Introduction
Colorectal cancer is one of the leading causes of
mortality worldwide, however, due to the development of minimally
invasive techniques, the majority of colorectal procedures can also
be performed using a laparoscopic approach, and the indications for
laparoscopic-assisted surgery have gradually expanded (1,2). A
number of available prospectively randomized trials and
meta-analyses of laparoscopic-assisted surgery for colorectal
cancer (3–8) reported that laparoscopic-assisted
colorectal surgery exhibited improved post-operative results,
including less pain, a smaller incision, a faster recovery of
gastrointestinal function, a shorter post-operative hospital stay
and similar long-term survival, compared with those of open
colorectal surgery (9–13). Therefore, laparoscopic-assisted
surgery has been widely accepted as an alternative to conventional
open surgery for colorectal cancer (14).
Despite the theoretical advantages of
laparoscopic-assisted surgery, it is not considered the standard
surgical treatment for colorectal cancer due to criticism
concerning its oncological stability (9,15). The
potential risks include port-site recurrence following curative
resection of the tumor and incomplete lymph node dissection. The
present study aimed to compare the clinical outcomes of
laparoscopic-assisted surgery versus open surgery for colorectal
cancer and investigate the oncological safety and potential
advantages and disadvantages of laparoscopic-assisted surgery for
colorectal cancer.
Materials and methods
Patients
The medical records of a total of 160 patients who
underwent surgery for tumor node metastasis (TNM) (16,17)
stage I-IIIC colorectal cancer between January 2009 and January
2013 at The Second Hospital of Dalian Medical University (Dalian,
China) were retrospectively analyzed. The medical records consisted
of 80 cases of laparoscopic-assisted surgery (the laparoscopic
group) and 80 cases of traditional open surgery (the open surgery
group). Patients were non-randomized, enrolled and allocated to
laparoscopic or conventional open surgery groups at the patients
discretion. The inclusion criteria were as follows: All patients
were diagnosed with colorectal cancer by pre-operative colonoscopy
and biopsy analysis. All patients who were confirmed with
colorectal cancer by physical examination [lung X-rays,
pre-operative upper abdominal ultrasonography and abdominal
computed tomography (CT)] exhibited no bowel obstruction or tumor
invasion of the surrounding adjacent or distant organs. The
exclusion criteria were as follows: Patients who required emergency
surgery due to serious complications, including acute colorectal
cancer obstruction or cancer perforation, cases with a history of
pre-operative chemoradiotherapy and major abdominal surgery, cases
with a previous history of abdominal surgery and cases in which a
curative resection could not be performed. Data were collected and
reviewed retrospectively, including patient demographics,
pre-operative clinical characteristics, surgical procedures,
pathological parameters, perioperative recovery and complications.
This study was approved by the Research Ethics Committee of Dalian
Medical University, and informed consent was obtained from all
participants.
Surgical technique
All surgeries were performed by the same team of
surgeons who had proven expertise in colorectal cancer procedures
and who perform >100 laparoscopic and open colorectal surgeries
annually. All patients received cefminox (2.0 g) intravenously at
the induction of general anesthesia for systemic antibiotic
prophylaxis. Additional pre-operative preparations were
standardized, following the course of traditional abdominal
surgeries. For conventional open surgery, the patients were placed
in the supine position or modified lithotomy position, and a
midline or right paramedian skin incision was performed. Open
procedures were performed according to standard techniques, which
were applied by the operating surgeon. For laparoscopic-assisted
surgery, the patient was placed in the modified lithotomy (supine)
and trendelenburg positions. A pneumoperitoneum was created by the
open method, and the CO2 pneumoperitoneum pressure was
set at 12–15 mmHg. In this study, five ports were used: An
umbilical port for the laparoscopic camera (CV180, Olympus
Corporation, Tokyo, Japan) and two ports each in the right and left
sides. For a right hemicolectomy, the surgeon and camera operator
stood to the left side of the patient, and for a left
hemicolectomy, the surgeon and camera operator stood to the right
side of the patient. The first assistant stood on the side opposite
to that of the surgeon. The retroperitoneum and right colon
mesocolon were divided, exposing the ventral aspect of the superior
mesenteric vein. The ileocolic vessels, right colic vessels and
midcolic vessels were identified in that order. The terminal ileum,
cecum and ascending colon were mobilized up to the hepatic flexure,
while the duodenum and right ureter were being protected. In the
left hemicolectomy, using the medial approach, the inferior
mesenteric artery was identified. An anastomosis was made by a
small laparotomy or by endoscopic intraluminal anastomosis.
Follow-up
One month after surgery and every 3 months
thereafter, a physical examination was performed and levels of
laboratory markers, such as serum carcinoembryonic antigen and
carbohydrate antigen 19.9, were assessed. At each patient visit,
symptoms were recorded and wound scars were examined. Either
ultrasonography or CT scans of the abdomen, in addition to chest
X-rays, were performed every 6 months, and a total colonoscopy was
performed every year. All patients were followed-up subsequent to
being discharged from the hospital. Survival was calculated in
months from the date of diagnosis to the date of mortality or to
the date of the last visit to the outpatient clinic. For patients
who did not visit the hospital, telephone interviews were
performed. The last date for follow-up was April 2013. Data
collected included local recurrence, distant metastasis and
survival.
Statistical analysis
All calculations were performed using SPSS software,
version 17.0 (SPSS, Inc., Chicago, IL, USA). Parametric variables
are expressed as the mean ± standard deviation (SD). Categorical
data are presented as the frequencies and percentage and were
compared by the χ2 test. Parametric and non-parametric
continuous data are presented as the mean ± SD and evaluated by
Student’s t-test and the Mann-Whitney U test, respectively. The
Kaplan-Meier method was used to calculate the survival data, and
differences were compared by the log-rank test. P<0.05 was
considered to indicate a statistically significant difference.
Results
Demographic and pre-operative clinical
characteristics
A total of 160 patients were enrolled and the
medical records were retrospectively analyzed in this study. Of the
surgeries performed during the study period, 80 cases were
laparoscopic-assisted colorectal resections and 80 cases were
conventional open surgeries. No statistically significant
differences were found in the majority of the demographic and
pre-operative clinical parameters between the two patient
populations (Table I).
 | Table IDemographic and pre-operative clinical
characteristics. |
Table I
Demographic and pre-operative clinical
characteristics.
| Characteristics | Laparoscopic
(n=80) | Open surgery
(n=80) | P-value |
|---|
| Gender | | | 0.265 |
| Male | 48 (60.0) | 41 (51.3) | |
| Female | 32 (40.0) | 39 (48.8) | |
| Age, years | 64.35±1.18 | 65.1±1.38 | 0.569 |
| BMI,
kg/m2 | 26.7±4.0 | 27.3±4.6 | 0.342 |
| Tumor location | | | 0.205 |
| Colon | 38 (47.5) | 47 (58.8) | |
| Rectum | 42 (52.5) | 33 (41.2) | |
| ASA
classification | | | 0.443 |
| I | 45 (56.3) | 43 (53.8) | |
| II | 23 (28.8) | 19 (23.8) | |
| III | 12 (15.0) | 18 (22.5) | |
| Pre-operative
comorbid diseases | | | |
| Hypertension | 7 (8.8) | 9 (11.3) | 0.598 |
| Coronary heart
disease | 4 (5.0) | 5 (6.3) | 0.732 |
| Diabetes | 11 (13.8) | 13 (16.3) | 0.658 |
| Hepatic
cirrhosis | 0 (0.0) | 1 (1.3) | 0.316 |
| Cerebral
infarction | 1 (1.3) | 2 (2.5) | 0.560 |
| Others | 2 (2.5) | 3 (3.8) | 0.650 |
Surgical procedures and pathological
parameters
No statistically significant differences were found
in the surgical procedures between the two groups (Table II). The resection margins were
similar in the two groups and none were found to be positive for
cancer cells. There were no significant differences in the number
of lymph nodes sampled, the total sample length or the TNM staging
(Table II). A significant
difference was observed in the length of surgery between the two
groups (201.7±6.91 min for laparoscopic vs. 177.2±7.2 min for open
surgery; P=0.015; Table III).
Moreover, a significantly lower level of blood loss was found
during laparoscopic-assisted surgery compared with open surgery
(P=0.002) (Table III). Only one
patient (1.25%) was converted from laparoscopic-assisted to open
surgery.
 | Table IISurgical procedures and pathological
parameters. |
Table II
Surgical procedures and pathological
parameters.
|
Procedure/parameter | Laparoscopic
(n=80) | Open surgery
(n=80) | P-value |
|---|
| Procedures |
| Right
hemicolectomy | 18 (22.5) | 21 (26.3) | 0.416 |
| Left
hemicolectomy | 5 (6.3) | 12 (15.0) | |
| Sigmoid
colectomy | 15 (18.8) | 14 (17.5) | |
| Low anterior
resection | 35 (43.8) | 26 (32.5) | |
| Abdominoperineal
resection | 5 (6.3) | 6 (7.5) | |
| Total
colectomy | 2 (2.5) | 1 (1.3) | |
| Conversion to open
surgery | 1 (1.3) | - | |
| Tumor size, cm | 4.87±0.21 | 5.24±0.24 | 0.251 |
| Proximal margin,
cm | 11.04±2.2 | 11.12±2.7 | 0.721 |
| Distal margin,
cm | 8.15±3.62 | 8.24±3.67 | 0.543 |
| Total sample
length, cm | 24±5.76 | 25.19±5.91 | 0.522 |
| No. of lymph nodes
sampled | 11.86±1.95 | 12.24±1.17 | 0.363 |
| Positive resection
margin | 0 (0) | 0 (0) | |
| TNM stage | | | 0.715 |
| I | 16 (20.0) | 15 (18.8) | |
| IIA | 12 (15.0) | 14 (17.5) | |
| IIB | 17 (21.3) | 16 (20.0) | |
| IIC | 2 (2.5) | 3 (3.8) | |
| IIIA | 2 (2.5) | 5 (6.3) | |
| IIIB | 22 (27.5) | 20 (25.0) | |
| IIIC | 9 (11.3) | 7 (8.8) | |
 | Table IIIIntraoperative data and
post-operative outcomes. |
Table III
Intraoperative data and
post-operative outcomes.
| Data/outcome | Laparoscopic
(n=80) | Open surgery
(n=80) | P-value |
|---|
| Surgery time,
min | 201.7±6.91 | 177.2±7.2 | 0.015 |
| Blood loss, ml | 97.25±9.97 | 221.3±37.46 | 0.002 |
| Time in days
to |
| First passing
flatus | 2.34±0.12 | 3.80±0.17 | <0.001 |
| First bowel
movement | 3.43±0.28 | 4.87±0.18 | 0.009 |
| Resume liquid
food | 3.66±0.15 | 4.34±0.19 | 0.015 |
| Walk
independently | 1.63±0.11 | 2.22±0.17 | 0.006 |
| Incision length,
cm | 5.0±0.18 | 19.9±0.62 | <0.001 |
| Hospital stay,
days | 9.7±0.59 | 11.36±0.67 | 0.007 |
| Treatment
costs |
| Surgery
expenditure, thousand yuan RMB | 8.1±3.1 | 3.9±1.1 | 0.003 |
| Post-surgical
costs, thousand yuan RMB | 9.6±3.7 | 10.8±6.5 | 0.372 |
| Total
hospitalization costs, thousand yuan RMB | 48.3±10.7 | 26.9±7.5 | <0.001 |
Perioperative recovery
The patients who underwent the laparoscopic-assisted
procedure showed a significantly faster recovery time than those
who underwent open surgery, namely, less time to first passing
flatus (P<0.001), first bowel movement (P=0.009), resuming a
liquid food diet (P=0.015) and walking independently (P=0.006)
(Table III). Compared with the
patients who underwent open surgery, laparoscopic-assisted
colorectal surgery notably caused less pain for patients resulting
in a lower requirement for analgesics (P=0.001) and a shorter
hospital recovery time (10.7±0.59 days for laparoscopic-assisted
vs. 12.36±0.67 days for open surgery; P=0.007). However,
laparoscopic-assisted colorectal surgery resulted in higher surgery
expenditure (P=0.003) and total hospitalization costs (P<0.001)
compared with open surgery (Table
III). There was no statistically significant difference in
post-surgical costs between the two groups (Table III).
Complications
No significant difference was found in the number of
adverse events during surgery between the laparoscopic and open
surgery groups (Table IV). The
majority of the intraoperative and post-operative complications
were minor in the two groups and almost all were due to wound
infection.
 | Table IVIntraoperative and post-operative
complications for colorectal cancer. |
Table IV
Intraoperative and post-operative
complications for colorectal cancer.
| Complications | Laparoscopic
(n=80) | Open surgery
(n=80) | P-value |
|---|
| Intraoperative
complications |
| Massive
hemorrhage | 1 (1.3) | 2 (2.5) | 0.560 |
| >1,000 ml |
| Organ injury | 1 (1.3) | 3 (3.8) | 0.311 |
| Others | 2 (2.5) | 1 (1.3) | 0.560 |
| Post-operative
complications |
| Anastomotic
hemorrhage | 2 (2.5) | 4 (5.0) | 0.405 |
| Abdominal
hemorrhage | 3 (3.8) | 5 (6.3) | 0.468 |
| Anastomotic
stenosis | 1 (1.3) | 0 (0.0) | 0.316 |
| Ileus | 1 (1.3) | 2 (2.5) | 0.560 |
| Intestinal
adhesion | 1 (1.3) | 1 (1.3) | 1.000 |
|
Enteroparalysis | 0 (0.0) | 1 (1.3) | 0.316 |
| Wound
infection | 3 (3.8) | 10 (12.5) | 0.053 |
| Lung
infection | 2 (2.5) | 4 (5.0) | 0.405 |
| Dysuria | 0 (0.0) | 1 (1.3) | 0.316 |
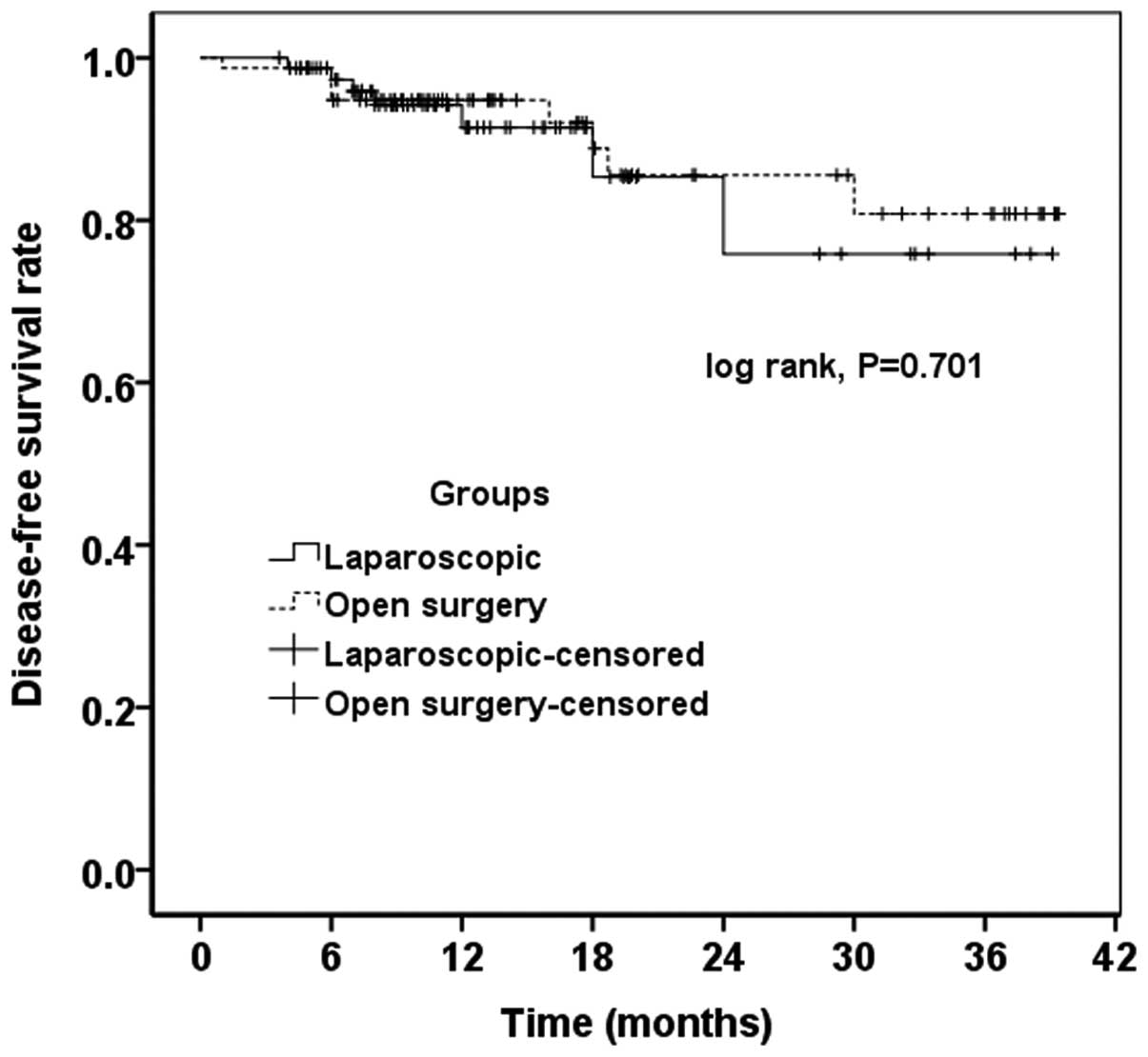
Recurrence and survival
No significant difference in the rate of recurrence
between the two groups was found (Table
V). The mean follow-up times were 17.5 and 18.2 months in the
laparoscopic and open surgery groups, respectively. According to
the results of the Kaplan-Meier analysis, the laparoscopic and open
surgery groups did not have significant differences in overall
survival (P=0.894) (Fig. 1) and
disease-free survival (P=0.701) rates (Fig. 2).
 | Table VLocal recurrence and distant
metastasis. |
Table V
Local recurrence and distant
metastasis.
|
Recurrence/metastasis | Laparoscopic
(n=80) | Open surgery
(n=80) | P-value |
|---|
| Local
recurrence |
| Anastomotic
recurrence | 2 (2.5) | 1 (1.3) | 0.560 |
| Pelvic
recurrence | 1 (1.3) | 2 (2.5) | 0.560 |
| Perineal
recurrence | 1 (1.3) | 0 (0.0) | 0.316 |
| Total | 4 (5.0) | 3 (3.8) | 0.699 |
| Distant
metastases |
| Liver
metastases | 1 (1.3) | 2 (2.5) | 0.560 |
| Lung
metastases | 1 (1.3) | 2 (2.5) | 0.560 |
| Extensive
abdominal metastasis | 1 (1.3) | 0 (0.0) | 0.316 |
| Total | 3 (3.8) | 4 (5.0) | 0.699 |
Discussion
Since Jacobs et al (18) completed the first
laparoscopic-assisted colectomy in the world, laparoscopic-assisted
surgery for colorectal cancer has been widely performed. Over the
past two decades, improvements have increasingly been made to the
laparoscopic-assisted resection of colorectal cancer. However,
laparoscopic-assisted colorectal surgery, which is the gold
standard treatment for colorectal cancer, has controversial
oncological stability. The present study compared and analyzed data
on patients with colorectal carcinoma who underwent
laparoscopic-assisted or conventional open surgery. The results
indicated that laparoscopic-assisted surgery had the clear
advantages of a minimally invasive surgery and comparable rates of
recurrence and survival compared with that of conventional open
surgery.
A number of previous studies (9,11,19–21)
reported that patients who underwent laparoscopic-assisted
colorectal cancer surgery possessed several advantages, including
less bleeding, less trauma, a faster recovery of bowel function and
a shorter hospital stay. In the present study, significant
improvements in post-operative recovery among laparoscopic-treated
patients were observed, with shorter times to first passing flatus
and ambulation, earlier resumption of a liquid food diet and a
shorter post-operative hospital stay. These results were consistent
with a number of domestic and foreign studies (22,23).
Thus, the advantages of minimally invasive surgery were
confirmed.
The post-operative hospital stay for the patients
who underwent the laparoscopic procedure ranged between 5 and 8
days in certain randomized controlled trials (12,24–25),
which was a shorter time than the 10.7 days reported in the present
study. Several confounding factors could have affected the
comparison of the hospital stay between the two groups, as well as
between studies. For example, certain variables, such as the
pre-operative health status of the patients and chemotherapy may
have extended the length of hospital stay for all patients. As
pre-operative comorbidities may affect post-operative recovery, and
patients could not be discharged until the end of the first regimen
of post-operative chemotherapy, such covariates were examined to
assess any substantial differences between the two groups.
The mean operating time of the laparoscopic
procedure versus open surgery varied among studies, with certain
studies reporting no differences between the two groups (11,26),
and others reporting a significantly longer time for the
laparoscopic procedure. This may be due to the higher complexity of
technical expertise involved in such techniques (27). In the present study, a longer
operating time was observed for the laparoscopic procedure compared
with open surgery, and this difference was significant. Therefore,
with the stabilization of the learning curve of the surgeon, the
operating time may be significantly reduced in the future.
Higher treatment costs were a relative disadvantage
in the laparoscopic group of the present study. Laparoscopic
colorectal surgery caused higher surgery expenditure (P=0.003) and
total hospitalization costs (P<0.001) compared with open
surgery. Kapritsou et al (28) found that the surgery costs in the
laparoscopic group were significantly higher than those in the open
surgery group. In addition, Steele et al (29) reported that the total
hospitalization costs in the laparoscopic group were significantly
higher than those in the open surgery group. We hypothesize that
the reason for the higher surgery expenditure and total
hospitalization costs in laparoscopic-assisted surgery is that
disposable endoscopic supplies and laparoscopic instruments are
more expensive overall.
The conversion rate of the present study was 1.3%,
which was notably lower than that reported in other studies, which
ranged between 15 and 30% (12,25,30–32).
The variation among studies may be due to the evolution of
operating skills over time, thus reducing the conversion rates in
the more recent studies. In addition, as the learning curve of the
technique was incorporated during the study period and the skills
were evolved during the conduct of the study, it is not unexpected
that the number of conversions was lower in the latter phase of the
present study.
The present study assessed the oncological safety by
examining the post-operative results, such as the resection margin
and the number of resected lymph nodes. The results indicated that
the laparoscopic-assisted procedural outcomes were comparable to
those achieved by open surgery. None of the resection margins were
found to be positive for cancer cells, as reported in the majority
of previous studies with data on resection margins (25,26,33–36).
The mean number of resected lymph nodes was 11.86±1.95 and
12.24±1.17 in the patients who underwent laparoscopic-assisted and
open surgery, respectively, thus confirming that there were no
differences in the number of lymph nodes harvested between the two
groups. These findings indicated that the oncological safety of the
laparoscopic-assisted surgery in the present study was comparable
to previous results (37,38).
The long-term outcomes of laparoscopic-assisted
surgery for colorectal cancer from three major multicenter trials
have not yet been determined (12,30,39).
In the present study, the follow-up outcomes, including rates of
local recurrence, distant metastasis, overall survival and
disease-free survival, were assessed over 1 year, and the median
follow-up time was ~17.9 months for each group. With regard to the
recurrence rate, patients who underwent laparoscopic-assisted
surgery displayed rates comparable to those who underwent open
abdominal surgery. The study revealed that the recurrence rate for
patients with colorectal cancer was lower than the prospective
trials, with ~3–7% and 17–19% local and distant recurrence rates,
respectively (7,13,24,38).
This may be associated with the small sample size and short
follow-up time. Furthermore, the follow-up time for all is ≤3
years, so the laparoscopic equipment used was relatively advanced,
therefore the surgery was relatively easy to perform. However, the
number of patients with recurrent colorectal cancer was similar in
the laparoscopic-assisted and open surgery groups of these studies,
and these results were comparable to the present study. Similar
overall and disease-free survival rates in the two groups confirmed
the long-term oncological safety of the laparoscopic approach
compared with open surgery. The long-term follow-up results
conducted in prospective studies were reviewed and the 3-year
survival rates were ~85% in almost all studies (13,24),
whereas in other previous studies they were significantly lower
(<70%) (26). With regard to the
5-year survival rate, a certain degree of controversy has been
found among different studies (data ranging between 65.3 and 77%)
(13,14). The present results were consistent
with those findings in which laparoscopic-assisted surgery appeared
to be equivalent to the open method.
The present study was limited in that the patients
were partially non-randomized into the two treatment arms. However,
as there were no differences in demographic data, we suggest that
this bias had a negligible affect on the results. In addition, the
mean follow-up time was short, which may cause deletions of the
long-term follow-up results; thus, we cannot provide a more
reliable basis with regard to the long-term outcomes.
In conclusion, the present results indicated that
laparoscopic-assisted surgery for colorectal cancer is a safe and
feasible approach. Laparoscopic-assisted colorectal cancer surgery
possessed the clear advantages of a minimally invasive surgery;
however, it also had certain disadvantages, including a longer
surgery time and higher surgery expenditure and hospitalization
costs. Laparoscopic-assisted colorectal cancer surgery had similar
rates of recurrence and survival compared with open surgery.
Acknowledgements
This study was supported by grants from the Chinese
National Natural Science Foundation Projects (grant nos. 81372669
and 31270867), the Chinese State Key Program in Basic Research
(grant no. 2012CB822103), the Science and Technology Planning
Project of Liaoning (grant no. 2012225020) and the Project of the
Chinese Ministry of Health (grant no. W2012RQ23).
References
|
1
|
Ku G, Tan IB, Yau T, Boku N, Laohavinij S,
Cheng AL, Kang YK and de Lima Lopes G Jr: Management of colon
cancer: resource-stratified guidelines from the Asian Oncology
Summit 2012. Lancet Oncol. 13:e470–e481. 2012.PubMed/NCBI
|
|
2
|
Miyajima N, Fukunaga M, Hasegawa H, Tanaka
J, Okuda J and Watanabe M; Japan Society of Laparoscopic Colorectal
Surgery. Results of a multicenter study of 1,057 cases of rectal
cancer treated by laparoscopic surgery. Surg Endosc. 23:113–118.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Abraham NS, Young JM and Solomon MJ:
Meta-analysis of short-term outcomes after laparoscopic resection
for colorectal cancer. Br J Surg. 91:1111–1124. 2004. View Article : Google Scholar
|
|
4
|
Aziz O, Constantinides V, Tekkis PP,
Athanasiou T, Purkayastha S, Paraskeva P, Darzi AW and Heriot AG:
Laparoscopic versus open surgery for rectal cancer: a
meta-analysis. Ann Surg Oncol. 13:413–424. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Neudecker J, Klein F, Bittner R, Carus T,
Stroux A and Schwenk W; LAPKON II Trialists. Short-term outcomes
from a prospective randomized trial comparing laparoscopic and open
surgery for colorectal cancer. Br J Surg. 96:1458–1467. 2009.
View Article : Google Scholar
|
|
6
|
Levy BF, Tilney HS, Dowson HM and Rockall
TA: A systematic review of postoperative analgesia following
laparoscopic colorectal surgery. Colorectal Dis. 12:5–15. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Colon Cancer Laparoscopic or Open
Resection Study Group. Buunen M, Veldkamp R, Hop WC, Kuhry E,
Jeekel J, Haglind E, Påhlman L, Cuesta MA, Msika S, Morino M, et
al: Survival after laparoscopic surgery versus open surgery for
colon cancer: long-term outcome of a randomised clinical trial.
Lancet Oncol. 10:44–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Braga M, Frasson M, Zuliani W, Vignali A,
Pecorelli N and Di Carlo V: Randomized clinical trial of
laparoscopic versus open left colonic resection. Br J Surg.
97:1180–1186. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Biondi A, Grosso G, Mistretta A,
Marventano S, Toscano C, Gruttadauria S and Basile F:
Laparoscopic-assisted versus open surgery for colorectal cancer:
short- and long-term outcomes comparison. J Laparoendosc Adv Surg
Tech A. 23:1–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Z, Ying X, Shen Y, Ye P, Pan W and Chen
H: Laparoscopic versus open surgery for rectal cancer: a clinical
comparative study. J Int Med Res. 40:1599–1607. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Sun J, Jiang T, Qiu Z, Cen G, Cao J, Huang
K, Pu Y, Liang H, Huang R and Chen S: Short-term and medium-term
clinical outcomes of laparoscopic-assisted and open surgery for
colorectal cancer: A single center retrospective case-control
study. BMC Gastroenterol. 11:852011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Clinical Outcomes of Surgical Therapy
Study Group. A comparison of laparoscopically assisted and open
colectomy for colon cancer. N Engl J Med. 350:2050–2059. 2004.
View Article : Google Scholar
|
|
13
|
Biondi A, Tropea A, Monaco G, Musumeci N,
Benfatto G and Basile F: Management of early rectal cancer: Our
clinical experience. G Chir. 32:34–36. 2011.(In Italian).
|
|
14
|
Lee JE, Joh YG, Yoo SH, Jeong GY and Kim
SH, Chung CS, Lee DG and Kim SH: Long-term outcomes of laparoscopic
surgery for colorectal cancer. J Korean Soc Coloproctol. 27:64–70.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tan KY and Konishi F: Long-term results of
laparoscopic colorectal cancer resection: current knowledge and
what remains unclear. Surg Today. 40:97–101. 2010. View Article : Google Scholar
|
|
16
|
Edge SB, Byrd DR, Compton CC, Fritz AG,
Greene FL and Trotti A: AJCC Cancer Staging Manual. 7th edition.
Springer; New York, NY: 2010
|
|
17
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM classification of malignant tumors. 7th edition. John Wiley
& Sons; Hoboken, NJ: 2009
|
|
18
|
Jacobs M, Verdeja JC and Goldstein HS:
Minimally invasive colon resection (laparoscopic colectomy). Surg
Laparosc Endosc. 1:144–150. 1991.PubMed/NCBI
|
|
19
|
Martel G, Crawford A, Barkun JS, Boushey
RP, Ramsay CR and Fergusson DA: Expert opinion on laparoscopic
surgery for colorectal cancer parallels evidence from a cumulative
meta-analysis of randomized controlled trials. PLoS One.
7:e352922012. View Article : Google Scholar
|
|
20
|
Chand M, Bhoday J, Brown G, Moran B and
Parvaiz A: Laparoscopic surgery for rectal cancer. J R Soc Med.
105:429–435. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Jayne DG, Thorpe HC, Copeland J, Quirke P,
Brown JM and Guillou PJ: Five-year follow-up of the Medical
Research Council CLASICC trial of laparoscopically assisted versus
open surgery for colorectal cancer. Br J Surg. 97:1638–1645.
2010.
|
|
22
|
Gong J, Shi DB, Li XX, Cai SJ, Guan ZQ and
Xu Y: Short-term outcomes of laparoscopic total mesorectal excision
compared to open surgery. World J Gastroenterol. 18:7308–7313.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McKay GD, Morgan MJ, Wong SK, Gatenby AH,
Fulham SB, Ahmed KW, Toh JW, Hanna M and Hitos K; South Western
Sydney Colorectal Tumor Group. Improved short-term outcomes of
laparoscopic versus open resection for colon and rectal cancer in
an area health service: a multicenter study. Dis Colon Rectum.
55:42–50. 2012. View Article : Google Scholar
|
|
24
|
Fleshman J, Sargent DJ, Green E, Anvari M,
Stryker SJ, Beart RW Jr, Hellinger M, Flanagan R Jr, Peters W and
Nelson H: Laparoscopic colectomy for cancer is not inferior to open
surgery based on 5-year data from the COST Study Group trial. Ann
Surg. 246:655–662. 2007.PubMed/NCBI
|
|
25
|
Guillou PJ, Quirke P, Thorpe H, Walker J,
Jayne DG, Smith AM, Heath RM and Brown JM: Short-term endpoints of
conventional versus laparoscopic-assisted surgery in patients with
colorectal cancer (MRC CLASICC trial): multicentre, randomised
controlled trial. Lancet. 365:1718–1726. 2005. View Article : Google Scholar
|
|
26
|
Kim HJ, Lee IK, Lee YS, Kang WK, Park JK,
Oh ST, Kim JG and Kim YH: A comparative study on the short-term
clinicopathologic outcomes of laparoscopic surgery versus
conventional open surgery for transverse colon cancer. Surg Endosc.
23:1812–1817. 2009. View Article : Google Scholar
|
|
27
|
Li JC, Leung KL, Ng SS, Liu SY, Lee JF and
Hon SS: Laparoscopic-assisted versus open resection of right-sided
colonic cancer - A prospective randomized controlled trial. Int J
Colorectal Dis. 27:95–102. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kapritsou M, Korkolis DP and Konstantinou
EA: Open or laparoscopic surgery for colorectal cancer: a
retrospective comparative study. Gastroenterol Nurs. 36:37–41.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Steele SR, Brown TA, Rush RM and Martin
MJ: Laparoscopic vs open colectomy for colon cancer: results from a
large nationwide population-based analysis. J Gastrointest Surg.
12:583–591. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Veldkamp R, Kuhry E, Hop WC, Jeekel J,
Kazemier G, Bonjer HJ, Haglind E, Pahlman L, Cuesta MA, Msika S, et
al; COlon cancer Laparoscopic or Open Resection Study Group
(COLOR). Laparoscopic surgery versus open surgery for colon cancer:
Short-term outcomes of a randomised trial. Lancet Oncol. 6:477–484.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
COLOR Study Group. A randomized clinical
trial comparing laparoscopic and open resection for colon cancer.
Dig Surg. 17:617–622. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Leung KL, Kwok SP, Lam SC, Lee JF, Yiu RY,
Ng SS, Lai PB and Lau WY: Laparoscopic resection of rectosigmoid
carcinoma: Prospective randomised trial. Lancet. 363:1187–1192.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhou ZG, Hu M, Li Y, Lei WZ, Yu YY, Cheng
Z, Li L, Shu Y and Wang TC: Laparoscopic versus open total
mesorectal excision with anal sphincter preservation for low rectal
cancer. Surg Endosc. 18:1211–1215. 2004. View Article : Google Scholar
|
|
34
|
Biondi A, Tropea A and Basile F: Clinical
rescue evaluation in laparoscopic surgery for hepatic metastases by
colorectal cancer. Surg Laparosc Endosc Percutan Tech. 20:69–72.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Ragusa M, Statello L, Maugeri M, Majorana
A, Barbagallo D, Salito L, Sammito M, Santonocito M, Angelica R,
Cavallaro A, et al: Specific alterations of the microRNA
transcriptome and global network structure in colorectal cancer
after treatment with MAPK/ERK inhibitors. J Mol Med (Berl).
90:1421–1438. 2012. View Article : Google Scholar
|
|
36
|
Goldstein NS: Lymph node recoveries from
2427 pT3 colorectal resection specimens spanning 45 years:
Recommendations for a minimum number of recovered lymph nodes based
on predictive probabilities. Am J Surg Pathol. 26:179–189.
2002.
|
|
37
|
Cawthorn SJ, Parums DV, Gibbs NM, A’Hern
RP, Caffarey SM, Broughton CI and Marks CG: Extent of mesorectal
spread and involvement of lateral resection margin as prognostic
factors after surgery for rectal cancer. Lancet. 335:1055–1059.
1990. View Article : Google Scholar
|
|
38
|
Park IK, Kim YH, Joh YG and Hahn KY:
Recurrence pattern after laparoscopic resection for colorectal
cancer: Analysis according to timing of recurrence and location of
primary tumor. J Korean Soc Coloproctol. 23:110–115. 2007.
View Article : Google Scholar
|
|
39
|
Jayne DG, Guillou PJ, Thorpe H, Quirke P,
Copeland J, Smith AM, et al: Randomized trial of
laparoscopic-assisted resection of colorectal carcinoma: 3-year
results of the UK MRC CLASICC Trial Group. J Clin Oncol.
25:3061–3068. 2007.PubMed/NCBI
|