Introduction
Rhabdomyosarcoma (RMS), a form of soft-tissue
sarcoma, is the most common type of extracranial neoplasm in
childhood and represents ~4.5% of childhood cancer (1). RMS shows a wide range of biological,
genetic and morphological characteristics and exhibits a diverse
clinical behavior. On the basis of histopathological criteria, RMS
in childhood is classified into the two main subtypes of embryonal
RMS (eRMS; 80%) and alveolar RMS (aRMS; 20%). Patients that present
with a localized tumor have a good prognosis. However, ~15% of
patients with RMS have high-risk stage IV diseases with bone marrow
(BM) involvement and a poor clinical outcome, with a three-year
survival rate of ~15% (1,2). The use of myeloablative chemotherapy
in combination with autologous BM or peripheral blood stem cell
transplantation rescue may be required to treat children with stage
IV disease (3,4). Thus, the detection of BM involvement
at diagnosis is critical for accurate staging and risk assessment.
In addition, minimal residual RMS cell assays may be used during
therapy to assess the early response to treatment and predict
relapse. Morphological screening of BM aspirates has been the gold
standard for a number of years. Considering a detection sensitivity
level of 1% tumor cells for cytological screening of BM samples, it
appears conceivable that morphological techniques alone lack the
sensitivity to monitor minimal residual disease (MRD). For this
reason, new methods have been developed. Detection of specific
transcripts, such as PAX3/7-FKHR, in RMS patients by reverse
transcription-polymerase chain reaction (PCR) has been previously
demonstrated (5–7). Although PCR amplification has a high
sensitivity, it is relatively complicated and time consuming in
application. It has also been shown that almost all eRMS and ~25%
of aRMS are translocation-negative, and PCR methods may not be used
for BM metastasis detection in such patients (5).
Flow cytometry (FCM) is widely used for the
diagnosis of leukemia and lymphoma, which has been previously shown
to increase the overall diagnostic accuracy in a number of studies.
FCM has a high sensitivity and is also commonly used to assess MRD
in leukemia. To date, few studies have been reported that evaluate
the utility of FCM immunophenotyping for the diagnosis of BM
metastasis in patients with RMS. Additionally, detection by FCM of
minimal residual RMS cells in the BM during therapy to enable an
evaluation of the efficacy of therapy has not been reported.
Previous studies have shown that RMS cells typically exhibit a
cluster of differentiation
(CD)45−/CD56+/CD90+/myogenin+
phenotype with variable expression of CD57, desmin, vimentin and
CD99 in fine-needle aspiration cytological specimens (8). Bozzi et al (9) previously reported that tricolor FCM
detection of the CD56+/CD90+/CD45−
immunophenotype is extremely useful for the diagnosis of BM
metastasis in patients with RMS, but the efficacy of FCM in
monitoring therapeutic effects during and/or after chemotherapy has
not been previously evaluated. In addition, neuroblastoma (NB) is
the most common type of non-hematopoietic metastatic tumor in
children and also has a
CD56+/CD90+/CD45− immunophenotype
(9–11). The formation of a differential
diagnosis of RMS from NB using this immunophenotype is difficult
with FCM. Previous studies have shown that ganglioside D2 (GD2) is
expressed by neuroectodermally-derived tumors, such as NB and
retinoblastoma (11–14). RMS cells are negative for GD2
(9,10). In the current study, a four-color
FCM assay was developed with a CD56/CD90/CD45/GD2 monoclonal
antibody cocktail to stage RMS and detect MRD in BM samples during
and/or after chemotherapy, to evaluate therapeutic efficacy.
Materials and methods
Patients and samples
Between November 2008 and December 2012, 27 cases of
children with RMS were diagnosed by histopathology at the
Children’s Hospital of Zhejiang University School of Medicine
(Hangzhou, China). The patients consisted of 13 females and 14
males, with a median age of six years (range, 1–14 years). The
primary sites included the genitourinary tract (n=8), the head and
neck (n=7), the trunk and extremities (n=5), the post-peritoneum
(n=4) and the pelvis (n=3). In total, 25 cases presented as the
embryonal type and two as the alveolar type in histopathology. In
addition, 32 BM and two cerebrospinal fluid (CSF) samples were
obtained from 11 patients with suspected metastasis and analyzed by
FCM in parallel to conventional diagnostic procedures at the time
of diagnosis or during treatment. The study was approved by the
local ethics committee and written informed consent was obtained
from the parents or guardians of each patient in accordance with
the Helsinki protocol. The treatment of RMS included multimodal
therapy combined with surgery (complete primary tumor excision and
lymph node removal), chemotherapy [vincristine (1.5
mg/m2 ), actinomycin D (12 μg/kg) and cyclophosphamide
(VAC; 300 mg/m2)] and radiation (40–50 Gy at 1.5–1.8
Gy/fraction) based on the Children’s Oncology Group (COG) protocol.
Chemotherapy was performed monthly.
Control group
BM samples were obtained from nine cases of
clinically or pathologically diagnosed non-neoplastic disease and
an additional 27 cases diagnosed with other types of clinical
neoplastic diseases, consisting of 12 cases of acute lymphocytic
leukemia and 15 cases of NB.
Morphological and immunochemical
evaluation
The aspirates of BM were smeared onto at least three
slides and then stained and evaluated using the May-Grünwald-Giemsa
(MGG) procedure. The CSF samples were centrifuged at 800 × g for 8
min. The supernatant was removed and the cell pellets were smeared
onto the three slides. Following air-drying, the slides were
stained with MCG and immunochemical stains. Immunoreactivities to
myogenic differentiation 1 (MyoD1) and desmin in BM biopsied
samples were used as specific RMS markers. Antibodies for MyoD1 and
desmin (Mouse anti-human MyoD1 and desmin monoclonal, respectively)
were obtained from DakoCytomation (Glostrup, Denmark). Samples were
stained immunochemically using the ChemMate™ Dako EnVision™
two-step system (horseradish peroxidase; DakoCytomation).
Appropriate positive and negative controls were set. Microscopic
examinations were performed by at least two experienced
pathologists.
Four-color FCM assay
The BM samples were adjusted to 1×107
mononuclear cells per ml. The cell pellets of CSF were resuspended
with 100 μl phosphate-buffered saline. Cell suspensions (100 μl)
were stained with GD2 conjugated with fluorescein isothiocyanate
(FITC) (supplied by Professor C. Patrick Reynolds of the Children’s
Hospital Los Angeles, Los Angeles, CA, USA), CD90-phycoerythrin
(PE), CD45-peridinin chlorophyll protein (PerCP) and
CD56-allophycocyanin (APC) (Becton-Dickinson, Franklin Lakes, NJ,
USA) for 30 min at 4°C in the dark. The background of non-specific
antibody uptake was evaluated by staining in parallel with
isotype-matched immunoglobulin (Ig)G2a-FITC, IgG1a-PE, CD45-PerCP
and CD56-APC (Becton-Dickinson). At least 50,000 events were
acquired and analyzed using the CellQuest (version 3.2) software of
the fluorescence-activated cell sorting calibur flow cytometer
(Becton-Dickinson) and FlowJo7.6 software (Tree star, Inc.,
Ashland, OR, USA), respectively.
The sensitivity of the four-color FCM assays was
evaluated using spiking experiments. The absolute RMS cells were
calculated in a BM sample from a patient with BM involvement by
providing the total white blood cell count and the percentage of
RMS cells from a complete/differential cell count. Next, the RMS
cells were diluted with the normal hematopoietic cells from the BM
at the following indicated proportions: 1, 0.1, 0.01 and 0.001%.
The dilutions from 1 to 0.001% were evaluated by FCM. FCM results
(positive or negative for malignancy) were compared with those from
BM and CSF cytology, and the diagnosis was established by the
clinicians.
Statistical analysis
Data are presented as the median when continuous and
as the absolute and relative frequency when categorical. The
diagnostic specificity of FCM was calculated by the χ2
test. McNemar’s test was used to evaluate the differences between
FCM and cytological study for the BM involvement of the tumor
cells. All statistical analyses were performed using the
Statistical Package for the Social Sciences (SPSS) software,
version 12.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Morphological and immunohistochemical
observations
A total of 11 cases with suspected metastasis at
diagnosis underwent BM examination. Smears from three cases showed
the feature of BM metastasis by RMS, and these patients were
determined as stage IV RMS based on the Intergroup RMS Study Group
(IRSG). All cases presented as the embryonal type. Clinical
characteristics in cases with BM metastasis are shown in Table I. The neoplastic cells in the BM
from these three cases demonstrated a similar morphology. Smear
preparations showed that a homogeneous population of the primitive
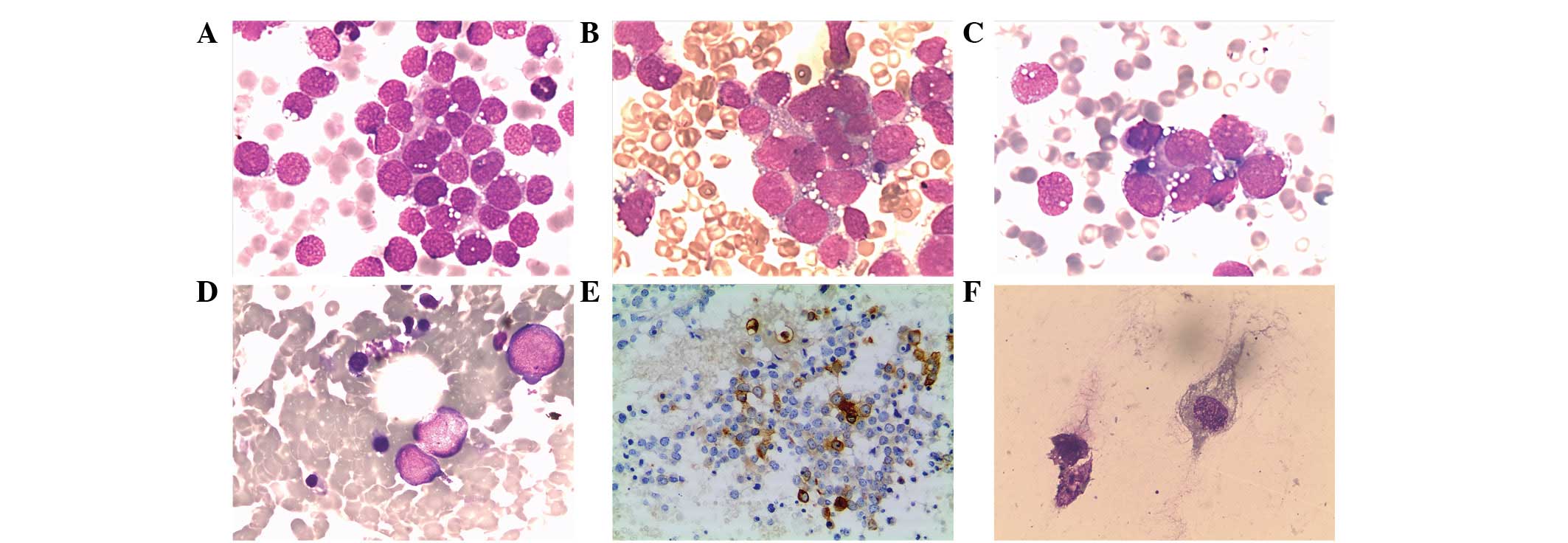
malignant cells gathered in small clusters (Fig. 1A–C). The cells demonstrated
cytoplasmic immunoreactivity with desmin, confirming the diagnosis
of RMS (Fig. 1E).
 | Table IClinical characteristics of four cases
of RMS with BM metastasis. |
Table I
Clinical characteristics of four cases
of RMS with BM metastasis.
| Patient no. | Gender | Age, years | Primary tumor
sites | Metastatic sites | Histological
subtype | Stage | Therapy | Chemotherapy | Survival period,
months | Follow-up |
|---|
| 1 | M | 8 | Head | BM | Embryonal | IV | S+R+C | VAC | 8 | Succumbed to
recurrence |
| 2 | M | 5 | Neck | BM | Embryonal | IV | S+R+C | VAC | 38 | Alive |
| 3 | M | 13 | Head (orbit) | BM | Embryonal | IV | S+R+C | VAC | 12 | Succumbed to
disease |
| 4 | F | 10 | Post-peritoneum | BMa | Embryonal | IV | S+R+C | VAC | 49 | Alive |
The treatment to RMS included multimodal therapy
with a combination of surgery, chemotherapy and radiation based on
the COG protocol. The three patients with BM involvement received
chemotherapy with the VAC regimen for 8–12 months following
surgery. The follow-up with morphological examination demonstrated
that RMS cells had not been found in the BM following the first
cycle of chemotherapy in all patients. One patient exhibited
repeated twitching and altered consciousness following 10 cycles of
chemotherapy. A lumbar puncture was performed when the patient was
admitted and a routine analysis of CSF showed a cell count of
90×106/l with 90% mononuclear cells. A smear examination
of the pellet of CFS showed a number of neoplastic cells with
vesicular nuclei and abundant densely eosinophilic scattered
cytoplasm (Fig. 1F). The cells were
positive for desmin that was compatible with RMS.
FCM immunophenotyping observations
Four out of the 11 BM samples obtained from 11
patients were positive for RMS cells by FCM at diagnosis, from
which, three cases were in accordance with the results of the
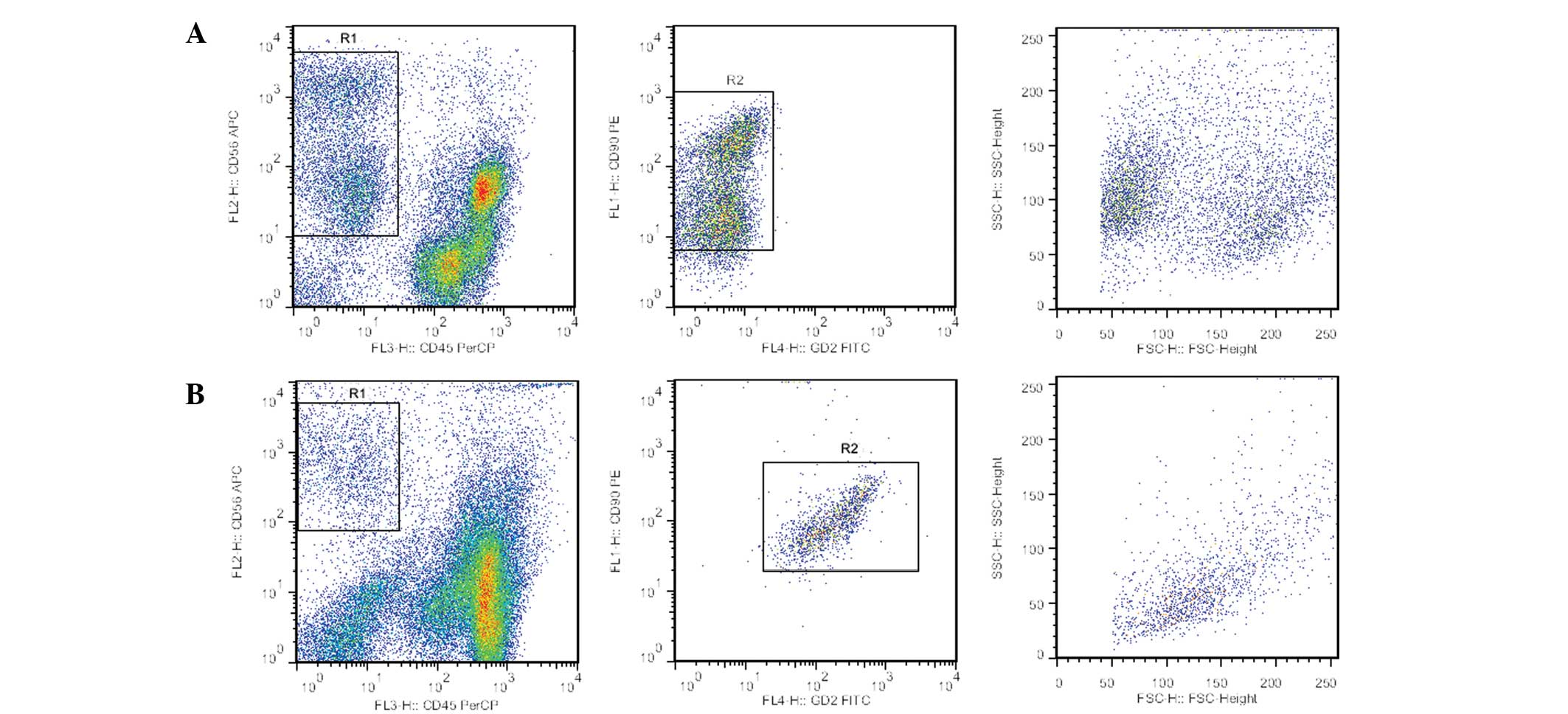
morphological evaluation. RMS cells demonstrated an immunophenotype
with positive CD90 and CD56 expression, but lacking expression of
CD45 and GD2 antigen (Fig. 2A). The
percentage of positive cells was 29.3, 12.3, 6.8 and 0.35% among
the total nucleated cells in these four cases, respectively. FCM
showed an extremely low percentage (0.35%) of RMS cells in the
morphologically-negative case. A retrospective morphological
examination of the BM smear from this case was carefully performed
due to its FCM positivity, and the results showed neoplastic cells
scattered in the background (Fig.
1D). This patient, previously stage II, had their staging
diagnosis modified to stage IV RMS by their clinician and were
administered stage IV treatment regimens. A total of 15 cases of
metastatic NB diagnosed in the BM specimens were examined by FCM.
The FCM results showed that the NB cells demonstrated a
CD56+/CD90+/CD45−/GD2+
expression immunophenotype (Fig.
2B). The analysis of the 21 BM samples obtained from nine
patients with non-neoplastic diseases and 12 patients with acute
lymphoblastic leukemia revealed no cells with the
CD56+/CD90+/CD45−/GD2−
or
CD56+/CD90+/CD45−/GD2+
phenotypes.
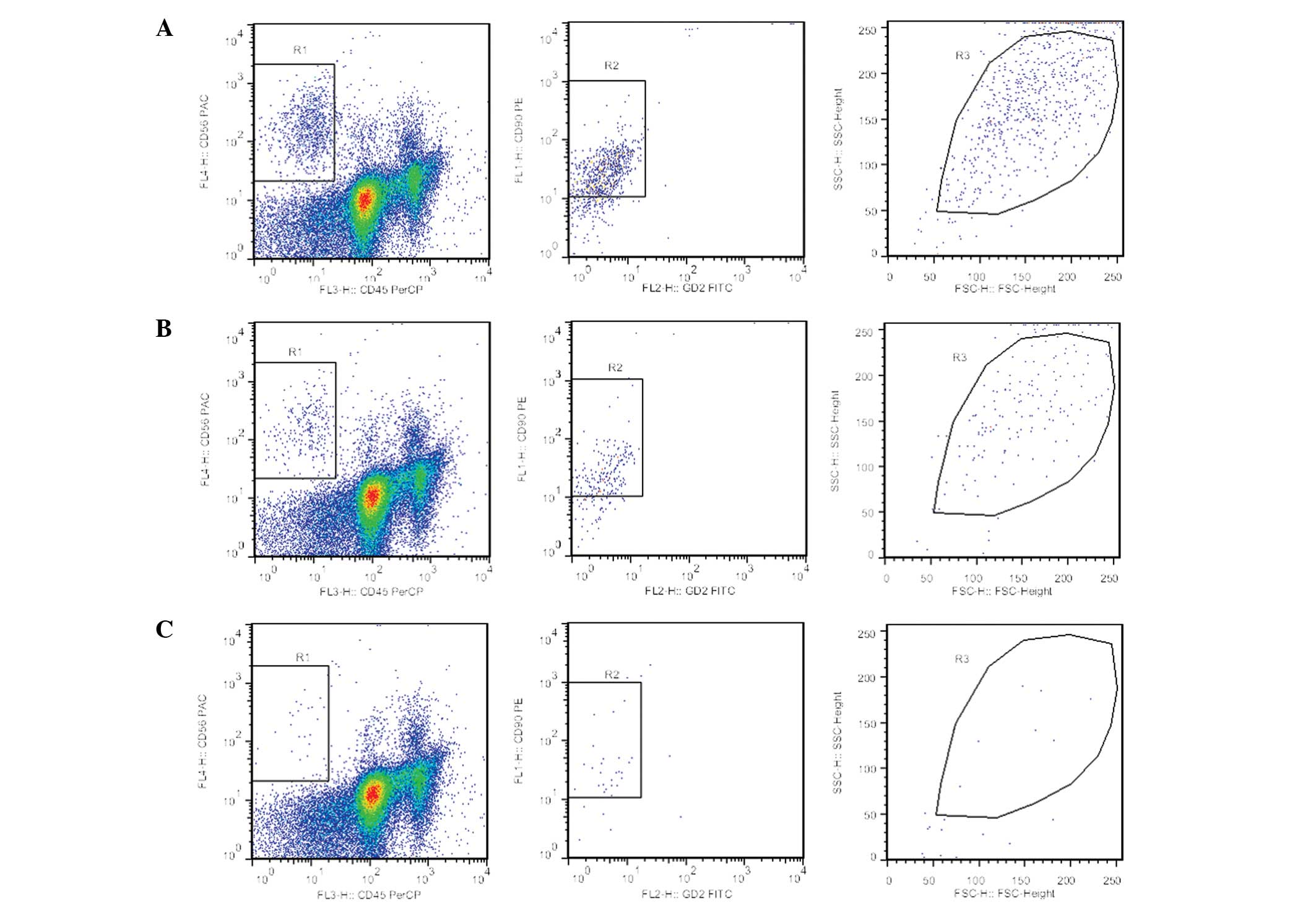
To evaluate the therapy response during treatment
and in order to predict a prognosis, RMS-MRD detection by FCM with
GD2-FITC/CD90-PE/CD45-PerCP/CD56-APC was established. The
sensitivity of the FCM assays was evaluated using spiking
experiments. The results showed that when 5×104 cells
were evaluated, one RMS cell in every 104 normal
mononuclear cells (0.01%) was detected (Fig. 3).
In four cases with positive BM at diagnosis, further
BM aspirates were obtained during treatment to detect MRD by FCM at
the interval of two courses of chemotherapy in order to evaluate
the efficacy of the therapy. Follow-up with FCM assays demonstrated
notable results (Fig. 4). In total,
two patients [patient (P)2 and P4] became MRD-negative in BM, as
determined by FCM following two and four cycles of chemotherapy,
and remained alive without evidence of disease with follow-up
periods of 38 and 49 months, respectively. The other two cases (P1
and P3) remained RMS MRD-positive despite receiving four courses of
chemotherapy with 8 and 12 months of follow-up, respectively. Among
them, one case (P1) ultimately experienced disease recurrence and
succumbed to uncontrolled disease progression eight months after
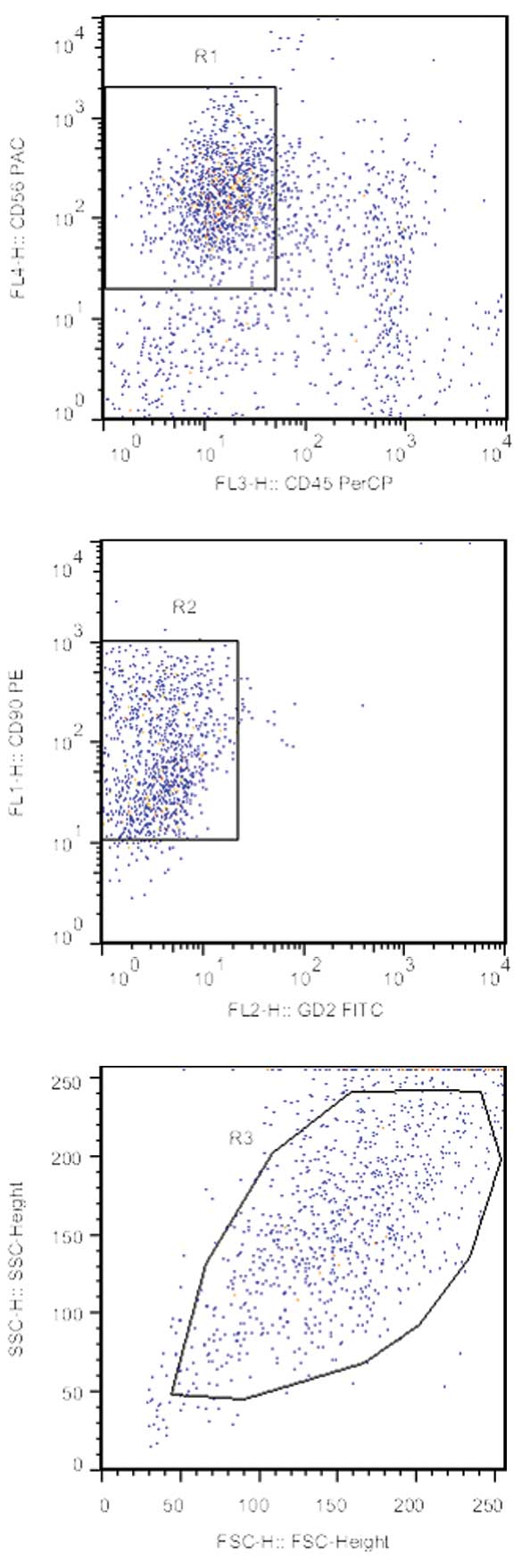
chemotherapy. The other case (P3) exhibited CSF metastasis of RMS
following 10 courses of chemotherapy, and continuous neoplastic
cells with the
CD56+/CD90+/CD45−/GD2−
phenotype remaining were observed in CSFs by FCM during treatment
(Fig. 5). The patient became
unconscious and subsequently succumbed to progressive disease
following two cycles of intrathecal chemotherapy.
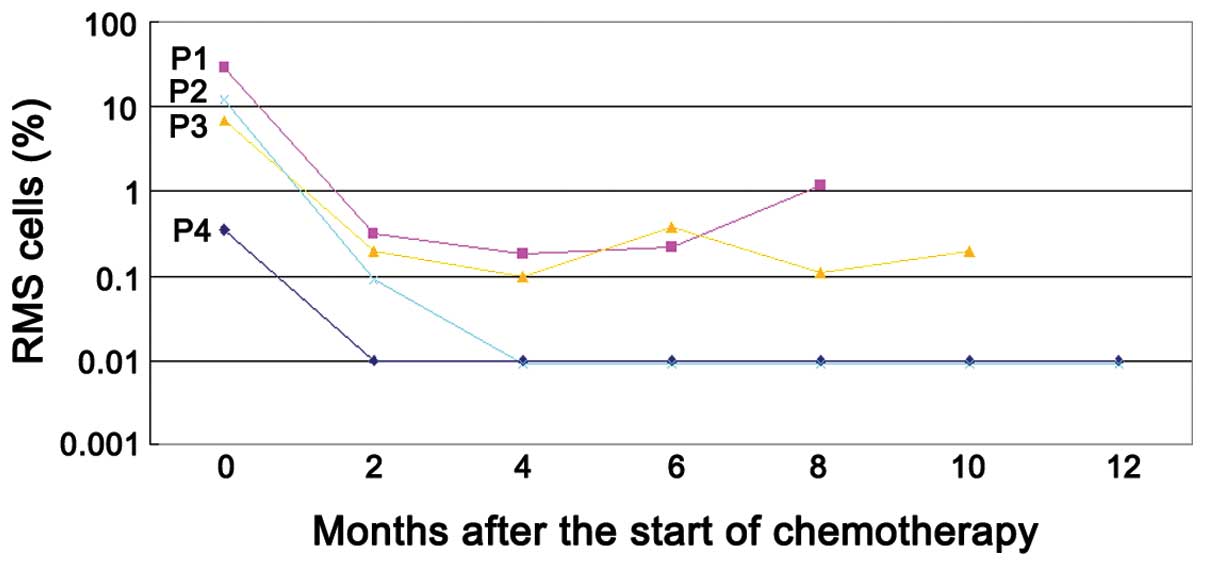 | Figure 4Detection of MRD by flow cytometry
(FCM) using a GD2-FITC, CD90-PE, CD45-PerCP and CD56-APC monoclonal
antibody combination in four patients with RMS. The cells with the
phenotype of >0.01%
CD56+/CD90+/CD45−/GD2−
expression in the bone marrow (BM) sample were considered positive.
Two patients (P2 and P4) became MRD-negative following two and four
cycles of chemotherapy and remained alive. The other two cases (P1
and P3) maintained their positivity despite receiving four courses
of chemotherapy and succumbed to progressive disease at 8 and 12
months, resepctively, following the initiation of chemotherapy.
RMS, rhabdomyosarcoma; MRD, minimal residual disease; P, patient;
FITC, fluorescein isothiocyanate; PE, phycoerythrin; PerCP,
peridinin chlorophyll protein; APC, allophycocyanin; CD, cluster of
differentiation. |
Five samples (14.7%) were positive for RMS onup
morphological examination. By FCM, 16 samples (47.1%) were positive
for RMS. A significant difference was identified between the two
methods (χ2=9.09; P<0.05). The specificity of FCM for
diagnosing RMS in BM and CSF samples was 100%.
Discussion
A proportion of children with RMS present with
disseminated disease, including BM involvement. In the present
study, 14.8% (4/27) of patients with RMS exhibited high-risk stage
IV diseases with BM involvement, similar to the results (15%)
previously reported by Dasgupta and Rodeberg (1). The detection of contaminating RMS
cells in the BM is important in clinical staging and risk
assessment. Cytological examination of the BM remains the gold
standard for RMS diagnosis, but has a limited sensitivity.
Furthermore, several studies have previously shown that RMS
presents with extensive BM involvement and mimics acute leukemia in
cytology (15,16). For several decades, FCM
immunophenotyping has been confirmed to be essential for the rapid
diagnosis, classification and monitoring of therapy in the majority
of hematological malignancies, including pediatric leukemias and
lymphomas. Conversely, it is rarely used to identify metastatic
cells of solid tumors in BM. An important exception is represented
by NB stage IV patients, in whom FCM has been used to detect
disease in BM during diagnostic or staging procedures (17–19).
However, few previous studies have performed an FCM analysis of
RMS, and the efficacy of FCM in monitoring therapeutic effects and
progress by detecting residual disease following chemotherapy has
not been evaluated.
The present study developed a four-color FCM assay
using GD2, CD90, CD45 and CD56 to detect RMS cells in BM. In total,
three RMS IRSG IV BM samples that had been determined to be
infiltrated by neoplastic cells using standard morphology,
exhibited the
CD56+/CD90+/CD45−/GD2−
phenotype. Cases were encountered where the aspirate samples were
positive by FCM while negative by morphology analysis. FCM
demonstrated the presence of an aberrant population of cells with
the
CD56+/CD90+/CD45−/GD2+
phenotype, which accounted for 0.35% of the cells of the case. This
case, previously stage II, was modified to stage IV RMS by a
clinician and was administered stage IV treatment regimens and
obtained a good clinical outcome. This discrepancy between the
cytological and FCM results may be explained by the difference in
the sensitivity of the methods used. In the present study, the FCM
assay had a sensitivity of 0.01% based on spiking experiments. In
general, the detection of malignant cells using a conventional
cytology method requires that a minimum of 5% of the neoplastic
cells in a sample are identified. The results of the current study
indicated that an FCM assay in RMS may identify a group of patients
who are at a high risk despite their cytomorphologically-negative
BM.
Follow-up examinations using FCM assays demonstrated
that two out of the four cytomorphically-negative cases remained
FCM-positive following four courses of chemotherapy. The patients
clinical outcomes were poor while the clinical outcomes for the
other two FCM-negative cases were good. Detection of MRD using FCM
may have prognostic or therapeutic implications in advanced RMS.
Further studies with follow-up for a larger number of cases are
required to validate the prognostic significance of MRD assessment
by FCM in children with RMS.
Notably, in the current study, FCM results showed
that the cells in the CSF from one patient demonstrated the
CD56+/CD90+/CD45−/GD2−
expression immunophenotype, indicating the presence of CSF
metastasis of RMS. This patient with a primary tumor site in the
orbit may have developed metastatic spread into the meninges. A
complete metastatic evaluation in orbital sarcoma patients
currently includes a lumbar puncture for CSF cytological analysis
(20). To date, few studies have
analyzed the application of FCM to the study of the diagnosis of
CSF metastasis in patients with RMS. Although a cytomorphological
examination of the CSF may allow an easy and quick identification
of RMS, a paucity of cells in the CSF and the presence of
degenerative changes in effusions may result in difficulty in the
specific categorization of neoplastic cells (21). Therefore, FCM analysis is extremely
useful for the diagnosis of CSF metastasis in patients with RMS,
which may also be useful during the follow-up of patients with a
high risk of metastasis.
NB is the most common type of extracranial malignant
solid tumor in children, accounting for 7–10% of all childhood
cancers. In total, ~60% of children and 80% of infants with NB are
stage IV at the time of diagnosis with BM or bone metastases
(13). Due to the relatively high
frequency of metastatic BM involvement in NB, the differential
diagnosis of RMS from NB is essential in clinical practice. NB and
RMS exhibit a similar morphology, characterized by small, round,
relatively undifferentiated cells that may often be morphologically
confused. NB and RMS exhibit a
CD45−/CD56+/CD90+ phenotype.
Previous studies have shown that GD2 is positively expressed by
neuroectodermally-derived tumors, such as NB, while it is negative
in RMS cells. GD2 may be a useful marker to differentiate between
NB and RMS. The results of the current study demonstrated that the
four-color FCM assay with the CD45/CD56/CD90/GD2 antibody cocktail
is useful in establishing a differential diagnosis between these
two malignancies. In the current study, none of the BM samples from
patients with non-neoplastic diseases were misdiagnosed as cancer
by FCM.
In conclusion, FCM may have a role not only in
staging and monitoring the effects of therapy, but also in
providing diagnostic confirmation of CSF metastasis in RMS. This
technique is simple, quick and cost effective and may be translated
to routine practice.
Acknowledgements
The authors would like to thank Ning Zhao and Baiqin
Qian at the Hematology-Oncology Laboratory in the Children’s
Hospital of Zhejiang University School of Medicine (Hangzhou,
China) for their excellent technical support. The present study was
supported in part by grants from the National Science and
Technology Support Program (no. 2013BAI01B03) and the Fund of
Zhejiang Province Innovation Team for Early Screening and
Intervention of Birth Defects (nos. 2010R50045 and
JSW2012-A010).
References
|
1
|
Dasgupta R and Rodeberg DA: Update on
rhabdomyosarcoma. Semin Pediatr Surg. 21:68–78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
McDowell HP, Foot AB, Ellershaw C, Machin
D, Giraud C and Bergeron C: Outcomes in paediatric metastatic
rhabdomyosarcoma: results of The International Society of
Paediatric Oncology (SIOP) study MMT-98. Eur J Cancer.
46:1588–1595. 2010. View Article : Google Scholar
|
|
3
|
Ohta H, Hashii Y, Yoshida H, et al:
Allogeneic hematopoietic stem cell transplantation against
recurrent rhabdomyosarcoma. J Pediatr Hematol Oncol. 33:e35–e38.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Palma J, Sasso DF, Dufort G, et al:
Successful treatment of metastatic retinoblastoma with high-dose
chemotherapy and autologous stem cell rescue in South America. Bone
Marrow Transplant. 47:522–527. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Sartori F, Alaggio R, Zanazzo G, et al:
Results of a prospective minimal disseminated disease study in
human rhabdomyosarcoma using three different molecular markers.
Cancer. 106:1766–1775. 2006. View Article : Google Scholar
|
|
6
|
Stegmaier S, Poremba C, Schaefer KL, et
al: Prognostic value of PAX-FKHR fusion status in alveolar
rhabdomyosarcoma: a report from the cooperative soft tissue sarcoma
study group (CWS). Pediatr Blood Cancer. 57:406–414. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Yang XL, Zhang SC, Zhang SW and Wang H:
Detection of PAX3/PAX7-FKHR fusion transcripts in rhabdomyosarcoma
and other small round cell tumors by 1-step reverse transcriptase
polymerase chain reaction: a novel tool for diagnosis and
differentiation. Ann Diagn Pathol. 16:107–111. 2012. View Article : Google Scholar
|
|
8
|
Gautam U, Srinivasan R, Rajwanshi A,
Bansal D and Marwaha RK: Comparative evaluation of flow-cytometric
immunophenotyping and immunocytochemistry in the categorization of
malignant small round cell tumors in fine-needle aspiration
cytologic specimens. Cancer. 114:494–503. 2008. View Article : Google Scholar
|
|
9
|
Bozzi F, Collini P, Aiello A, et al: Flow
cytometric phenotype of rhabdomyosarcoma bone marrow metastatic
cells and its implication in differential diagnosis with
neuroblastoma. Anticancer Res. 28:1565–1569. 2008.PubMed/NCBI
|
|
10
|
Ferreira-Facio CS, Milito C, Botafogo V,
et al: Contribution of multiparameter flow cytometry
immunophenotyping to the diagnostic screening and classification of
pediatric cancer. PLoS One. 8:e555342013. View Article : Google Scholar
|
|
11
|
Sethuraman C, Simmerson M, Vora AJ and
Cohen MC: Flowcytometric immunophenotyping in the diagnosis of
pediatric lymphoma: how reliable is it and how can we optimize its
use? J Pediatr Hematol Oncol. 32:298–303. 2010. View Article : Google Scholar
|
|
12
|
Swerts K, De Moerloose B, Dhooge C, et al:
Detection of residual neuroblastoma cells in bone marrow:
comparison of flow cytometry with immunocytochemistry. Cytometry B
Clin Cytom. 61:9–19. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Matthay KK, George RE and Yu AL: Promising
therapeutic targets in neuroblastoma. Clin Cancer Res.
18:2740–2753. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Shen H, Tang Y, Xu X and Tang H: Detection
of the GD2+/1CD56+/CD45− immunophenotype by flow cytometry in
cerebrospinal fluids from a patient with retinoblastoma. Pediatr
Hematol Oncol. 30:30–32. 2013.
|
|
15
|
Stall JN and Bailey NG: Metastatic
alveolar rhabdomyosarcoma to the bone marrow mimicking acute
leukemia. Blood. 120:36322012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Jelić-Puskarić B, Rajković-Molek K, Raić
L, Batinić D, Konja J and Kardum-Skelin I: Rhabdomyosarcoma with
bone marrow infiltration mimicking hematologic neoplasia. Coll
Antropol. 34:635–639. 2010.PubMed/NCBI
|
|
17
|
Cai JY, Tang YJ, Jiang LM, Pan C, Chen J
and Tang JY: Prognostic influence of minimal residual disease
detected by flow cytometry and peripheral blood stem cell
transplantation by CD34+ selection in childhood advanced
neuroblastoma. Pediatr Blood Cancer. 49:952–957. 2007.PubMed/NCBI
|
|
18
|
Esser R, Glienke W, Bochennek K, et al:
Detection of neuroblastoma cells during clinical follow up:
advanced flow cytometry and rt-PCR for tyrosine hydroxylase using
both conventional and real-time PCR. Klin Padiatr. 223:326–331.
2011. View Article : Google Scholar
|
|
19
|
Cai JY, Pan C, Tang YJ, et al: Minimal
residual disease is a prognostic marker for neuroblastoma with bone
marrow infiltration. Am J Clin Oncol. 35:275–278. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Raney B, Huh W, Hawkins D, et al: Outcome
of patients with localized orbital sarcoma who relapsed following
treatment on Intergroup Rhabdomyosarcoma Study Group (IRSG)
Protocols-III and -IV, 1984–1997: a report from the Children’s
Oncology Group. Pediatr Blood Cancer. 60:371–376. 2013.PubMed/NCBI
|
|
21
|
Ahluwalia MS, Wallace PK and Peereboom DM:
Flow cytometry as a diagnostic tool in lymphomatous or leukemic
meningitis: ready for prime time? Cancer. 118:1747–1753. 2012.
View Article : Google Scholar : PubMed/NCBI
|