Introduction
The majority of primary bladder tumours are
transitional cell (urothelial) tumours. Cases of squamous cell
carcinoma, primary adenocarcinoma or small cell carcinoma are
encountered much less frequently (1). Haemangiopericytoma (HPC) of the
bladder, which is a tumour originating from the vascular pericytes
of Zimmermann, is exceedingly rare and carries uncertain malignant
potential. HPC mostly arises in the lower extremities,
retroperitoneum and head and neck area (2–4). To
the best of our knowledge, only eight cases of HPC of the bladder
have been previously reported in the English literature (2–9). The
rarity of HPC of the bladder makes it difficult to predict the
clinical behaviour and determine the appropriate treatment of this
tumour. The current study reports a case of recurrent primary HPC
of the bladder. The clinical and histological features, and the
treatment and prognosis of this tumour are discussed together with
a review of the literature.
Case report
A 48-year-old female patient was admitted to the Sir
Run Run Shaw Hospital (Hangzhou, China) due to a large mass in the
bladder identified during a health examination in June 2011. The
patient exhibited no mass-related symptoms, such as pain, gross
haematuria or urinary irritation. Seven years prior to admission,
the patient underwent surgical resection of a bladder mass, which
was diagnosed as HPC of the bladder. However, the patient did not
attend regular follow-up examinations after the surgery. The
patient was otherwise healthy. Computed tomography (CT) scans
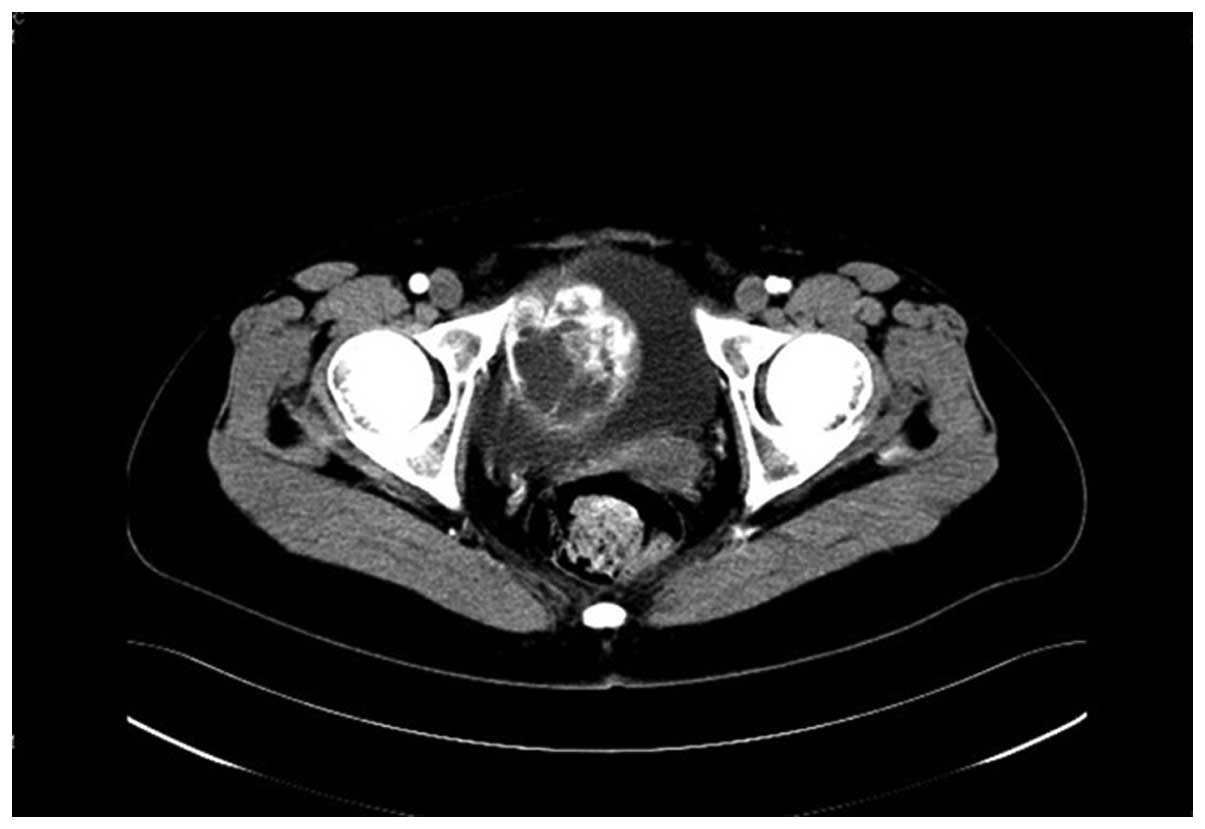
showed a well-defined, heterogeneously enhancing 6.2×5.0-cm solid
cystic mass in the bladder (Fig.
1). Cystoscopy showed extrinsic compression of the right
bladder wall, but the bladder mucosa was normal. The physical
examination was unremarkable. No lymph node or distant metastasis
was found. Following a thorough pre-operative evaluation, the
patient underwent complete excision of the tumour and a partial
cystectomy. The tumour was 6.5×5.0×4.0 cm in size, with an intact
external surface. The cut surface of the tumour was grey-white,
with a medium texture.
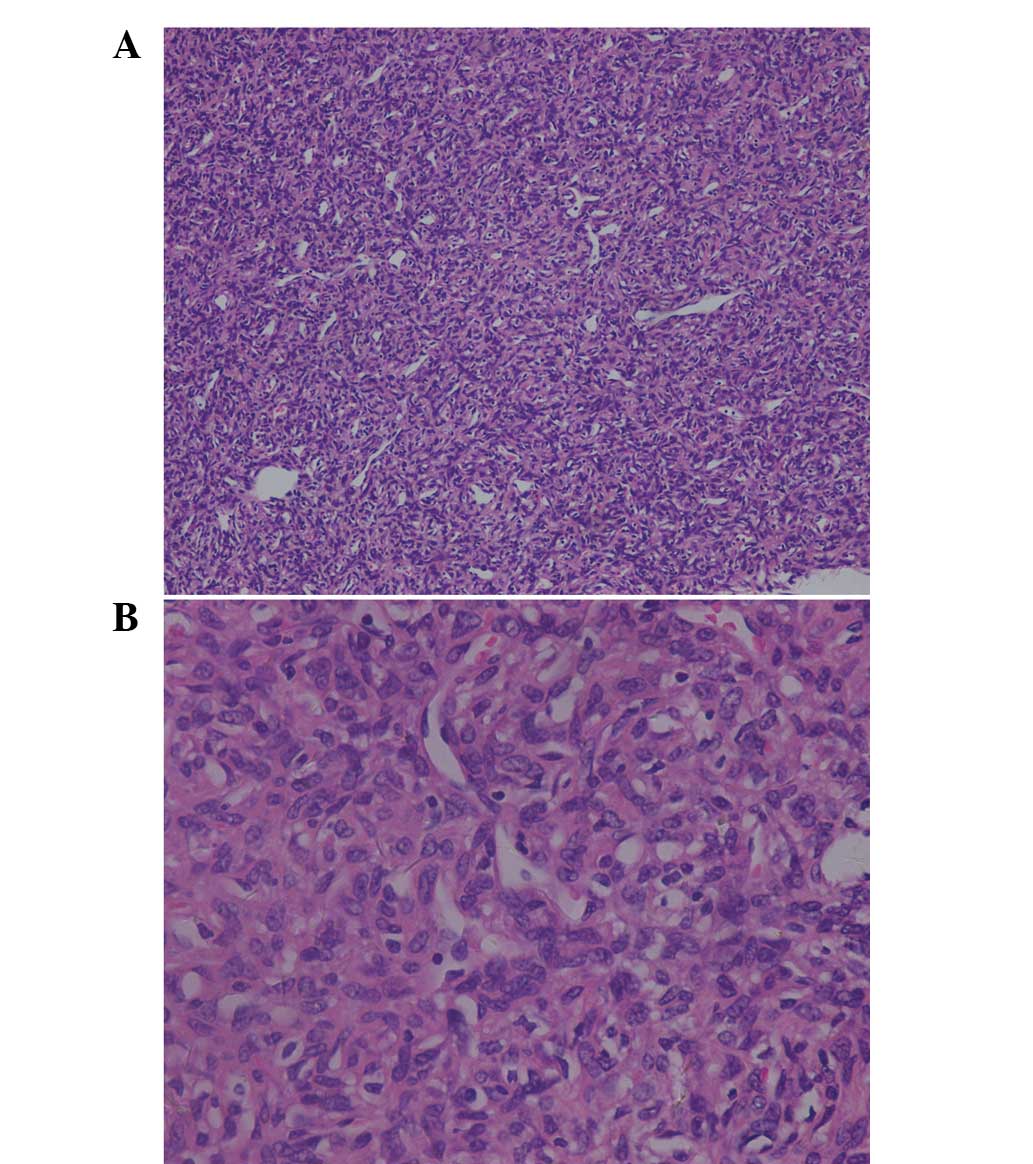
The histopathological examination revealed a
neoplasm consisting of spindle-shaped cells, which were arranged
around the vasculature, with a ‘staghorn’ configuration (Fig. 2). The images captured were
hypercellular and showed cells that exhibited oval nuclei. Mitosis
was rare and necrosis was not present. The neoplastic cells
exhibited marked positivity for Bcl-2 and were negative for cluster
of differentiation (CD)34, CD31, factor VIII, epithelial membrane
antigen, S100, chromogranin A, cytokeratin (CK)7 and CK19. The
proliferation marker, Ki-67, was positive in <5% of the tumour
cells. The histopathological diagnosis was HPC of the bladder and
surgical margins were noted to be tumour-negative.
The post-operative recovery was uneventful, but the
patient refused further adjuvant radiotherapy. To date, the patient
has been followed up regularly with no evidence of recurrence or
metastasis. Written informed consent was obtained from the family
of the patient.
Discussion
HPC is a rare soft-tissue tumour, first described by
Stout and Murray in 1942 (10). HPC
was considered to originate from the pericytes, a specific cell
type that surround the capillary vessels. However, according to the
World Health Organization for the classification of tumours of soft
tissues and bone, the term ‘haemangiopericytoma’ may be used to
refer to a variety of tumours, which have the presence of a
thin-walled branching ‘staghorn’ vascular pattern and resemble
cellular areas of solitary fibrous tumours (11). An accurate histopathological
assessment determines the definitive diagnosis of HPC. There is not
always clarity in the prediction of the clinical behaviour of HPC
and this does not always correlate with the histopathological
features of the tumour either. There is variation between studies
with regard to the histopathological criteria for malignancy, and
strict universal criteria have not yet been identified (12).
HPC in the urinary bladder is extremely rare. To the
best of our knowledge, only eight cases of HPC of the bladder have
been previously reported in the English literature (Table I) (2–9). In
these cases and including the current study, the mean age of the
patients at the time of diagnosis is 48.5 years (range, 29–72
years), with a predominance of females (six vs. two). The mean size
of the tumours is 8 cm (range, 2.5–12 cm) and the clinical features
of the patients are not characteristic. Urinary symptoms, such as
haematuria and frequency, were noted in four patients and three
patients had pain associated with the masses. Hypoglycaemia,
attributed to the extensive metabolism of glucose within the
tumour, was present in one case (6). In one patient, anaemia and weight loss
were the reasons for hospitalisation (5). However, the patient of the current
study had no complaints associated with the tumour.
 | Table ICharacteristics of patients with HPC
of the bladder previously reported in the English literature,
together with the current case (n=9). |
Table I
Characteristics of patients with HPC
of the bladder previously reported in the English literature,
together with the current case (n=9).
| First author/s
(year) | Age, years | Gender | Size, cm | Symptoms | Treatment | Follow-up |
|---|
| Sezhian et al
(2007) | 52 | F | 12 | Anaemia and weight
loss | Total cystectomy and
an ileal conduit | Well at two
years |
| Kibar et al
(2006) | 45 | M | 4 | Left groin pain,
vague suprapubic discomfort and urinary frequency | Partial cystectomy
and adjuvant radiotherapy | Well at two
years |
| Soran et al
(2007) | 72 | F | ~12 | Symptoms of
hypoglycaemia | Local palliative
radiotherapy | Succumbed at three
years |
| Bagchi et al
(1993) | NA | NA | NA | NA | NA | NA |
| Burgess et al
(1993) | 29 | F | 10 | Right lower abdominal
pain | Excision of the
lesion | Well at six
months |
| Sutton et al
(1989) | 30 | F | 6 | Acute urinary
retention | Complete
transurethral resection | Recurrence at two
years |
| Prout and Davis
(1977) | 40 | M | 12 | Right groin pain,
urinary frequency, dysuria and a right lower quadrant mass | Excision of the
lesion and chemotherapy for metastases | Metastases at nine
years |
| Baumgartner et
al (1976) | 72 | F | 2.5 | Intermittent painless
total haematuria with urinary frequency and vague suprapubic
discomfort | Partial cystectomy
with ligation of the ureter, hysterectomy and bilateral
salpingo-oophorectomy | Succumbed at three
days from a pulmonary embolism caused by tumour thrombi |
| Current case | 48 | F | 6.5 | No symptoms | Partial
cystectomy | Well at two
years |
Imaging is important in the diagnosis and management
of HPC by demonstrating the vascular nature of the tumour and
revealing the exact source of its blood supply, its size and its
association with the adjacent organs. However, no characteristic
signs of HPC have been recognised on ultrasonography, CT scan or
magnetic resonance imaging. Commonly, previous studies have
depicted a large mass, but with no pathognomonic features.
Cystoscopy may reveal no intravesical pathology, but evidence of
compression of the bladder wall.
The clinical and biological behaviour of HPC is
variable and unpredictable. En bloc resection remains the
cornerstone of therapy for curative intent (13). The surgeon must be as radical as
possible to avoid incomplete tumour resection and a high frequency
of relapse. Open surgery was used in seven of the patients in the
previous studies, with the exception of one case, reported by
Sutton et al, in which the patient underwent a transurethral
resection and the tumour recurred two years later (4). Since HPC originates in the bladder
wall, we do not recommend the transurethral approach for HPC of the
bladder due to the fear of incomplete resection. An appropriate
first surgical treatment must be selected to obtain a complete view
of the mass. For tumours exhibiting evident criteria for
malignancy, adjuvant radiotherapy should be considered (13). Radiotherapy is reserved as the
adjuvant therapy in cases of incompletely excised lesions and
recurrent and inoperable tumours. Radiotherapy was used in two of
the previously reported cases and the authors considered
radiotherapy effective for preventing recurrence and controlling
the hypoglycaemic syndrome (2,6).
Systemic chemotherapy may be employed for metastasis and
recurrence. However, standard and effective chemotherapeutic
regimens have yet to be established (14). Previously, one patient with HPC of
the bladder received chemotherapy for metastases, but did not
benefit from the treatment (7). In
other HPCs in various locations, chemotherapy does not appear to be
an effective adjunct therapy (15,16).
The outcomes of the previously reported cases were
quite different: One patient succumbed three days after surgery
from a pulmonary embolism caused by tumour thrombi; lung metastasis
was noted in a patient nine years after surgery; and three patients
developed local recurrence following the initial surgery (after
seven years in two cases). Since recurrence and metastasis may
occur after a number of years, lifelong regular follow-up is
necessary. To date, the current patient has been followed for two
years and no evidence of local recurrence or metastasis has been
identified.
HPC of the bladder is an extremely rare tumour with
unpredictable clinical and biological behaviour. Radical surgical
excision is considered to be the cornerstone of treatment.
Radiotherapy is reserved as the adjuvant therapy in cases of
incompletely excised lesions and for recurrent or inoperable
tumours. The efficacy of classical chemotherapy appears
disappointing. In addition, since recurrence and metastasis may
occur after a number of years, lifelong regular follow-up is
necessary.
References
|
1
|
Lott S, Lopez-Beltran A, Montironi R,
MacLennan GT and Cheng L: Soft tissue tumors of the urinary
bladder. Part II: malignant neoplasms. Hum Pathol. 38:963–977.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Kibar Y, Uzar AI, Erdemir F, Ozcan A,
Coban H and Seckin B: Hemangiopericytoma arising from the wall of
the urinary bladder. Int Urol Nephrol. 38:243–245. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Burgess NA, Hudd C and Matthews PN: Two
cases of haemangiopericytoma. Br J Urol. 71:238–239. 1993.
View Article : Google Scholar
|
|
4
|
Sutton R, Hopper IP and Munson KW:
Haemangiopericytoma of the bladder. Br J Urol. 63:548–549. 1989.
View Article : Google Scholar
|
|
5
|
Sezhian N, Rimal D, Velchuru VR, Thapa SR
and Suresh G: Haemangiopericytoma of the bladder. Am J Clin Oncol.
30:6602007. View Article : Google Scholar
|
|
6
|
Soran H, Younis N, Joseph F, Hayat Z,
Zakhour H and Scott A: A case of haemangiopericytoma-associated
hypoglycaemia: Beneficial effect of treatment with radiotherapy.
Int J Clin Pract. 60:1319–1322. 2006. View Article : Google Scholar
|
|
7
|
Bagchi AG, Dasgupta A and Chaudhury PR:
Haemangiopericytoma of urinary bladder. J Indian Med Assoc.
91:211–212. 1993.PubMed/NCBI
|
|
8
|
Prout MN and Davis HL Jr:
Hemangiopericytoma of the bladder after polyvinyl alcohol exposure.
Cancer. 39:1328–1330. 1977. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Baumgartner G, Gaeta J, Wajsman Z and
Merrin C: Hemangiopericytoma of the Urinary Bladder: a case report
and review of the literature. J Surg Oncol. 8:281–286. 1976.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Stout AP and Murray MR:
Hemangiopericytoma: A vascular tumor featuring Zimmerman’s
pericytes. Ann Surg. 116:26–33. 1942.
|
|
11
|
Enzinger FM and Weiss SW: Soft Tissue
Tumors. 5th edition. Mosby; New York, NY: 2008
|
|
12
|
Fountoulakis EN, Papadaki E, Panagiotaki
I, Giannikaki E, Lagoudianakis G and Bizakis J: Primary
haemangiopericytoma of the parapharyngeal space: an unusual tumour
and review of the literature. Acta Otorhinolaryngol Ital.
31:194–198. 2011.PubMed/NCBI
|
|
13
|
Krishnan M, Kumar KS and Sowmiya T:
Hemangiopericytoma - the need for a protocol-based treatment plan.
Indian J Dent Res. 22:4972011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Aliberti C, Benea G, Kopf B and De Giorgi
U: Hepatic metastases of hemangiopericytoma: contrast-enhanced MRI,
contrast-enhanced ultrasonography and angiography findings. Cancer
Imaging. 6:56–59. 2006. View Article : Google Scholar
|
|
15
|
Enzinger FM and Smith BH:
Hemangiopericytoma. An analysis of 106 cases. Hum Pathol. 7:61–82.
1976.
|
|
16
|
McMaster MJ, Soule EH and Ivins JC:
Hemangiopericytoma. A clinicopathologic study and long-term follow
up of 60 patients. Cancer. 36:2232–2244. 1975. View Article : Google Scholar : PubMed/NCBI
|