (Fig. 2B). These
radiographic findings suggested malignancy; thus, CT-guided
biopsies were performed. Pathologically, cluster of differentiation
(CD) 10, CD20, CD79a and terminal deoxynucleotidyl transferase
(TdT) antigens indicated atypical lymphocytes infiltrating the left
ilium and adjacent soft tissue.
Subsequent BM aspiration from the anterior and
posterior left ilium revealed infiltration of monotonous blast
cells (anterior, 71.6%; posterior, 99.6%) (Fig. 1C). Immunophenotyping identified
expression of CD10, CD19, CD22, CD24, cytoplasmic (cy) CD22,
cyCD79a, cy-TdT, human leukocyte antigen-DR, CD34, CD99, CD38 and
CD58 antigens in the blasts. Taken together, these findings
suggested a diagnosis of isolated extramedullary bone relapse and
subsequent BM relapse of B-precursor ALL. Chemotherapy following
the Japanese Pediatric Leukemia/Lymphoma Study Group Protocol,
ALL-R08, for S2-risk ALL (vincristine, methotrexate,
L-asparaginase, cytarabine, 6-mercaptopurine, vindesine, ifosfamide
and daunorubicin), induced complete clinical and cytogenetic
remission (unpublished data). This study was approved by the ethics
committee of the University of Tokyo, Tokyo, Japan (no. 2701).
Written informed consent was obtained from the patient’s
family.
Discussion
The patient described in the current study presented
with severe intermittent and migrating bone pain of long duration,
claudication and fever, but no hematological abnormalities. These
findings suggested that isolated bone relapse had developed prior
to clinical manifestation of BM relapse of ALL. The radiographic
findings showed multifocal invasion of the bone and surrounding
soft tissues, which also corresponded with the possibility of
initial isolated bone tissue invasions.
Notably, repeated BM aspiration in different
sections of the ilium revealed no diffuse homogenous involvement in
the BM. This suggested that the leukemic cells had infiltrated the
BM in a localized manner mimicking a solid tumor at the time of
initial BM relapse. Extramedullary tissue infiltration as a solid
tumor is a common feature of a subset of acute myeloid leukemia
(3), but is rarely observed in ALL.
In addition, isolated bone relapse occurring during complete BM
remission in childhood ALL is less common (4). Furthermore, localized ALL relapse in
BM is extremely rare (5).
Therefore, these features could cause diagnostic difficulty.
FDG-PET has been widely used in the evaluation of
various malignancies, but clinical application in cases with
leukemia remains limited (6). Thus,
the present case demonstrates the diagnostic value of FDG-PET for
detection of focal localization in leukemia.
The differential diagnoses of cases with multiple
bone invasions are as follows: Metastases of solid tumor,
osteosarcoma, Langerhans cell histiocytosis, osseous lymphoma,
bacterial osteomyelitis, chronic recurrent multifocal osteomyelitis
(CRMO) and autoinflammatory disease. CRMO is one of the most
important differential diagnoses of multiple bone invasions due to
its similarity in radiographic imaging to our case (7). Although morphologically CRMO is a
reactive condition (8), cases of
CRMO following ALL (9) and osseous
lymphoma following CRMO (10) have
been reported. It was hypothesized that common genetic factors may
predispose patients with ALL to CRMO and lymphoblastic
proliferations.
In the present case, CRMO may have been an
alternative diagnosis based on the radiographic images, but a
patient history of ALL suggested the possibility of relapse. BM
aspiration or other tissue biopsy is necessary, but not sufficient,
to rule out suspected malignancy. Relapsed or primary hematological
neoplasms must not be excluded based on the results of a single BM
aspiration; radiographic imaging must also be utilized in the
differential diagnosis of cases with multiple bone lesions, even in
the presence of apparently normal blood cell counts.
Notably, no bone metastasis was detected in the
initial episode of ALL in the present case. At the time of relapse,
the leukemic cells may have infiltrated multiple bone and soft
tissue sites mimicking lymphoma or other solid tumors without
hematological changes in peripheral blood. This suggests that the
leukemic cells acquired affinity to the bone rather than the BM.
The molecular characteristics of the primary and relapsed leukemic
cells are likely to have differed. Since identifying the molecular
features of relapsed clones may shift the therapeutic target,
evaluating the molecular differences between primary and relapsed
leukemic cells is important not only biologically but also
clinically. Further investigation will improve the understanding of
the mechanisms involved in differentiation and clonal evolution of
leukemic cells.
In conclusion, isolated bone relapse in childhood
ALL is uncommon. The use of imaging investigation may be useful if
a patient in remission develops bone pain without presenting with
abnormal findings of BM aspiration, in order to assess whether the
patient has relapsed. Our report provides an improved understanding
of the development and diagnosis of isolated bone relapse in
childhood ALL
References
|
1
|
Inaba H, Greaves M and Mullighan CG: Acute
lymphoblastic leukemia. Lancet. 381:1943–1955. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pui CH, Carroll WL, Meshinchi S and Arceci
RJ: Biology, risk stratification, and therapy of pediatric acute
leukemias: an update. J Clin Oncol. 29:551–565. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Klco JM, Welch JS, Nguyen TT, et al: State
of the art in myeloid sarcoma. Int J Lab Hematol. 33:555–565. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Murray JC, Gmoser DJ, Barnes DA, et al:
Isolated bone relapse during hematologic remission in childhood
acute lymphoblastic leukemia: report of a metatarsal relapse and
review of the literature. Med Pediatr Oncol. 23:153–157. 1994.
View Article : Google Scholar
|
|
5
|
Endo T, Sato N, Koizumi K, et al:
Localized relapse in bone marrow of extremities after allogeneic
stem cell transplantation for acute lymphoblastic leukemia. Am J
Hematol. 76:279–282. 2004. View Article : Google Scholar
|
|
6
|
Su K, Nakamoto Y, Nakatani K, et al:
Diffuse homogeneous bone marrow uptake of FDG in patients with
acute lymphoblastic leukemia. Clin Nucl Med. 38:e33–e34. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Khanna G, Sato TS and Ferguson P: Imaging
of chronic recurrent multifocal osteomyelitis. Radiographics.
29:1159–1177. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
King SM, Laxer RM, Manson D and Gold R:
Chronic recurrent multifocal osteomyelitis: a noninfectious
inflammatory process. Pediatr Infect Dis J. 6:907–911. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Abril JC, Castillo F, Loewinsonh AF, et
al: Chronic recurrent multifocal osteomyelitis after acute
lymphoblastic leukaemia. Int Orthop. 18:126–128. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jellicoe P and Hopyan S: Can chronic
recurrent multifocal osteomyelitis predispose to lymphoma of bone?
A case report. J Pediatr Orthop B. 17:329–332. 2008. View Article : Google Scholar : PubMed/NCBI
|