Introduction
Treatment outcome of childhood acute lymphoblastic
leukemia (ALL) has evidently improved, but the prognosis of
high-risk (HR) ALL remains unsatisfactory (1). Refinement of risk stratification is
required to improve survival by providing intensive treatment to
patients at HR of relapse. The clinical outcome of ALL is known to
be associated with variable factors, such as demographics,
immunophenotype, cytogenetic features and early treatment response
(2). In addition, the ability of
individual patients to metabolize antileukemic drugs appears to be
involved in the prognosis of ALL, but the knowledge of
pharmacological features of leukemic cells in childhood ALL is
largely limited (3).
Hematological toxicity is the most frequent
dose-limiting side effect of combination chemotherapy in the
treatment of childhood ALL. The severity of each case of acute
hematological toxicity is highly variable despite use of the same
regimen. Chemotherapy-induced leukopenia may be a biological
measure of drug activities and disease control (4,5). The
response of leukemic cells to chemotherapy depends on the level of
active drugs reaching the target and the sensitivity to these
drugs. These factors also affect the response of non-malignant
hematopoietic cells. The availability of active drugs is influenced
by pharmacokinetic parameters. In part, sensitivity to the drugs is
affected by genetic predisposition, which produces a similar effect
in tumor and normal cells, but is also modified by tumor-specific
mutations (6).
The association between less chemotherapy-induced
leukopenia and poor clinical outcome has been previously reported
for several malignancies, including lung cancer, breast cancer,
osteosarcoma and Hodgkin lymphoma (6–12).
This provides additional prognostic information that may be used to
further refine patient stratification and risk-directed therapy.
However, the prognostic role of chemotherapy-induced leukopenia in
childhood ALL has not been elucidated.
Conventional treatment for ALL consists of
induction, consolidation, reinduction and maintenance elements.
Cytotoxic agents, dose levels and severity of myelosuppression are
significantly different between treatment courses. This makes it
difficult to define the measure of hematological toxicity compared
with the treatment and to evaluate the prognostic significance of
chemotherapy-induced leukopenia. In the ALL-BFM 95 HR protocol for
childhood ALL, the consolidation phase consists of a series of
intensive treatment courses (block therapy), with an interval of
three to four weeks between blocks (13). This repetition of treatments with
relatively similar intensity is suitable for the evaluation of
chemotherapy-induced leukopenia. Therefore, to investigate the
association of leukopenia early in the course of treatment with
treatment outcomes of childhood ALL, the current study analyzed ALL
patients treated according to the ALL-BFM 95 HR protocol following
induction therapy.
Materials and methods
Study population
In total, 19 patients (age range, 1–18 years)
consecutively diagnosed with ALL between November 2003 and
September 2010 were studied and uniformly treated according to the
ALL-BFM 95 HR protocol following induction therapy at the
University of Tokyo Hospital (Tokyo, Japan), Saitama Children’s
Medical Center (Saitama, Japan) and Gunma Children’s Medical Center
(Shibukawa, Japan).
Children diagnosed with ALL were enrolled in the
Tokyo Children’s Cancer Study Group (TCCSG) L99-1502 study between
November 2003 and January 2005, and in the L04-16 study between May
2005 and September 2010 (14). The
patients were stratified into the following three risk groups:
Standard-risk (SR), intermediate-risk (IR) and HR. The initial
stratification was based on presenting features (age and white
blood cell count prior to initiating treatment) and leukemic blasts
in peripheral blood on day eight following prednisolone monotherapy
(Tables I and II). The patients were finally stratified
based on cytogenetic observations and bone marrow status examined
following remission induction therapy. Following induction therapy,
patients assigned to the HR group were treated with block
chemotherapy regimen of the ALL-BFM 95 HR protocol. Patients who
did not achieve remission and those with the Philadelphia
chromosome or 11q23 rearrangements (with the exception of MLL/ENL
in the L04-16 study) were scheduled for allogeneic stem cell
transplantation and were excluded from the present study.
 | Table IRisk stratification (the TCCSG
L99-1502 study). |
Table I
Risk stratification (the TCCSG
L99-1502 study).
| A, B-lineage ALL |
|---|
|
|---|
| Years |
|---|
|
|
|---|
| Initial risk | 1–6 | 7–9 | ≥10 |
|---|
| Initial leukocyte
count, ×109/l |
| <20 | SR | IR | IR |
| 20–49 | IR | IR | IR |
| 50–99 | IR | IR | HR |
| ≥100 | HR | HR | HR |
|
| B, B-lineage ALL |
|
| Days |
|
|
| Day 8 risk | 1 SR | 1 IR | 1 HR |
|
| Day 8 PB
blasts/μl |
| 0 | SR | IR | IR |
| 1–999 | SR | IR | HR |
| ≥1,000 | IR | HR | Allo-SCT |
|
| C, T-lineage ALL |
|
| Day 8 risk | | | All patients |
|
| Day 8 PB
blasts/μl |
| 0 | | | IR |
| 1–999 | | | HR |
| ≥1,000 | | | Allo-SCT |
 | Table IIRisk stratification (the TCCSG L04-16
Study). |
Table II
Risk stratification (the TCCSG L04-16
Study).
| A, B-lineage ALL |
|---|
|
|---|
| Years |
|---|
|
|
|---|
| Initial risk | 1–6 | 7–9 | ≥10 |
|---|
| Initial leukocyte
count, ×109/l |
| <20 | SR | IR | IR |
| 20–49 | IR | IR | IR |
| 50–99 | IR | IR | HR |
| ≥100 | HR | HR | HR |
|
| B, B-lineage ALL |
|
| Days |
|
|
| Day 8 risk | 1 SR | 1 IR | 1 HR |
|
| Day 8 PB
blasts/μl |
| 0–999 | SRa | IRa | HR |
| ≥1,000 | HR | HR | Allo-SCT |
|
| C, T-lineage ALL |
|
| Day 8 risk | | | All patients |
|
| Day 8 PB
blasts/μl |
| 0–999 | | | HR |
| ≥1,000 | | | Allo-SCT |
The data regarding chemotherapeutic dosage, dates of
administration and leukocyte counts were retrieved from the
electronic patient databases of the hospitals involved. The parents
of all patients provided written informed consent for the
treatment. The current study was approved by the Ethics Committee
of the University of Tokyo Hospital.
Treatment protocols
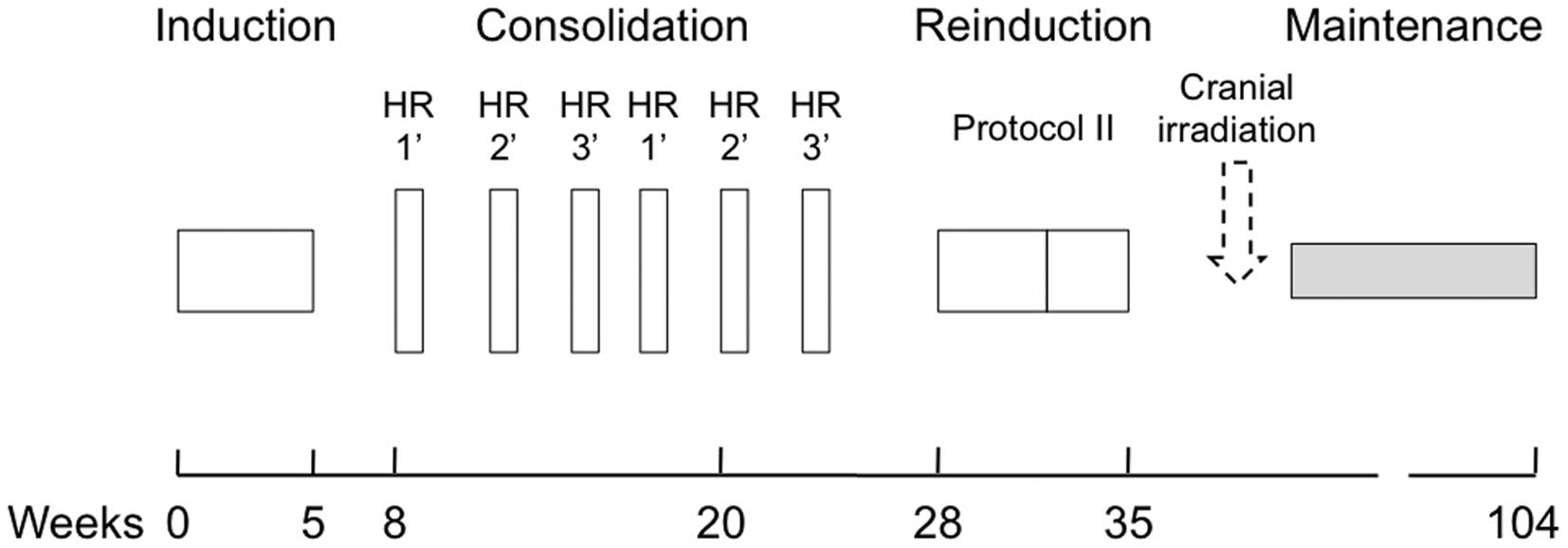
An outline of the treatment regimens is shown in
Fig. 1 and the details of each
treatment aspect are provided in Table III (13). Following TCCSG induction therapy,
patients were uniformly treated according to the ALL-BFM 95 HR
protocol; the patients continued on an intensive rotational
consolidation schedule consisting of three separate six-day pulses
of high-dose chemotherapy, which were each administered twice.
Patients were treated according to the reinduction protocol II
following the consolidation phase.
 | Table IIIDetails of the treatment regimens. |
Table III
Details of the treatment regimens.
| Therapy | Details |
|---|
| Induction |
| Day 8 SR | Pred, 60
mg/m2 × 5 weeks; VCR, 1.5 mg/m2 on weeks 1–5;
Pirarubicin, 20 mg/m2 on weeks 3 and 4; and L-asp, 6,000
U/m2 3 times a week on weeks 2–4 |
| Day 8 IR and HR | Pred, 60
mg/m2 × 5 weeks; VCR, 1.5 mg/m2 on weeks 1–5;
DNR, 25 mg/m2 2 times a week on weeks 2 and 5; CY 1,000
mg/m2 on weeks 2 and 5; and L-asp 6,000 U/m2
3 times a week on weeks 2–4 |
| HR-1′ (two
cycles) | Dex, 20
mg/m2 × 5 days; MTX, 5 g/m2 on day 1; CY, 200
mg/m2 once on day 2 and twice on days 3 and 4; Ara-C, 2
g/m2 twice on day 5; and L-asp, 25,000 U/m2
on day 6 (VCR 1.5 mg/m2 on day 1 and 6 only in the
second cycle) |
| HR-2′ (two
cycles) | Dex, 20
mg/m2 × 5 days; Vindesine, 3 mg/m2 on days 1
and 6; MTX, 5 g/m2; IFO, 800 mg/m2 once on
day 2 and twice on days 3 and 4; DNR, 30 mg/m2 on day 5;
and L-asp, 25,000 U/m2 on day 6 |
| HR-3′ (two
cycles) | Dex, 20
mg/m2 × 5 days; Ara-C, 2 g/m2 twice on days 1
and 2; VP-16, 100 mg/m2 once on day 3 and twice on days
4 and 5; and L-asp 25,000 U/m2 on day 6 |
| Protocol II |
| First half | Dex, 10
mg/m2 × 14 days; VCR, 1.5 mg/m2 on days 8,
15, 22 and 29; ADR, 30 mg/m2 on days 8, 15, 22 and 29;
and L-asp, 10,000 U/m2 on days 3, 8, 16 and 21 |
| Second half | 6MP, 60
mg/m2 × 14 days; CY, 1 g/m2 on day 36; and
Ara-C, 75 mg/m2 × 4 consecutive days for 2 weeks |
| Cranial
irradiation |
| Maintenance | 6MP/MTX until week
104 |
| Total number of IT
therapies | 10–17 |
Granulocyte colony-stimulating factor (G-CSF) was
administered in certain patients with febrile neutropenia and
occasionally used prophylactically when severe and prolonged
neutropenia was predicted.
Leukocyte count
Blood examination was routinely performed several
times a week. The minimum leukocyte count during each course of
chemotherapy was recorded. The minimum leukocyte count was averaged
over the first three courses of the consolidation phase. The mean
was used as the measure of hematological toxicity for each patient.
The leukocyte count during the induction phase was excluded from
the analysis, since disease status markedly affected the leukocyte
count until remission was achieved.
Study outcomes
To assess the correlation between leukocyte nadir
and disease control, relapse-free survival (RFS) from the
initiation of chemotherapy was selected as the endpoint.
Statistical analysis
RFS curves were calculated by the Kaplan-Meier
method and were compared by means of the log-rank test in a
univariate analysis. The minimum leukocyte count in treatment
courses with or without the use of G-CSF was compared with the
Mann-Whitney U test to assess the effect of G-CSF on leukocyte
nadir.
All statistical tests were two-tailed and P<0.05
was considered to indicate a statistically significant difference.
Statistical analyses were performed using R software (R Foundation
for Statistical Computing, Vienna, Austria).
Results
Patient characteristics
In total, 22 patients were assigned to the HR group
on day eight in remission induction therapy. One patient in the SR
group on day eight was stratified into the HR group due to residual
leukemic infiltration in the liver and frontal bone, although,
hematological remission was achieved. Finally, 23 patients were
stratified into the HR group. Of these, four received allogeneic
stem cell transplantation and were excluded from the analysis; one
with the Philadelphia chromosome and three with a poor response to
prednisolone. The remaining 19 patients were uniformly treated
according to the ALL-BFM 95 HR protocol and included in the
analysis. The median age was 11 years (range, 1–18 years) and eight
(42%) patients were female. All patients were treated with the same
dose per body surface area in the consolidation phase with the
exception of L-asparaginase, which was not administered to two
patients in the third course due to anaphylaxis. Detailed patient
characteristics are shown in Table
IV.
 | Table IVCharacteristics of the study
population. |
Table IV
Characteristics of the study
population.
| No. | Age, years | Gender |
Immunophenotype | Initial WBC,
×109/l | Day 8 PB
blast/μl | Risk group | Mean minimum
WBC/μl | Outcome |
|---|
|
|---|
| Day 1 | Day 8 |
|---|
| 1 | 12 | M | B | 81 | 63 | HR | HR | 1,167 | RFS |
| 2 | 11 | F | B | 581 | 632 | HR | HR | 450 | Relapsed |
| 3 | 14 | M | T | 279 | 14 | HR | HR | 433 | RFS |
| 4 | 8 | F | B | 7.2 | 7,684 | IR | HR | 367 | RFS |
| 5 | 9 | M | T | 430 | 116 | HR | HR | 500 | Relapsed |
| 6 | 12 | F | B | 8.2 | 1,269 | IR | HR | 733 | Relapsed |
| 7 | 14 | M | T | 1.5 | 0 | HR | HR | 367 | RFS |
| 8 | 7 | M | T | 259 | 20 | HR | HR | 433 | Relapsed |
| 9 | 12 | F | T | 147 | 247 | HR | HR | 233 | RFS |
| 10 | 15 | M | T | 42 | 0 | HR | HR | 433 | RFS |
| 11 | 6 | F | T | 11 | 0 | HR | HR | 233 | RFS |
| 12a | 3 | F | B | 8.5 | 825 | SR | SR | 667 | RFS |
| 13 | 7 | F | B | 12 | 76 | HR | HR | 300 | RFS |
| 14 | 11 | F | B | 21 | 12,802 | IR | HR | 200 | RFS |
| 15 | 13 | M | T | 126 | bND | HR | HR | 633 | Relapsed |
| 16 | 10 | M | B | 539 | 7 | HR | HR | 467 | RFS |
| 17 | 13 | M | B | 53 | 81 | HR | HR | 200 | RFS |
| 18 | 6 | M | T | 28 | 28 | HR | HR | 467 | RFS |
| 19 | 6 | M | T | 65 | 459 | HR | HR | 300 | RFS |
| Median | 11 | | | 52.9 | 69.5 | | | 433 | |
| IQR | 6–13 | | | 8.5–278.6 | 7–825 | | | 233–633 | |
Treatment outcome
Of the 19 patients, five suffered a relapse: Two
during consolidation, one during reinduction and two during
maintenance therapy. The median follow-up period of relapse-free
patients was 51.5 months (range, 10–85 months). The mean minimum
leukocyte count was calculated for the first three courses of the
consolidation phase, with the exception of two patients who
relapsed during the third course of the consolidation phase; their
mean minimum leukocyte count was calculated for the first two
courses of the consolidation phase. The median of the mean minimum
leukocyte count was 433/μl (range, 200–1,167/μl).
Of the 19 patients, 13 received G-CSF at least once
during treatment. The minimum leukocyte count was not significantly
different between the courses with and without the use of G-CSF
(P=0.367; Mann-Whitney U test).
Prognostic factors
RFS curves were compared by means of the log-rank
test in a univariate analysis (Table
V). Variables included age, gender, immunophenotypes of
leukemic blasts (B- or T-lineage), initial leukocyte count,
response to prednisolone monotherapy and the mean minimum leukocyte
count. Patients were divided at the median values for age, initial
leukocyte count and the mean minimum leukocyte count. The risk of
relapse was significantly higher in patients with a mean minimum
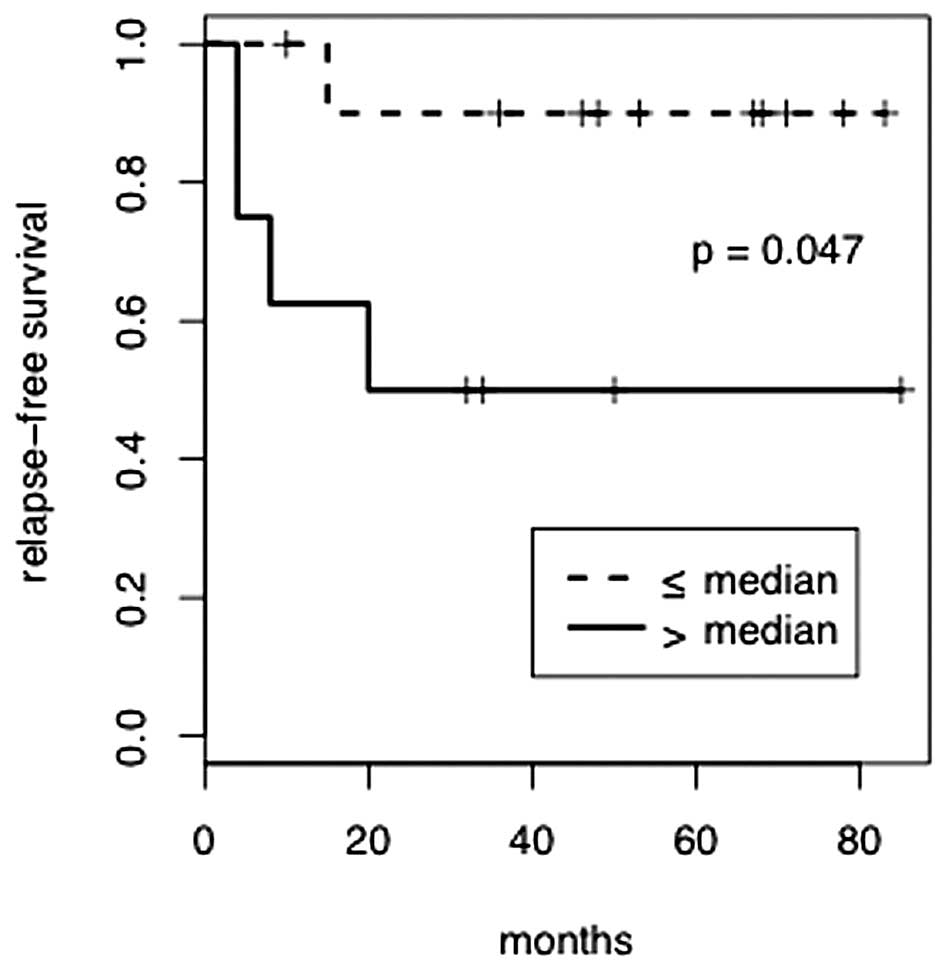
leukocyte count above the median (hazard ratio, 6.61; P=0.047).
Fig. 2 shows RFS according to the
severity of leukopenia. No other factors were significantly
associated with the risk of relapse.
 | Table VResults of univariate analysis by
log-rank test. |
Table V
Results of univariate analysis by
log-rank test.
| Variables | n | HR (95% CI) | P-value |
|---|
| Age, years | | | |
| ≤11 | 11 | 1 | |
| >11 | 8 | 1.26
(0.21–7.40) | 0.800 |
| Gender | | | |
| Female | 8 | 1 | |
| Male | 11 | 1.07
(0.18–6.37) | 0.940 |
| Immunophenotype of
leukemic blasts | | | |
| B-lineage | 9 | 1 | |
| T-lineage | 10 | 1.37
(0.23–8.02) | 0.730 |
| Initial leukocyte
count, ×109/l | | | |
| ≤52.9 | 10 | 1 | |
| >52.9 | 9 | 5.45
(0.85–35.0) | 0.083 |
| Response to
prednisolone monotherapy (day 8 PB blast of <1,000/μl) | | | |
| Good | 16 | 1 | |
| Poor | 3 | 1.38
(0.14–13.8) | 0.770 |
| Mean minimum
leukocyte count/μl | | | |
| ≤433 | 11 | 1 | |
| >433 | 8 | 6.61
(1.04–42.1) | 0.047 |
Discussion
The severity of acute hematological toxicity varies
considerably in childhood ALL despite the use of the same
chemotherapy. The current study analyzed patients with ALL in the
same risk group and showed that patients with low hematological
toxicity during chemotherapy exhibited a higher rate of relapse. HR
of relapse was identified by low hematotoxicity in the first half
of the consolidation phase. Early identification of the HR
population enables us to intensify treatment in these patients.
Low hematological toxicity has been reported to be
associated with a poorer outcome of other malignancies (6–12).
This association is predicted to be evident in acute leukemia,
considering the common origin of leukemic blasts and normal
hematopoietic cells. Previously, Han et al showed that a
leukocyte nadir of >1,200/μl in induction chemotherapy is
associated with poor overall survival in adult patients with acute
myeloid leukemia (AML), although, no statistically significant
difference was identified (15).
This is consistent with the observations of the current study. On
the other hand, previous studies have reported that patients with
severe hematological toxicity and a slow rate of myeloid recovery
in induction chemotherapy exhibit a poor clinical outcome in adult
AML and childhood ALL (15,16). The mechanism underlying this
association is unclear, but leukemic blasts in bone marrow are
likely to affect the leukocyte count until remission and rate of
myeloid recovery following induction therapy. In the present study,
chemosensitivity of non-malignant hematopoietic cells were
evaluated following remission induction, when the effect of
residual leukemic cells may almost be ignored.
A false association between leukopenia and treatment
outcome may have been established, since more severe leukopenia was
predicted, as the patients had prolonged survival and received more
treatment courses. In the present cohort, 17 of the 19 patients
completed all three courses of the first half of the consolidation
phase. The remaining two patients who relapsed in the third course
also received two out of three courses. Low hematological toxicity
could not be fully explained by a reduced number of chemotherapy
courses.
The results of the present study indicated that
leukopenia may be used as a biomarker for effective chemotherapy
dose, supporting the theory of individualizing chemotherapy dosage
based on hematological toxicity (17). Patients with low acute hematological
toxicity may be rapid metabolizers of cytotoxic agents. Considering
that the hematopoietic cells of these patients exhibit low
sensitivity to cytotoxic agents, corresponding leukemic blasts may
also demonstrate low sensitivity to the drugs. Whether the outcome
of these patients may be improved by dose-escalation must be
prospectively studied in a large clinical trial.
The current study was unable to evaluate the
influence of other possible prognostic factors by multivariate
analysis, as the number of patients was too small. However,
patients in the present cohort were stratified into the same risk
group and were roughly adjusted for the conventional factors,
including age, leukocyte count at diagnosis, immunophenotypes of
leukemic blasts and early treatment response. This may be one of
the reasons why these factors were not associated with relapse. In
addition, chemotherapy-induced leukopenia is unlike the
conventional risk factors, since it reflects the response of normal
hematopoietic cells, but not tumor cells. Leukocyte nadir is thus
predicted to be an independent prognostic factor. Further
investigation in a larger cohort is required to assess this
possibility.
In conclusion, the degree of chemotherapy-induced
leukopenia was found to correlate with RFS in child patients with
ALL. Trials exploring intrapatient dose escalation are
warranted.
Acknowledgements
The current study was supported by institutional and
departmental sources at the Department of Pediatrics, the
University of Tokyo Hospital, Japan.
References
|
1
|
Pui CH and Evans WE: Treatment of acute
lymphoblastic leukemia. N Engl J Med. 354:166–178. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Pui CH, Robison LL and Look AT: Acute
lymphoblastic leukaemia. Lancet. 371:1030–1043. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Vrooman LM and Silverman LB: Childhood
acute lymphoblastic leukemia: update on prognostic factors. Curr
Opin Pediatr. 21:1–8. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Kvinnsland S: The leucocyte nadir, a
predictor of chemotherapy efficacy? Br J Cancer. 80:16811999.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Gurney H: How to calculate the dose of
chemotherapy. Br J Cancer. 86:1297–1302. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Di Maio M, Gridelli C, Gallo C, et al:
Chemotherapy-induced neutropenia and treatment efficacy in advanced
non-small-cell lung cancer: a pooled analysis of three randomised
trials. Lancet Oncol. 6:669–677. 2005.PubMed/NCBI
|
|
7
|
Banerji U, Ashley S, Coward J, et al: The
association of chemotherapy induced neutropenia on treatment
outcomes in small cell lung cancer. Lung Cancer. 54:371–377. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Carpenter JT Jr, Maddox WA, Laws HL,
Wirtschafter DD and Soong SJ: Favorable factors in the adjuvant
therapy of breast cancer. Cancer. 50:18–23. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Saarto T, Blomqvist C, Rissanen P, Auvinen
A and Elomaa I: Haematological toxicity: a marker of adjuvant
chemotherapy efficacy in stage II and III breast cancer. Br J
Cancer. 75:301–305. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Poikonen P, Saarto T, Lundin J, Joensuu H
and Blomqvist C: Leucocyte nadir as a marker for chemotherapy
efficacy in node-positive breast cancer treated with adjuvant CMF.
Br J Cancer. 80:1763–1766. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Cortes EP, Holland JF, Wang JJ, et al:
Amputation and adriamycin in primary osteosarcoma. N Engl J Med.
291:998–1000. 1974. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Brosteanu O, Hasenclever D, Loeffler M and
Diehl V; German Hodgkin’s Lymphoma Study Group. Low acute
hematological toxicity during chemotherapy predicts reduced disease
control in advanced Hodgkin’s disease. Ann Hematol. 83:176–182.
2004.PubMed/NCBI
|
|
13
|
Möricke A, Reiter A, Zimmermann M, et al:
Risk-adjusted therapy of acute lymphoblastic leukemia can decrease
treatment burden and improve survival: treatment results of 2169
unselected pediatric and adolescent patients enrolled in the trial
ALL-BFM 95. Blood. 111:4477–4489. 2008.
|
|
14
|
Manabe A, Ohara A, Hasegawa D, et al:
Significance of the complete clearance of peripheral blasts after 7
days of prednisolone treatment in children with acute lymphoblastic
leukemia: the Tokyo Children’s Cancer Study Group Study L99-15.
Haematologica. 93:1155–1160. 2008.PubMed/NCBI
|
|
15
|
Han HS, Rybicki LA, Thiel K, et al: White
blood cell count nadir following remission induction chemotherapy
is predictive of outcome in older adults with acute myeloid
leukemia. Leuk Lymphoma. 48:1561–1568. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Laughton SJ, Ashton LJ, Kwan E, Norris MD,
Haber M and Marshall GM: Early responses to chemotherapy of normal
and malignant hematologic cells are prognostic in children with
acute lymphoblastic leukemia. J Clin Oncol. 23:2264–2271. 2005.
View Article : Google Scholar
|
|
17
|
Jordan SD, Poole CJ, Archer VR, Steven NM
and Burton A: A retrospective evaluation of the feasibility of
intrapatient dose escalation as appropriate methodology for
dose-ranging studies for combination cytotoxic regimens. Cancer
Chemother Pharmacol. 52:113–118. 2003. View Article : Google Scholar
|