Introduction
Malignant obstructive jaundice is prevalent in
periampullary carcinoma, cholangiocarcinoma, gallbladder carcinoma,
and in metastatic lymph nodes of the hepatic hilum and
hepatoduodenal ligament. In the majority of patients, malignant
obstructive jaundice is incurable with a poor prognosis (1–3). As a
result, biliary drainage is considered an important palliative
treatment which relieves high serum bilirubin-related symptoms and
provides patients with the opportunity to receive additional
therapies, including surgery, chemotherapy and local treatment
(4,5).
Percutaneous transhepatic biliary drainage (PTBD)
and metallic stent insertion are established methods used for the
relief of malignant biliary obstruction. Although the effectiveness
of PTBD has been reported and discussed in a number of previous
studies, predictors able to differentiate between a good and poor
prognosis have not been established (6–8).
Furthermore, the role of biliary drainage prior to subsequent
treatment remains controversial. Notably, the possible benefits of
preoperative biliary drainage (PBD) for cholangiocarcinoma and
pancreatic cancer are debated (9,10).
However, the potential beneficial effects of chemotherapy on
patient survival following PTBD have been shown in several studies
(11–13). Therefore, it is necessary to
elucidate which features patients possess in order to ensure the
maximum possible benefits for survival.
In the present study, 102 consecutive patients who
had undergone PTBD and stenting were reviewed, with the aim to
evaluate the technical and clinical success and complications of
the procedure, and to analyze patient survival to identify
potential prognostic factors.
Materials and methods
Patients
Between December 2009 and February 2011, 102
patients suffering from malignant obstructive jaundice received
PTBD in the Department of Radiology (Shanghai Cancer Center,
Shanghai, China). The clinical data of all patients were
retrospectively studied. Patients or their families provided
written informed consent.
Data collection
Following approval by the internal review board,
electronic clinical records of all patients were reviewed. Age,
gender, primary cause of obstruction, obstruction level, Bismuth
type, serum bilirubin levels prior to and following drainage,
complications associated with intervention, additional treatments
and survival, were analyzed. Technical and clinical success were
recorded, in addition to any complications. Successful placement of
the catheter into the correct position and bilirubin drainage, was
taken to indicate technical success. Clinical success was defined
by decreases in serum bilirubin levels of >20% within 7 days
after drainage, compared with the bilrubin level prior to the
procedure (14). Complications were
divided into major and minor spectra according to the report
standards and quality improvement guidelines for percutaneous
transhepatic cholangiography, biliary drainage and percutaneous
cholecystostomy from the Society of Interventional Radiology
(15,16). Major complications included sepsis
or cholangitis, hemorrhage requiring blood transfusion, abscess
formation, peritonitis, cholecystitis, pancreatitis, pneumothorax,
pneumonia, pleural infection and mortality. Minor complications
included self-limiting hemorrhage, bilovenous fistulae and
subcapsular biloma. Subsequent treatments included palliative
surgery, systemic chemotherapy and transarterial chemoinfusion and
embolization. Palliative surgery included Whipple-based regional
pancreatectomy. The regimens applied in systemic chemotherapy were
gemcitabine for pancreatic carcinoma and cholangiocarcinoma, and
epirubicin plus cisplatin plus fluorouracil for gastric cancer. The
chemoagents employed for transarterial chemoinfusion for
hepatocellular carcinoma were doxorubicin and cisplatin.
Following intervention, the serum bilirubin levels
of all patients were followed up on day 15 or later. For patients
having received additional treatments, clinical records were
tracked and telephone interviews were employed to check patient
survival and general performance. Survival was calculated by the
number of days since PTBD until mortality or until the study time
limit of October 2012.
Drainage procedure
PTBD and stenting were performed by at least two
experienced interventional radiologists. Guided by sonography and
X-ray fluorescence, an 8-French external pigtail nephrostomy
catheter was placed proximal to the occlusion site to reduce damage
to the liver parenchyma and tract system. Following a period of
drainage, cholangiography was performed to investigate whether
biliary wall edema was compromised by stent insertion. For patients
with a life expectancy of <6 months, metallic stents were used
following informed patient consent. MTN-DA self-expandable metallic
stents [Micro-Tech (Nanjing) Co., Ltd, Nanjing, China] were used.
Following successful stent placement, an 8-French external pigtail
catheter was placed proximal to the stent for drainage and frequent
flushing. The catheter was tapped after 2 days and was removed when
proper drainage was confirmed, according to bilirubin levels and
clinical findings.
Statistical analysis
The Wilcoxon-signed rank test was used for
comparison of changes in bilirubin levels prior to and following
PTBD. A Student’s t-test was used to analyze the means between two
groups. Fisher’s exact test was employed to compare clinical
success rate stratified by clinical characters. Kaplan-Meier
survival curves calculated the cumulative survival rates.
Differences from curves were tested by the log-rank test.
Univariate analysis was used to screen for potential candidate
variables for multivariate analysis. Multivariate analysis was
undertaken using the Cox’s proportional hazard model. All
statistical analysis was performed using SPSS version 16.0 for
Windows (SPSS Inc., Chicago, IL, USA). P<0.05 was considered to
indicate a statistically significant difference.
Results
Patient characteristics
The study cohort consisted of 66 males and 36
females with a median age of 63.50 years (range, 29–84 years). The
primary causes of jaundice included pancreatic carcinoma (n=48;
47.1%), carcinoma of the papilla of Vater and duodenal cancer
(n=11; 10.8%), gallbladder tumor (n=8; 7.9%), cholangiocarcinoma
(n=6; 5.8%), hepatocellular carcinoma (n=8; 7.8%), lymph node
metastasis (n=15; 14.7%) and digestive tract invasion (n=6; 5.9%).
In total, 18 (17.7%) patients developed proximal bile duct
obstruction, which was categorized by Bismuth type as follows: Type
I, n=15; type II, n=1; type III, n=1; type IV, n=1. In total, 37
(36.3%) patients received additional treatments following PTBD. Of
these, 10 patients received surgery, 12 patients received
chemotherapy and 10 patients received local therapy. In addition,
65 patients (63.7%) received symptomatic support treatments.
Effectiveness of PTBD
Bile ducts were successfully drained in all patients
with a 100% technical success rate. In the study group, 82 patients
(80.4%) received PTBD only and metallic stents were inserted in 20
individuals (19.6%). Unfortunately, due to the limited number of
stent cases, analysis of stent patency was abandoned.
Serum bilirubin levels were recorded prior to and
following intervention. The mean baseline bilirubin level was
285.4±136.7 μmol/l (median, 267.3 μmol/l). After 7 days of
drainage, levels fell to 192.0±128.5 μmol/l (median, 161.3 μmol/l).
On day 15, mean bilirubin levels fell to 140.8±120.2 μmol/l
(median, 106.5 μmol/l). The decrease in bilirubin levels pre- and
post-procedure was statistically significant at the two time-points
(Wilcoxon signed-rank test, P<0.001).
Overall clinical success was achieved in 78 cases,
with a 76.5% success rate. In total, 20 patients (19.6%) exhibited
a mild decrease and 4 patients (3.9%) experienced increases in the
bilrubin level following PTBD. Among these, 3 patients succumbed to
proximal bile duct obstruction. A total of 2 patients succumbed to
cancer cachexia 53 and 28 days after PTBD. One patient succumbed to
liver rupture caused by catheter displacement on day 18 after PTBD,
having accidentally stretched the catheter out of place by ~5 cm
which led to subsequent bleeding along the catheter.
However, in week 2 after intervention, a further 8
subjects achieved successful drainage. The relationship between
successful drainage and clinical features was analyzed and is shown
in Table I. Age, gender,
obstruction level and serum bilirubin levels pre-intervention were
not associated with successful drainage. However, the presence of
liver metastasis was associated with a lower chance of clinical
success (70.6 vs. 79.4%; P=0.033).
 | Table IUnivariate analysis of the
relationship between successful clinical drainage and patient
characteristics. |
Table I
Univariate analysis of the
relationship between successful clinical drainage and patient
characteristics.
| Bilirubin, n (%) | |
|---|
|
| |
|---|
| Features | ≥20% decrease | Increase or minor
change | P-value |
|---|
| Age, years |
| >70 | 19 (67.9) | 9 (32.1) | 0.295 |
| ≤70 | 59 (79.7) | 15 (20.3) | |
| Gender |
| Male | 46 (69.7) | 20 (30.3) | 0.052 |
| Female | 32 (88.9) | 4 (11.1) | |
| Obstruction
level |
| Hilar | 13 (72.2) | 5 (27.8) | 0.760 |
| Non-hilar | 65 (77.4) | 19 (22.6) | |
| Liver metastases |
| Present | 24 (70.6) | 10 (29.4) | 0.033 |
| Absent | 54 (79.4) | 14 (20.6) | |
| Bilirubin prior to
PTBD, μmol/l |
| >342 | 26 (81.2) | 6 (18.8) | 0.616 |
| ≤342 | 52 (76.5) | 18 (23.5) | |
Complications
A total of 2 patients succumbed to their conditions
within 30 days of receiving PTBD. However, the patient who
succumbed to invasive primary tumor progression was not included in
data for major complications, as the cause of mortality was not
drainage procedure-related. Minor complications occurred in 2
patients (1.9%), which presented as self-limiting hemorrhages and
major complications were observed in 6 patients (5.9%). In total, 3
patients developed sepsis and presented with shivering and high
fever during the 1–3- day period following catheter insertion.
Hemoculture showed positive results in 2 cases and antibiotics
against anaerobic bacteria were injected. The remaining patient
with a negative blood culture experienced relief from the high
fever and shivering 30 min after onset. One patient suffered from
pancreatitis and received conservative therapy. The remaining 2
patients exhibited symptoms of pneumonia and pleural infection.
Conservative therapy lasted for 3–5 days and was effective for the
final 2 patients.
Thus, the overall complication rate was 7.8% (8/102
patients) and the procedure-related 30-day mortality rate was 0.9%
(1/102 patients).
Survival
By the cut-off date, 96 patients had succumbed to
various conditions, 5 patients were still alive and 1 patient was
lost to follow-up. The overall median survival time following PTBD
was 185 days (95% CI, 159–211 days). The 6-month and 1-year
survival rates were 43 and 14%, respectively.
Univariate analysis revealed three factors that
significantly affected patient survival. These were patient age
(χ2, 4.003; P=0.04), bilirubin levels following
treatment (χ2, 5.139; P=0.02) and whether the patient
had received additional treatment (χ2, 15.459; P=0.00).
Cox’s regression results indicated that the absence of additional
therapy was a risk factor for poorer survival following PTBD
(Table II).
 | Table IIMultiple Cox regression analysis of
factors independently associated with survival following PTBD. |
Table II
Multiple Cox regression analysis of
factors independently associated with survival following PTBD.
| Variables | OR | 95% CI | P-value |
|---|
| Age ≤70 years | 1.263 | 0.734–2.171 | 0.399 |
| Serum bilirubin ≤68.4
μmol/l following PTBD | 1.215 | 0.797–1.853 | 0.365 |
| Recieved successive
therapies | 2.323 | 1.465–3.685 | 0.000 |
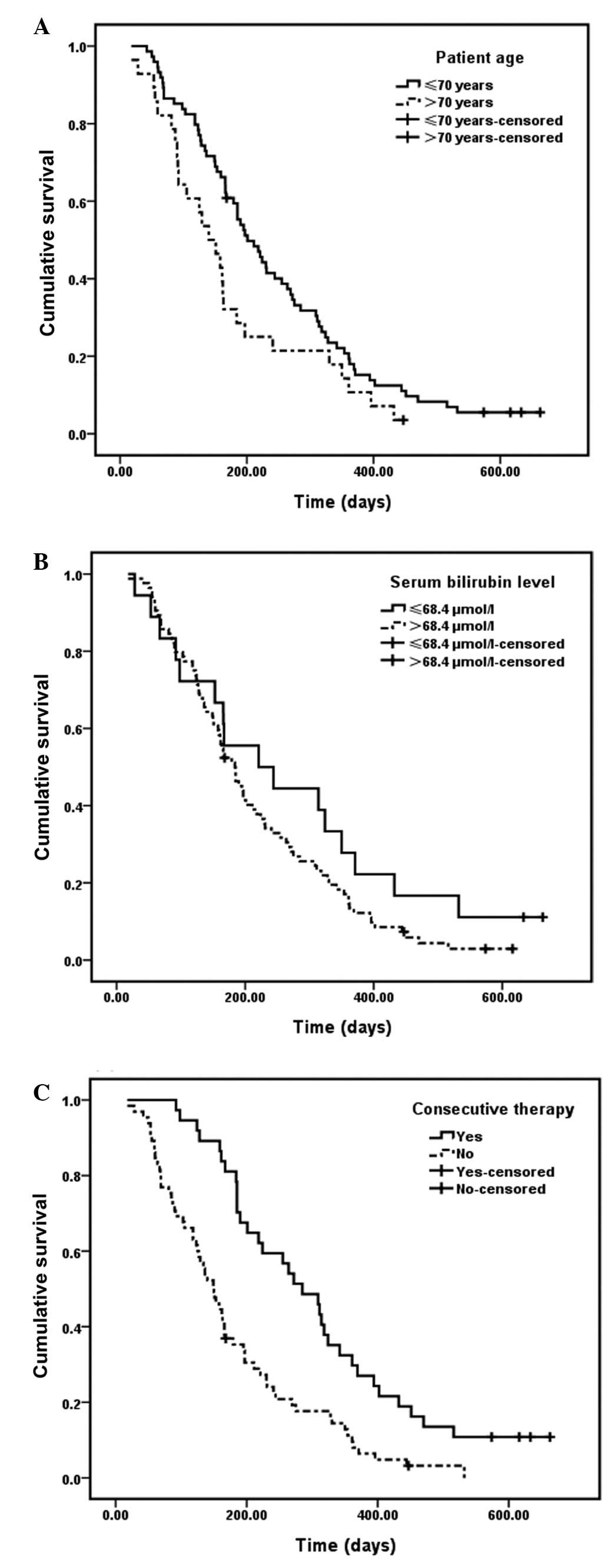
The estimated median survival periods based on
patient age of >70 or ≤70 years were 140 (95% CI, 97–183 days)
and 201 days (95% CI, 161–241 days), respectively. Kaplan-Meier
analysis showed that patients of >70 years old suffered from
significantly shorter survival times following PTBD (P=0.04)
(Fig. 1A).
Patients with post-drainage serum bilirubin levels
≤68.4 μmol/l had a median survival time of 244 days (95% CI,
166–322 days). In patients with serum bilirubin levels >68.5
μmol/l, the median survival time was 184 days (95% CI, 155–213
days). Log-rank survival analysis indicated a statistically
significant difference between the two groups (serum bilirubin,
≤68.4 vs. >68.5 μmol/l; P=0.01) (Fig. 1B).
Patients receiving additional treatments, in the
form of chemotherapy, palliative surgery and other local types,
exhibited a significantly longer survival span of 285 days (95% CI,
218–352 days) compared with 150 days (95% CI, 123–177 days) for
those without subsequent treatment (log-rank test, P=0.00)
(Fig. 1C).
However, obstruction levels (P=0.46) and the
presence of liver metastases (P=0.06) were not found to demonstrate
a statistically significant relationship with patient survival.
Discussion
Malignant biliary obstruction is often caused by
external compression from lymph node metastases or internal
stricture from neoplasms. Percutaneous biliary drainage and
stenting are established and well reported methods used to relieve
jaundice. Clinical success rates vary between 75 and 98% in various
reports (17). In the present
study, a slightly lower successful drainage rate (76.5%) was
observed. The underlying cause may be the relatively higher
base-line bilirubin levels of the present group. However, the
bilirubin levels on day 15 after drainage showed a >20% decline
in a further 8 patients, with an 84.3% clinical success rate. This
is in accordance with previous literature.
Unsatisfactory clinical success rates have been
found to be associated with liver metastases (18). Data of the current study
consistently show that the presence of liver metastases is
accompanied by a lower success rate for poor liver reserve and
advanced systemic disease. However, patient age, high bilirubin
levels prior to intervention and obstruction levels were generally
not found to be associated with clinical success rate.
The complication rate of PTBD from previous studies
ranges between 8 and 42% (19) and
the 30-day mortality rate ranges between 2 and 19.8% (17,20).
The overall complication rate (7.8%) and in-hospital mortality rate
(0.9%) of the present study compared favorably to these.
Antibiotics are not routinely applied prior to biliary procedures
in our center due to a lack of reliable evidence in favor of their
use (21). However, the incidence
of sepsis (2.9%) in the present study is similar to data from Clark
et al, in which all patients received prophylaxis (2%)
(22).
The majority of malignant biliary obstruction
patients suffered from a poor prognosis, due to advanced metastases
and/or a poor general health status. A 185-day median survival time
was observed in the present study, which appeared longer than
intervals of 79–104 days reported in previous studies (13,23,24).
By contrast, participants of the present study received
chemotherapy, surgery, transarterial chemoinfusion and
embolization, which may account for the prolonged survival rates
observed. There have been specific potential predictors discussed
in previous literature, including patient age, performance status,
tumor histology type, obstruction level, liver metastasis, serum
bilirubin level following PTBD and chemotherapy following drainage.
However, results are controversial. Unlike the results of Migita
et al (13) and Gwon et
al (24), with bilrubin levels
of 2 mg/dl, the present study the present study observed bilrubin
levels of 68.4 μmol/l (4 mg/dl). This contradiction may have arisen
due to three factors. Firstly, study subjects presented a
heterogeneous group of diseases, among which the progressiveness is
complex. Secondly, as described, the baseline levels of the present
study group are relatively high (285.4 vs. 145 and 172.7 μmol/l;
the present study vs. the results of Migita et al (13) and Gwon et al (24), respectively). Therefore, 7 days may
not be long enough for patient bilirubin levels to return to a
lower level. Log-rank analysis of serum bilirubin levels 2 weeks
after drainage revealed a significantly longer survival time [244
(median overall survival time in patients with bilrubin levels
<4 mg/dl) and 166 days (median overall survival time of patients
with bilrubin levels >4 mg/dl); 95% CI, 190–298 and 140–192
days, respectively; P=0.007) in patients with bilirubin levels
returning to <4 mg/dl. Finally, additional treatments
administered to either group of patients were comparable (35 vs.
44% for bilirubin levels >68.4 μmol/l and ≤68.4 μmol/l,
respectively; Fisher’s exact test, P=0.432). Thus, additional
therapies may prolong patient survival time, regardless of the
degree by which the post-drainage bilirubin level is reduced.
High serum bilirubin levels often provide
contraindications for surgery, chemotherapy, radiotherapy and local
methods, including transarterial chemoembolization and radio
frequency ablation for poor liver reserve. A reduction in bilirubin
levels following PTBD offers the possibility for patients to
receive radical antitumor therapies. However, patients with high
bilirubin levels should only receive supportive care. (4,25). The
importance of additional therapies on survival is highlighted in
the present study, as previously documented. Migita et al
(13) observed a prolonged survival
period in patients with metastatic gastric cancer who received
chemotherapy following PTBD, and chemotherapy was observed to be
tolerable and associated with an acceptable quality of life.
However, the necessity of PBD has been queried by numerous studies,
including a multicenter, randomized trial (26). This concluded that PBD increases
post-surgery complications in pancreatic head cancer patients.
However, the debate remains. Considering that an endoscopic method
was used for the trial, the percutanous pathway may alternatively
be analyzed. Furthermore, considering that surgery complications
were evaluated, other aspects may be analyzed, for example
mortality and survival time. Percutaneous drainage has been
recommended in a recent study for PBD (27). PBD showed no effect on the mortality
rate in jaundiced patients with hilar cholangiocarcinoma (28). In the present study, a markedly
increased survival time was observed in patients having received
surgery following biliary drainage. However, patients having
received subsequent treatment exhibited a good performance status
and relatively fewer advanced tumors. These imbalanced clinical
backgrounds may affect analysis of survival times. Therefore,
randomized control trials are essential for evaluating the
potential benefits of successive treatment on survival.
The present study undoubtedly holds certain
limitations, including the retrospective design and the
heterogeneity of primary tumors. In addition, the effect of various
treatment methods on survival rate were mixed. Thus, types which
are ‘harmful’ to survival may not be exposed. However, this issue
may be addressed in future studies. In conclusion, PTBD is a safe
and effective way to relieve jaundice caused by malignant tumors.
The utilization of subsequent radical therapies following drainage
is likely to increase patient survival.
Acknowledgements
This study was supported by grants from the National
Nature Science Foundation fund of China (no. 30670596) and the
Shanghai Science and Technology Foundation fund (no.
11nm0504000).
References
|
1
|
Berberat PO, Künzli BM, Gulbinas A, et al:
An audit of outcomes of a series of periampullary carcinomas. Eur J
Surg Oncol. 35:187–191. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Henson DE, Schwartz AM, Nsouli H and
Albores-Saavedra J: Carcinomas of the pancreas, gallbladder,
extrahepatic bile ducts, and ampulla of vater share a field for
carcinogenesis: a population-based study. Arch Pathol Lab Med.
133:67–71. 2009.
|
|
3
|
Hatzaras I, George N, Muscarella P, Melvin
WS, Ellison EC and Bloomston M: Predictors of survival in
periampullary cancers following pancreaticoduodenectomy. Ann Surg
Oncol. 17:991–997. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Thornton RH, Ulrich R, Hsu M, et al:
Outcomes of patients undergoing percutaneous biliary drainage to
reduce bilirubin for administration of chemotherapy. J Vasc Interv
Radiol. 23:89–95. 2012. View Article : Google Scholar
|
|
5
|
Iacono C, Ruzzenente A, Campagnaro T,
Bortolasi L, Valdegamberi A and Guglielmi A: Role of preoperative
biliary drainage in jaundiced patients who are candidates for
pancreatoduodenectomy or hepatic resection: highlights and
drawbacks. Ann Surg. 257:191–204. 2013. View Article : Google Scholar
|
|
6
|
Brown KT and Covey AM: Management of
malignant biliary obstruction. Tech Vasc Interv Radiol. 11:43–50.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tsuyuguchi T, Takada T, Miyazaki M, et al:
Stenting and interventional radiology for obstructive jaundice in
patients with unresectable biliary tract carcinomas. J
Hepatobiliary Pancreat Surg. 15:69–73. 2008. View Article : Google Scholar
|
|
8
|
Robson PC, Heffernan N, Gonen M, et al:
Prospective study of outcomes after percutaneous biliary drainage
for malignant biliary obstruction. Annal Surg Oncol. 17:2303–2311.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nagino M, Takada T, Miyazaki M, et al:
Preoperative biliary drainage for biliary tract and ampullary
carcinomas. J Hepatobiliary Pancreat Surg. 15:25–30. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Morris-Stiff G, Tamijmarane A, Tan YM, et
al: Pre-operative stenting is associated with a higher prevalence
of post-operative complications following pancreatoduodenectomy.
Int J Surg. 9:145–149. 2011. View Article : Google Scholar
|
|
11
|
Chu KM, Law S, Branicki FJ and Wong J:
Extrahepatic biliary obstruction by metastatic gastric carcinoma. J
Clin Gastroenterol. 27:63–66. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Makino T, Fujitani K, Tsujinaka T, et al:
Role of percutaneous transhepatic biliary drainage in patients with
obstructive jaundice caused by local recurrence of gastric cancer.
Hepatogastroenterology. 55:54–57. 2008.
|
|
13
|
Migita K, Watanabe A, Yoshioka T,
Kinoshita S and Ohyama T: Clinical outcome of malignant biliary
obstruction caused by metastatic gastric cancer. World J Surg.
33:2396–2402. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Schmassmann A, von Gunten E, Knuchel J,
Scheurer U, Fehr HF and Halter F: Wallstents versus plastic stents
in malignant biliary obstruction: effects of stent patency of the
first and second stent on patient compliance and survival. Am J
Gastroenterol. 91:654–659. 1996.
|
|
15
|
Sacks D, McClenny TE, Cardella JF and
Lewis CA: Society of Interventional Radiology clinical practice
guidelines. J Vasc Interv Radiol. 14:S199–S202. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saad WEA, Wallace MJ, Wojak JC, Kundu S
and Cardella JF: Quality improvement guidelines for percutaneous
transhepatic cholangiography, biliary drainage, and percutaneous
cholecystostomy. J Vasc Interv Radiol. 21:789–795. 2010. View Article : Google Scholar
|
|
17
|
van Delden OM and Laméris JS: Percutaneous
drainage and stenting for palliation of malignant bile duct
obstruction. Eur Radiol. 18:448–456. 2008.PubMed/NCBI
|
|
18
|
Inal M, Akgul E, Aksungur E, Demiryurek H
and Yagmur O: Percutaneous self-expandable uncovered metallic
stents in malignant biliary obstruction. Complications, follow-up
and reintervention in 154 patients. Acta Radiol. 44:139–146. 2003.
View Article : Google Scholar
|
|
19
|
Indar AA, Lobo DN, Gilliam AD, et al:
Percutaneous biliary metal wall stenting in malignant obstructive
jaundice. Eur J Gastroenterol Hepatol. 15:915–919. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tapping CR, Byass OR and Cast JE:
Percutaneous transhepatic biliary drainage (PTBD) with or without
stenting-complications, re-stent rate and a new risk stratification
score. Eur Radiol. 21:1948–1955. 2011. View Article : Google Scholar
|
|
21
|
Beddy P and Ryan JM: Antibiotic
prophylaxis in interventional radiology - anything new? Tech Vasc
Interv Radiol. 9:69–76. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Clark CD, Picus D and Dunagan WC:
Bloodstream infections after interventional procedures in the
biliary tract. Radiology. 191:495–499. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Brountzos EN, Ptochis N, Panagiotou I,
Malagari K, Tzavara C and Kelekis D: A survival analysis of
patients with malignant biliary strictures treated by percutaneous
metallic stenting. Cardiovasc Intervent Radiol. 30:66–73. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Gwon DI, Ko GY, Sung KB, et al: Clinical
outcomes after percutaneous biliary interventions in patients with
malignant biliary obstruction caused by metastatic gastric cancer.
Acta Radiol. 53:422–429. 2012. View Article : Google Scholar
|
|
25
|
Weston BR, Ross WA, Wolff RA, et al: Rate
of bilirubin regression after stenting in malignant biliary
obstruction for the initiation of chemotherapy: how soon should we
repeat endoscopic retrograde cholangiopancreatography? Cancer.
112:2417–2423. 2008. View Article : Google Scholar
|
|
26
|
van der Gaag NA, Rauws EAJ, van Eijck CH,
et al: Preoperative biliary drainage for cancer of the head of the
pancreas. New Engl J Med. 362:129–137. 2010.
|
|
27
|
Singh A and Lee JH: Self-expanding metal
stents for preoperative biliary drainage in patients receiving
neoadjuvant therapy for pancreatic cancer. J Gastrointest Oncol.
3:304–305. 2012.
|
|
28
|
Farges O, Regimbeau JM, Fuks D, et al:
Multicentre European study of preoperative biliary drainage for
hilar cholangiocarcinoma. Br J Surg. 100:274–283. 2013. View Article : Google Scholar : PubMed/NCBI
|