Introduction
Neuroendocrine tumors (NETs) are a rare type of
tumor, originating from dispersed neuroendocrine cells distributed
throughout the body. These cells have the ability to synthesise,
store and secrete neurohormones, neurotransmitters and
neuromodulators and can be either functioning or non-functioning.
Functioning NETs may lead to carcinoid syndrome due to the
secretion of serotonin and other vasoactive hormones including
gastrin, insulin, glucagon and somatostatin. The symptoms of NETs
may lead to misdiagnosis as may manifest in the abdominal organs in
addition to the neuroendocrine system, thereby complicating patient
treatment. Therefore researchers are attempting to diagnose this
tumor type early in order to commence treatment sooner. This tumor
type predominantly affects the gastrointestinal tract and pancreas
rather than the liver. Primary hepatic neuroendocrine carcinomas
(PHNEC) are a rare tumor type which are reported to have an
incidence of 1.5/100,000 (1),
accounting for <1% of all malignancies (2). Alternatively, due to carcinoid
syndrome the symptoms of PHNEC may be varied and different to those
for primary hepatocellular carcinoma (HCC) or those of other liver
tumors. This feature and the rarity of PHNEC make it difficult to
diagnose the PHNEC early and accurately prior to performing
biopsies or tumor resectioning. To date, PHNEC is often
misdiagnosed prior to performing a pathological examination.
Computed tomography (CT) and magnetic resonance imaging (MRI) are
emerging as two clinically usefully techniques in oncology. These
techniques may be used for diagnosis, treatment monitoring and
pathophysiologic understanding of PHNEC. However, it is often
impossible to diagnose PHNEC accurately by relying solely on CT or
MRI and pathological examination is currently the gold standard for
diagnosis. To improve knowledge and diagnosis of PHNEC, the present
study analyzed the imaging results and pathological features of
nine pathologically diagnosed cases.
Materials and methods
Clinical materials
Nine cases of PHNEC diagnosed by pathological
examination following surgery and biopsy between 2007 and 2012 were
collected from the Second Xiangya Hospital of Central South
University (Changsha, China). Patients included six males and three
females aged between 24 and 66 years with an average age of 46.2
years. Tumor marker serum analysis showed the nine patients to be
α-fetoprotein (AFP)-negative. Samples were also cancer antigen 125
(CA125)-, carbohydrate antigen 19-9 (CA19-9)-, carcinoembryonic
antigen (CEA)- and neuron-specific enolase (NSE)-negative in six
cases. One patient was HBsAg-positive. Six patients exhibited
symptoms of abdominal pain and distension, two had alimentary tract
bleeding, two had anorexia and exhibited weight loss and one
patient had jaundice. This study was approved by the Ethics
Commitee of The Second Xiangya Hospital of Central South University
(Changsha, China). All patients provided written informed
consent.
Equipment and methods
The nine patients were subjected to computed
tomography (CT) scanning. Eight were subjected to 64-slice spiral
CT (Siemens Corporation, Munich, Germany). Patients were tested by
plain and dynamic contrast-enhanced scanning of the liver with a
matrix of 512×512, layer depth of 5 mm, layer distance of 5 mm and
a vision field of 25×25 to 38×38 cm, using iohexol (dose, 1.5
ml/kg; speed of injection, 2.5 ml/sec) as a contrast medium.
Arterial, portal venous and delayed phase scans were performed at
25–30 sec, 50 sec and 5 min respectively for all phases, following
injection of the patients with contrast medium. The other patient
was subjected to LightSpeed 16 Slice CT (GE Healthcare, Little
Chalfont, UK). The patient was tested by dynamic CT scanning of the
liver with a matrix of 512×512, layer depth of 5 mm, layer distance
of 5 mm and a vision field of 50×50 cm, using iohexol as a contrast
medium (dose, 80 ml; speed of injection,3.5 ml/sec).
Two patients were subjected to magnetic resonance
imaging (MRI) testing using the 1.5T Signal Twin Speed MRI scanner
(GE Healthcare). The parameters for T1-weighted image (T1WI) were
listed as follows: Echo time (TE), minimum 1.6 msec; repetition
time (TR), 150 msec; matrix, 256×160; number of excitations (NEX),
2.00; field of view (FOV), 36×36 cm; layer depth, 5 mm; layer
distance, 1.5 mm; and fast spoiled gradient-recalled
echo-sequence cross-section scanning during a respiratory cycle.
The parameters for T2-weighted image (T2WI) were as follows: TE,
102 msec; TR, 4,000 msec; matrix, 256×256; NEX, 4.00; FOV, 36×36
cm; layer depth, 5 mm; layer distance, 1.5 mm; and fat suppression
fast recovery fast spin echo sequence with respiratory triggering
technique transverse and cross-section scanning. The two patients
were injected with gadolinium diethylene-triamine pentaacetic acid
as contrast medium at a dose of 0.1 ml/kg. The parameters used for
enhancement scanning were: Axial FAME ASSET sequence; TE in phase;
TR, 20 msec; FOV, 38×38 cm; NEX, 1.0; layer depth, 4 mm; layer
distance, 0 mm; and matrix 256×160. This was performed using
transverse scanning.
Pathology techniques
Four patients were subjected to surgical biopsy,
while the remaining five patients were subjected to CT-guided
puncture biopsy. Samples were cut into slices and stained with
hematoxylin-eosin and other immunohistochemical staining agents,
including chromogranin A (CgA), synaptophysin (Syn), NSE,
cytokeratin (CK), hepatocytic antigen (HPC), CEA, AFP, epithelial
membrane antigen (EMA) and vimentin (Vim) (Maxin-Bio, Fuzhou,
China).
Results
Imaging results
CT results
CT scanning revealed single or multiple masses in
the livers of the patients, with a maximum diameter of 1–10 cm.
These hepatic masses were shown by plain CT to be of low density.
The single tumor masses exhibited clear boundaries, whereas the
multiple masses exhibited unclear boundaries and an uneven density.
A liquefied necrotic area of lower density was observed in the
center of certain larger lesions. Furthermore, the arterial phase
of dynamic enhancement CT showed uneven or annular enhancement of
the tumor margins. The venous portal phase showed consistent or
declined enhancement in the tumor masses, whilst the delayed phase
showed light enhancement.
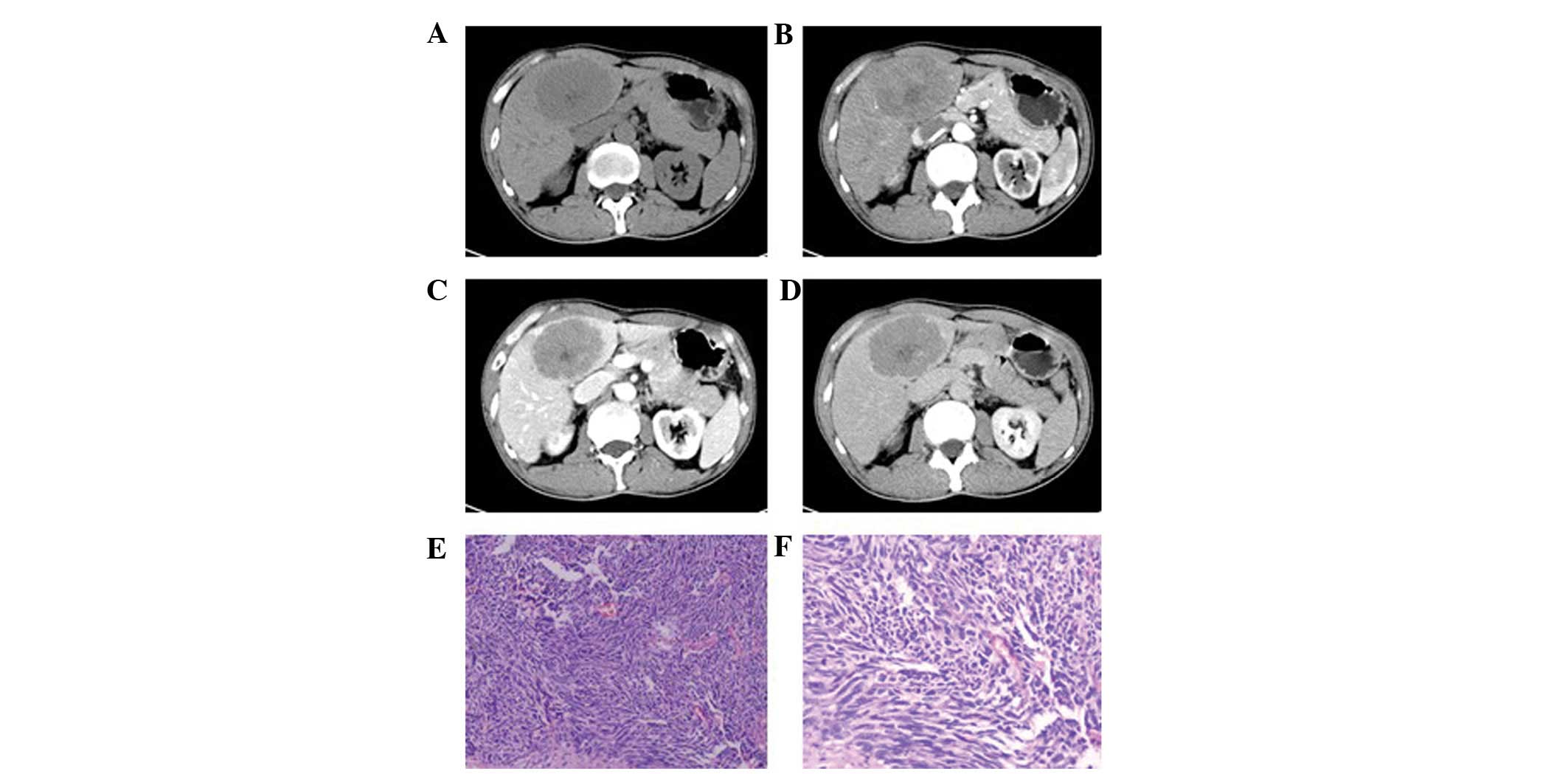
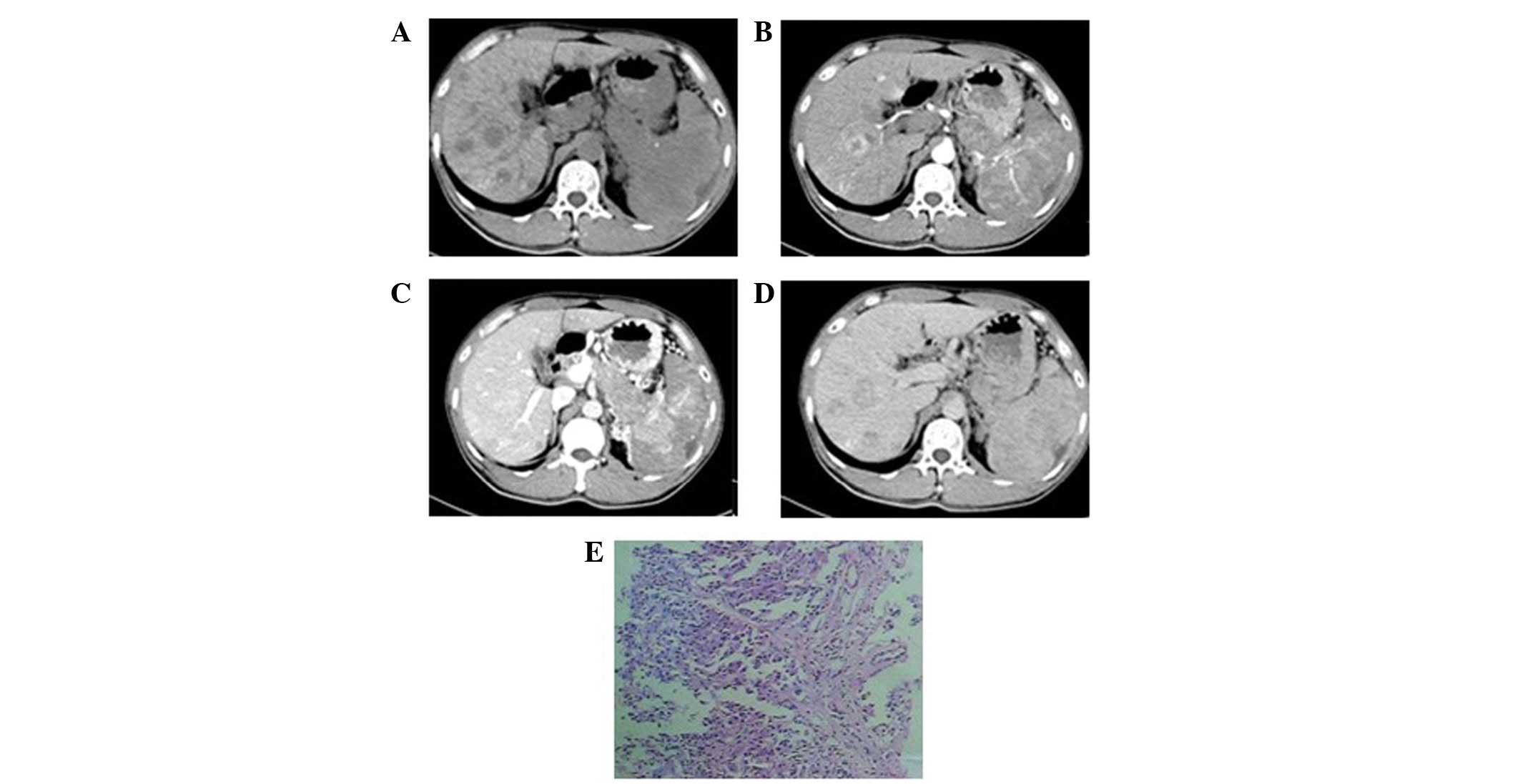
One patient exhibited a single mass in the left
hepatic lobe with a maximum cross-section size of 6.5×5.0 cm, clear
boundaries and an uneven density (Fig.
1). A liquefied necrotic area in the center of the focus was
observed. Enhancement scanning revealed a mild and uneven
enhancement at the edge of the mass in the arterial phase, which
declined in the portal venous and delayed phases.
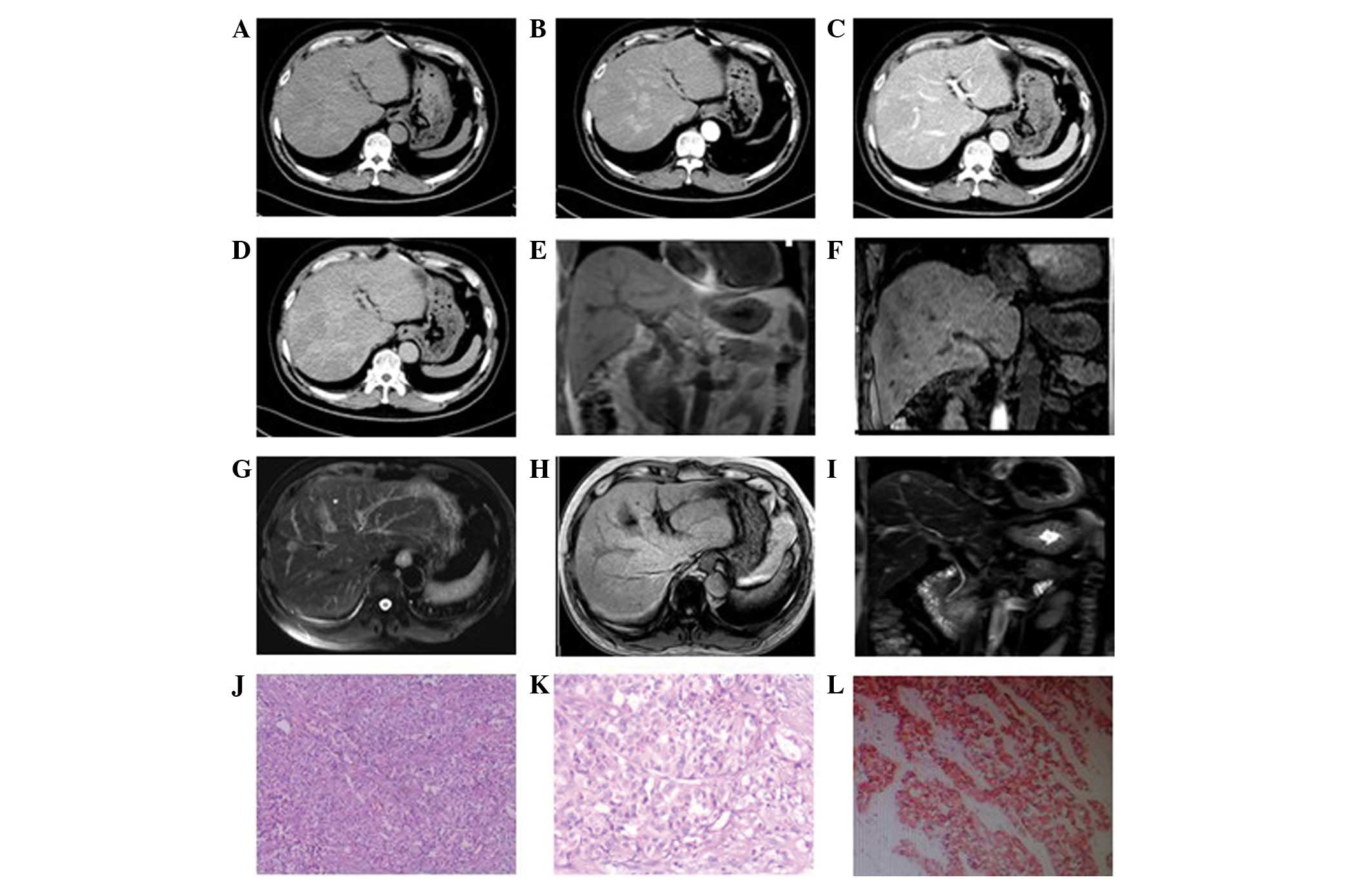
In the eight patients exhibiting multiple
intrahepatic lumps, the foci were of various sizes. In four
patients, the diameters of the largest foci were >7 cm whilst in
the remaining four, the diameters were <2 cm. Plain CT scan
showed low-density lesions. The four cases with larger masses had a
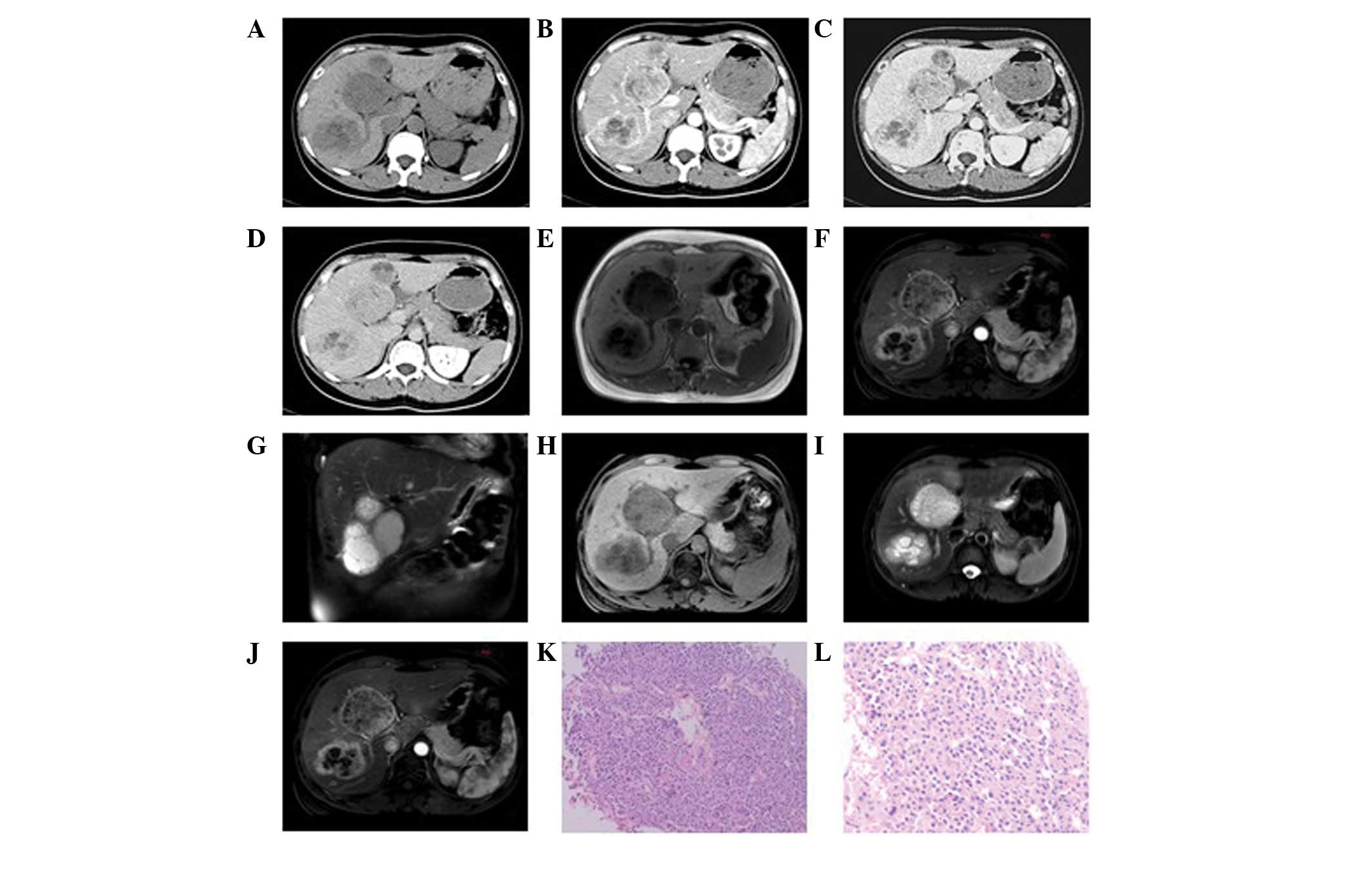
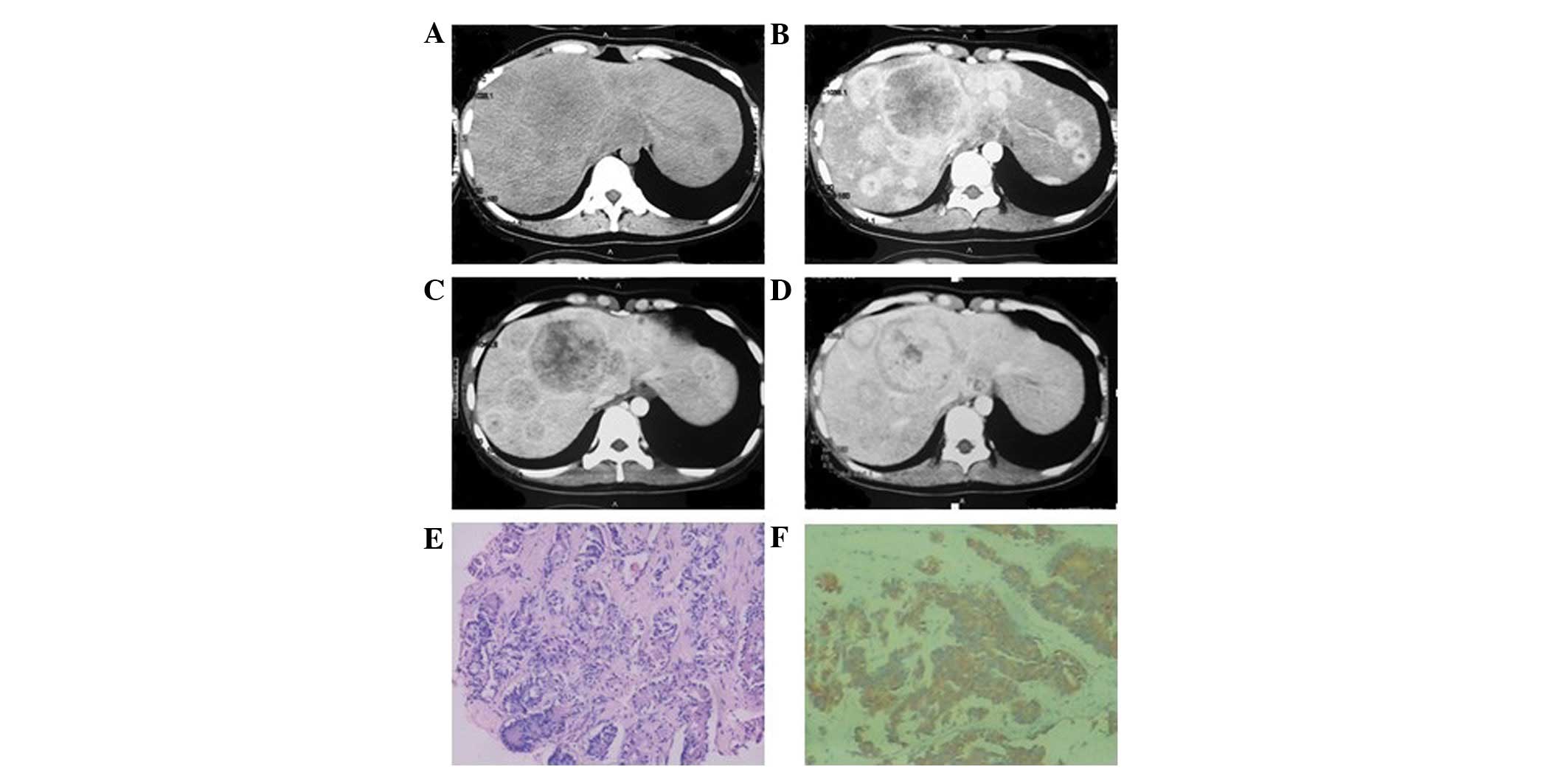
lower density liquefied necrotic area (Figs. 2A, 3A and 4A)
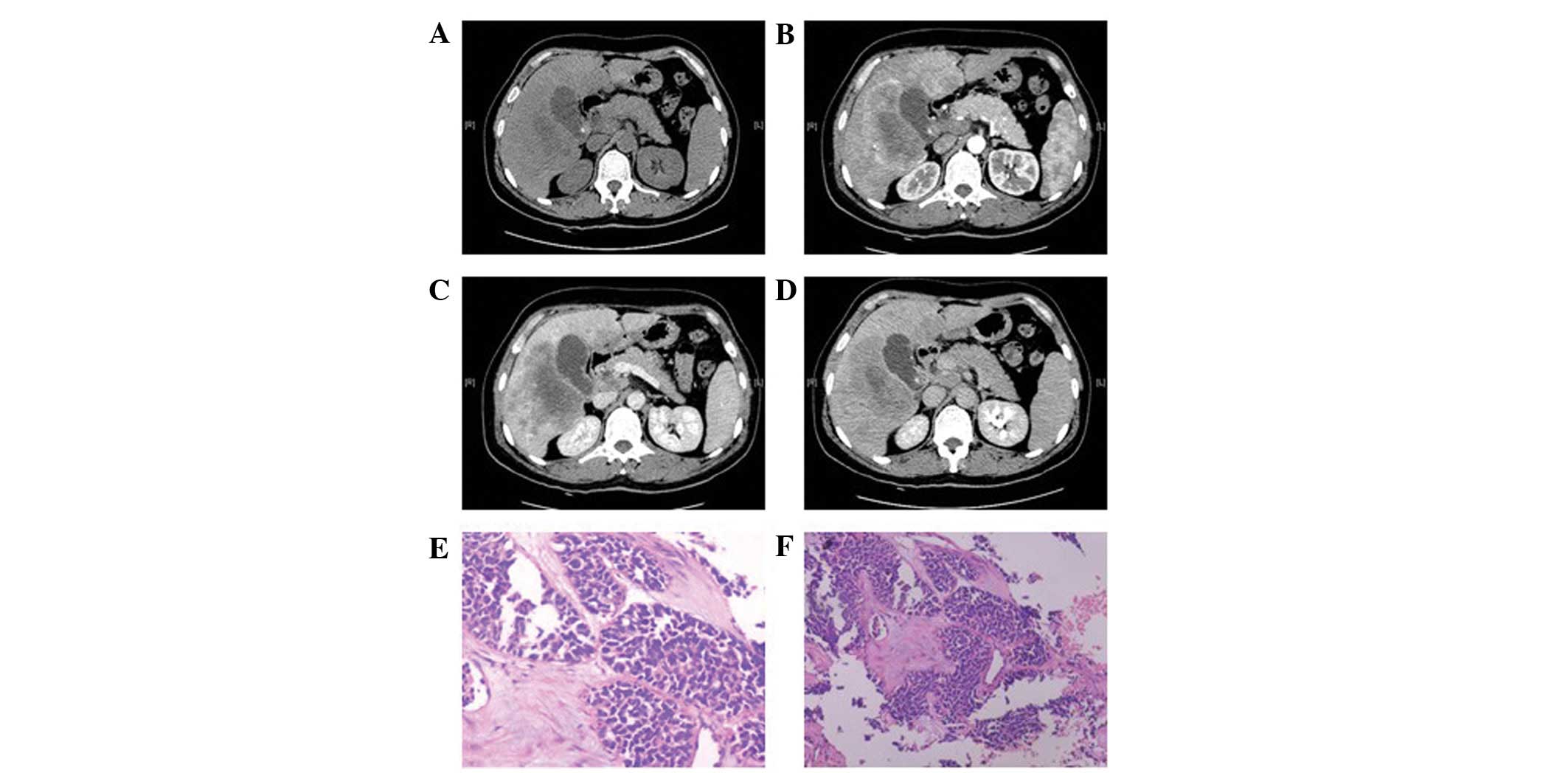
while the four cases with smaller foci had a relatively uniform
density (Fig. 5A). The boundaries
of the foci were unclear in six patients (Figs. 3 and 5), while they were clear in the other two
cases (Fig. 2). All foci showed
uneven enhancement in the arterial phase, six of which exhibited
annular enhancement (Figs.
2B–4B). In six patients, the
degree of enhancement was found to decline in the portal venous
phase (Figs. 2C, 3C and 4C)
and the delayed phase (Figs. 2D,
3D and 4D). In two patients, the enhanced area was
enlarged in the portal venous phase (Fig. 5C) and the enhancement extended over
a long period of time. The degree of enhancement in the delayed
phase (Fig. 5D) declined in all
cases, and the density of the foci was generally uniform. In one
patient (Fig. 6), the foci were not
observed in plain scanning but were clearly observed in the
arterial phase and absent in the portal venous and delayed
phases.
In this group, CT scanning was able to predict the
pathological changes and, therefore, the malignant changes.
However, a definitive diagnosis depends on pathological
examination.
MRI results
Two patients were subjected to MRI. In one case
(Fig. 2E–J), multiple long T1 and
T2 signal foci were observed in the liver, which were nodular,
lumpy and significantly enhanced. Another patient (Fig. 6E–I) exhibited multiple intrahepatic
nodules that were not clearly visible in T1- or T2-weighted images.
However, a relatively long signal was observed in T2-weighted fat
suppression and T1 enhancement images. In addition, the foci were
unevenly enhanced in T1 enhancement scanning.
Pathology
A number of different types of neuroendocrine tumor
were observed in this study, including carcinoid tumors, a
well-differentiated neuroendocrine carcinoma and a poorly
differentiated neuroendocrine carcinoma. Among these were two cases
of carcinoid tumors (Fig. 3E and
3F), three cases of well-differentiated neuroendocrine
carcinomas (Figs. 2K and 5E) and four cases of poorly-differentiated
neuroendocrine carcinomas (Figs.
1F, 4E and 6J–L). Pathological results showed that the
tumor cells were morphologically diverse and that a number of tumor
cells formed vessel-like arrangements, with similar morphological
features and of little interstitial substance (Figs. 2K, 3E
and 3F). Other tumor cells were uniformly small- to medium in
size with unclear cytoplasmic boundaries. Additionally, their
nuclei were round or regular in shape and arranged irregularly,
clustered and flakily or like a chrysanthemum (Fig. 6J–L). The poorly differentiated
cancer cells were smaller and with less cytoplasm than the
well-differentiated cells. Their nuclei were angular,
trachychromatic and karyokinesis was observed (Figs. 1F and 4E). In addition, neuroendocrine granules
were observed by electron microscopy (H-7500 Electron Microscope,
Hitachi, Tokyo, Japan). The immunohistochemical staining in these
tumor cells was positive for CgA and Syn and negative for CEA, HPC
and AFP (Table I).
 | Table IImmunohistochemistry results of the
nine patients. |
Table I
Immunohistochemistry results of the
nine patients.
| Patient no. | CgA | Syn | CK | CEA | HPC | NSE | AFP | Vim |
|---|
| 1 | ++ | ++ | + | − | − | | − | |
| 2 | + | + | − | − | | | | − |
| 3 | ++ | + | | | − | | | − |
| 4 | ++ | ++ | + | − | − | | − | − |
| 5 | + | + | | − | | + | − | + |
| 6 | + | + | + | | − | + | | |
| 7 | + | + | | | − | | − | |
| 8 | + | + | ++ | + | | | − | |
| 9 | ++ | ++ | − | − | | + | − | − |
Discussion
Neuroendocrine carcinomas most commonly develop in
the gastrointestinal tract and pancreas. Those seen in the liver
are metastasized from primary carcinomas of the gastrointestinal
tract or pancreas in the majority of cases, and primary
neuroendocrine carcinomas originating from the liver itself are
rare (3). Symptoms and signs are
not obvious in the early stages of this disease type. A number of
patients may exhibit nonspecific symptoms, including abdominal pain
and distension, whilst several suffer from carcinoid syndrome
(4). The condition worsens with
progression of the disease, with a number of manifestations,
including symptoms caused by tumors compressed in adjacent organs,
dyspepsia, weight loss and fatigue. Patients usually deny histories
of hepatitis or cirrhosis (5) and
exhibit AFP-negative serum levels. In addition, conventional tumor
markers, for example CEA, CA125 and CA19-9, are usually
negative.
Neuroendocrine tumors originating from
neuroendocrine cells may be divided into two types, neurological
and epithelial (6). Hepatic
neuroendocrine tumors fall into the epithelial subtype but there
are conflicting opinions, as it has been hypothesized that hepatic
neuroendocrine tumors are formed by proliferation of neuroendocrine
cells dispersed within the hepatic biliary epithelium (7). According to the latest World Health
Organization criteria (2010) (8),
neuroendocrine carcinomas may be subcategorized into three groups:
Well-differentiated neuroendocrine tumors (carcinoid), moderately
differentiated neuroendocrine carcinomas (atypical carcinoid) and
poorly differentiated neuroendocrine carcinomas (small cell
neuroendocrine carcinoma).
Pathological results, particularly
immunohistochemical results, are required for the definitive
diagnosis of a neuroendocrine carcinoma. It is generally accepted
that positive expression of CgA, Syn and NSE represents definite
evidence for diagnosis (9–11). Among the immunohistochemical
results, CgA and Syn staining were positive in the nine patients,
which was consistent with the aforementioned studies. NSE testing
was performed in three patients, all of which were positive. All
nine patients demonstrated negative results for CEA, HPC and AFP,
whereas the majority of CK results were positive. Irregular data
were obtained in Vim and EMA tests. However, in contrast to the
immunohistochemical results, serum NSE tests were negative in all
six patients tested, which indicated that NSE was expressed in
tumor cells but not in serum. Therefore, this mechanism must be
investigated further.
It is difficult to distinguish primary hepatic
neuroendocrine carcinomas from metastases using pathological
evidence alone. Therefore, clinical features are also important for
this clarification. Thorough testing prior to surgery, examination
during surgery and follow-ups after surgery are important in
determining whether a primary focus is present outside the liver
(12).
Neuroendocrine carcinomas are receiving considerable
attention and pathological examination techniques, particularly the
applications of electron microscopy and immunohistochemistry, are
being developed rapidly. Although imaging methods, including CT and
MRI, are unable to definitively diagnose neuroendocrine carcinomas
(13,14), they remain useful for the
preliminary diagnosis of a tumor. This is particularly true when
combined with clinical features and other supporting information,
for example no patient history of hepatic cirrhosis and an absence
of tumor markers, i.e., AFP (3).
The patients of the present study exhibited single
and multiple low-density masses with undefined boundaries, the
majority of which had uniform density. Liquefaction necrosis was
observed in the center of specific larger lesions.
Contrast-enhanced CT scanning showed annular enhancement or
small-sized heterogeneous enhancement in the arterial phase. The
area of enhancement enlarged in the portal venous phase and the
density remained almost constant in this phase. There was mild
enhancement in the delayed phase. These results are consistent with
previous observations (15,16).
In the present study, one patient exhibited lesions
only in the left lobe of the liver, while the other eight exhibited
multiple lesions in the whole liver, indicating diffuse growth of
the tumor. In addition, the majority of the tumors showed annular
enhancement. Two patients showed enlargement of the hepatic portal
lymph nodes; one showed abdominal lymph node enlargement and the
other showed retroperitoneal lymph node enlargement. Portal vein
tumor thrombi and cirrhosis were not observed in any of the
patients. CT scanning was unable to conclusively diagnose hepatic
neuroendocrine carcinomas due to its non-specificity. However, it
was able to clearly reveal the internal structure, blood supply,
association with neighboring organs and metastasis of the tumor.
This indicates that CT may improve preliminary differential
diagnosis and determine whether a mass is primary or secondary, the
correct treatment and the prognosis.
Two of the nine patients were examined by MRI and
showed marginally different appearances. One showed low signal
lesions at T1WI whilst the other did not show any abnormal signal
in the same MRI sequence. The two patients showed high signal
lesions at T2WI, consistent with previous studies (17–20).
This neuroendocrine carcinoma tumor type should be
distinguished from primary hepatocellular carcinoma (HCC)
irrespective of whether single or multiple in number. A typical HCC
often accompanies hepatitis and hepatic cirrhosis, and the majority
of patients have elevated serum AFP levels. In addition, HCCs show
well-defined enhancement in the arterial phase and rapid loss of
enhancement during the portal venous and delayed phases known as
the ‘quick in and out of contrast medium’. These factors are
important for differential diagnosis. However, specific
neuroendocrine carcinomas, with diffuse lesions, are difficult to
distinguish from diffuse HCCs. Multiple lesions with ring-shaped
enhancements are commonly found, which should be distinguished from
metastatic tumors by clinical and immunohistochemical analyses. In
addition, HCC should be distinguished from focal nodular
hyperplasia (FNH). FNH has a lower degree of enhancement and
approximately one-third of FNH patients exhibit a central fibrous
scar in lesions, showing a star-shaped low-density shadow in plain
and enhanced scanning.
Early detection and resection of primary lesions is
the preferred treatment for the improvement of survival rate. For
patients without indications for surgery, hepatic artery
chemotherapy embolism is a preferred non-operative treatment that
results in a good prognosis (21).
Radiofrequency ablation, cryotherapy, microwaving and anhydrous
alcohol injection also exert therapeutic effects (3,22).
Liver transplantation is considered in special cases (23,24).
Previous studies have indicated that interferon and somatostatin,
as well as its analogs, may be used in the treatment of carcinomas
(25,26).
As with other malignant tumors, early detection and
treatment are the key to achieving a good prognosis. The prognosis
is associated with a number aspects, including pathological type,
degree of differentiation, size and boundary of the tumor,
metastasis and the physical status of the patient.
References
|
1
|
Dogra VS and Poblete J: Metastatic
carcinoid tumor in the liver. J Clin Ultrasound. 21:639–641. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Newton JN, Swerdlow AJ, dos Santos Silva
IM, Vessey MP, Grahame-Smith DG, Primatesta P and Reynolds DJ: The
epidemiology of carcinoid tumors in England and Scotland. Br J
Cancer. 70:939–942. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yalav O, Ülkü A, Akçam TA, Demiryürek H
and Doran F: Primary hepatic neuroendocrine tumor: five cases with
different preoperative diagnoses. Turk J Gastroenterol. 23:272–278.
2012.PubMed/NCBI
|
|
4
|
Shetty PK, Baliqa SV, Balaiah K and Gnana
PS: Primary hepatic neuroendocrine tumor: an unusual cystic
presentation. Indian J Pathol Microbiol. 53:760–762. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ishida M, Seki K, Tatsuzawa A, et al:
Primary hepatic neuroendocrine carcinoma coexisting with
hepatocellular carcinoma in hepatitis C liver cirrhosis: report of
a case. Surg Today. 33:214–218. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Zheng W, Hua H and Shi W: Primary hepatic
neuroendocrine carcinoma: report of 2 cases. J Pract Oncol.
25:459–460. 2010.
|
|
7
|
Gravante G, De Liguori Carino N, Overton
J, Manzia TM and Orlando G: Primary carcinoids of the liver: a
review of symptoms, diagnosis and treatments. Dig Surg. 25:364–368.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Endo S, Dousei T, Yoshikawa Y, et al:
Gastric neuroendocrine tumors in our institutions according to the
WHO 2010 classification. Int Surg. 97:335–339. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sundin A, Eriksson B, Bergström M,
Långström B, Oberg K and Orlefors H: PET in the diagnosis of
neuroendocrine tumors. Ann NY Acad Sci. 1014:246–257. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Campana D, Nori F, Piscitelli L,
Morselli-Labate AM, Pezzilli R, Corinaldesi R and Tomassetti P:
Chromogranin A: is it a useful marker of neuroendocrine tumors? J
Clin Oncol. 25:1967–1973. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Pilichowska M, Kimura N, Ouchi A, et al:
Primary hepatic carcinoid and neuroendocrine carcinoma:
clinicopathological and immunohistochemical study of five cases.
Pathol Int. 49:318–324. 1999. View Article : Google Scholar
|
|
12
|
Sano K, Kosuge T, Yamamoto J, Shimada K,
Takayama T, Yamasaki S and Makuuchi M: Primary hepatic carcinoid
tumors confirmed with long-term follow-up after resection.
Hepatogastroenterology. 46:2547–2550. 1999.PubMed/NCBI
|
|
13
|
van der Hoef M, Crook DW, Marincek B and
Weishaupt D: Primary neuroendocrine tumors of the liver: MRI
features in two cases. Abdom Imaging. 29:77–81. 2004.PubMed/NCBI
|
|
14
|
Kong W, Qiu Y, Zhang W, Jun C, Zhu X, Qiu
J and Ding Y: Diagnosis of primary hepatic carcinoid tumor: report
of one case. Chinese-German J Clin Oncol. 7:673–675. 2008.
View Article : Google Scholar
|
|
15
|
Kumbasar B, Kamel IR, Tekes A, Eng J,
Fishman EK and Wahl RL: Imaging of neuroendocrine tumors: accuracy
of helical CT versus SRS. Abdom Imaging. 29:696–702. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Schwartz G, Colanta A, Gaetz H, Olichney J
and Attiyeh F: Primary carcinoid tumors of the liver. World J Surg
Oncol. 6:912008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fenoglio LM, Severini S, Ferrigno D, et
al: Primary hepatic carcinoid: a case report and literature review.
World J Gastroenterol. 15:2418–2422. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zhu Z, Zhao X and Zhou C: Imaging features
of primary hepatic endocrine carcinoma. Zhongguo Yi Xue Ying Xiang
Ji Shu. 4:721–723. 2010.(In Chinese).
|
|
19
|
Ren Y, Wang S, Chen J and Li Y: Primary
hepatic neuroendocrine carcinoma: report of 4 cases. Radiol
Practice. 26:687–688. 2011.
|
|
20
|
Takayasu K, Muramatsu Y, Sakamoto M, et
al: Findings in primary hepatic carcinoid tumor: US, CT, MRI, and
angiography. J Comput Assist Tomogr. 16:99–102. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Touloumis Z, Delis SG, Triantopoulou C,
Giannakou N, Avgerinos C and Dervenis C: Primary hepatic carcinoid;
a diagnostic dilemma: a case report. Cases J. 1:3142008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mazzaglia PJ, Berber E, Milas M and
Siperstein AE: Laparoscopic radiofrequency ablation of
neuroendocrine liver metastases: a 10-year experience evaluating
predictors of survival. Surgery. 142:10–19. 2007.
|
|
23
|
Fewick SW, Wyatt JI, Toogood GJ and Lodqe
JP: Hepatic resection and transplantation for primary carcinoid
tumors of the liver. Ann Surg. 239:210–219. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gurung A, Yoshida EM, Scudamore CH, Hashim
A, Erb SR and Webber DL: Primary hepatic neuroendocrine tumor
requiring live donor liver transplantation: case report and concise
review. Ann Hepatol. 11:715–720. 2012.
|
|
25
|
Modlin IM, Kidd M, Latich I, Zikusoka MN
and Shapiro MD: Current status of gastrointestinal carcinoids.
Gastroenterology. 128:1717–1751. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Iwao M, Nakamuta M, Enjoji M, et al:
Primary hepatic carcinoid tumor: case report and review of 53
cases. Med Sci Monit. 7:746–750. 2001.PubMed/NCBI
|