Introduction
Breast cancer is the most common type of cancer
among females, and is the second leading cause of cancer-related
mortality worldwide (1). Current
treatment for estrogen-receptor (ER)-positive tumors (>60% of
breast cancers) includes surgery, whereby gross tumors are removed,
which is followed by treatment with drugs. Drug treatments include
aromatase inhibitors and antiestrogens, such as tamoxifen, which
target the hormone dependence that is demonstrated by these tumors
(2). However, such drugs have
little efficacy against certain types of breast cancer cells,
including human epidermal growth factor receptor 2
(HER-2)/neu-overexpressing breast cancers. The HER-2/neu oncogene
is the second member of the HER (also known as ErbB) family
(3). More than 30% of breast
cancers were identified to exhibit HER-2/neu overexpression, which
is considered a predictive marker of resistance to tamoxifen
therapy. Aberrant activation of the HER-2 receptor is closely
associated with increased metastatic potential and resistance to
chemotherapeutic agents (4,5). Activation of receptor tyrosine
kinases, such as phosphatidylinositide 3-kinase (PI3K) and protein
kinase B (Akt), which have an intrinsic ability to phosphorylate
tyrosine residues in their cytoplasmic domains, results in the
activation of nuclear transcription factors that induce cell growth
and inhibit apoptosis (4).
Therefore, inhibition of HER-2/neu has become an important
therapeutic target for human breast cancers.
Chrysin (5,7-dihydroxyflavone, ChR) is a naturally
occurring, biologically active flavone that has been demonstrated
to inhibit cell proliferation and induce apoptotic cell death in a
variety of cancer cells, including breast cancer (6–8). Due
to poor oral bioavailability, ChR may not be successfully used as a
dietary flavonoid for cancer chemotherapeutics (9). We previously demonstrated that the
effect of 8-bromo-7-methoxychrysin (BrMC) on the inhibition of
proliferation and induction of apoptosis in a colon cancer cell
line, HT-29, and a gastric cancer cell line, SGC-7901, was stronger
than that of ChR (10). BrMC also
exhibited effective inhibition of proliferation and induction of
apoptosis in colon, gastric and liver cancer cells (11–13).
However, whether BrMC inhibits the cell growth of
HER-2/neu-overexpressing breast cancers has not yet been
determined.
In the present study, the effectiveness of BrMC
against two human breast cancer cell lines exhibiting high levels
of HER-2/neu expression was investigated.
Materials and methods
Cell culture and reagents
The human breast cancer cell lines, MDA-MB-453 and
BT-474 (which endogenously overexpress the HER-2/neu oncogenes) and
MCF-7 (low HER-2 expression), as well as the human breast cell
line, MCF-10A (low HER-2 expression), were used in this study. The
cell lines were obtained from the Cell Bank of the Chinese Academy
of Sciences (Shanghai, China), and cells were grown in Dulbecco’s
Modified Eagle’s medium (DMEM), with F-12 nutrient mixture,
supplemented with 10% heat-inactivated fetal bovine serum (FBS), 2
mM glutamine, and 1% penicillin-streptomycin-neomycin (all
purchased from Gibco-BRL, Grand Island, NY, USA) at 37°C in a
humidified incubator with 5% CO2. BrMC was synthesized
as described previously (10).
Cultures were harvested and monitored for changes in cell number by
counting cell suspensions using a hemocytometer (Model 1280,
Shanghai Qiujing Biochemical Reagent and Instrument Co., Ltd.,
Shanghai, China) with a phase-contrast microscope (BX41, Olympus
Optical Co., Ltd., Tokyo, Japan).
Cell viability assay
Cells were seeded in a 96-well plate at a density of
0.5×104 cells/well and treated with serum-free medium
(Gibco-BRL) for 24 h, followed by treatment with various
concentrations of experimental agents (Akt inhibitor LY294002:
Calbiochem, La Jolla, CA, USA; proteasome inhibitor MG132: Bio
Vision, Inc., Mountain View, CA, USA), which were added to each
well and cultured for 24 h, followed by incubation with media
containing 0.5 mg/ml
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide
(Sigma-Aldrich, St. Louis, MO, USA) for 4 h. The supernatant was
removed following centrifugation at 1000 × g for 5 min. Finally,
100 μl dimethyl sulfoxide (Amresco Company, Solon, OH, USA) was
added and the absorbance at the 570-nm wavelength (A570)
was measured using an enzyme-labeling instrument (ELX-800; Bio-Tek
Instruments, Inc., Shanghai, China). Cell viability was calculated
as follows: Cell viability (%) = (A570 of treated
cells/A570 of untreated cells) ×100.
Western blot analysis
MDA-MB-453 or BT-474 cells (1.5×106
cells/10-cm dish) were incubated with various concentrations of
BrMC for 24 h. After incubation, the cells were washed once in
phosphate-buffered saline (PBS), detached, pooled and centrifuged
at 1500 × g for 5 min. The cell pellets were subsequently suspended
in 100 μl lysis buffer (Sigma-Aldrich; 10 mM Tris-HCl, pH 8.0; 320
mM sucrose; 1% Triton X-100; 5 mM ethylenediaminetetraacetic acid;
2 mM dithiothreitol; and 1 mM phenylmethylsulfonyl fluoride). The
suspensions were kept on ice for 20 min and centrifuged at 15000 ×
g for 30 min at 4°C. Total protein content was determined with the
Bio-Rad protein assay reagent (Bio-Rad, Hercules, CA, USA) using
bovine serum albumen (Gibco-BRL) as a standard. Protein extracts
were reconstituted in sample buffer [Sigma-Aldrich; 62 mM Tris-HCl,
2% sodium dodecyl sulfate (SDS), 10% glycerol, 5%
β-mercaptoethanol], and the mixture was boiled at 97°C for 5 min.
Equal amounts (50 μg) of denatured protein samples were loaded into
each lane, separated by SDS-polyacrylamide gel electrophoresis on
an 8–15% polyacrylamide gradient gel and transferred onto
polyvinylidene difluoride membranes overnight. The membranes were
blocked with 5% non-fat dried milk in PBS containing 1% Tween-20
(Sigma-Aldrich) for 1 h at room temperature, and subsequently
incubated with primary antibodies [rabbit polyclonal antibodies
against cyclinE, p27KIP, p21CIP, CDK1, CDK2,
and β-actin were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA). Mouse monoclonal antibodies against HER
2/neu (p185), HER/neu, p-PI3K, PI3K, p-Akt, Akt, β-catenin,
p-GSK-3β, GSK-3β, cyclin D1 and CDK4 were obtained from Cell
Signaling Technology, Inc. (Danvers, MA, USA)]. for 2 h and either
horseradish peroxidase-conjugated goat anti-rabbit or anti-mouse
antibodies overnight. The signal intensity was then measured using
a chemiluminescent detection system (Pierce Biotechnology, Inc.,
Rockford, IL, USA).
Colony formation assay
Anchorage-independent growth was determined by
colony formation in soft agar (14). The assay was performed in six-well
plates (1×104 cells/well) with a base layer containing
0.5% agar in DMEM containing 10% FBS, 1 mM glutamine, 100 units
penicillin and 100 μg/ml streptomycin. This layer was overlaid with
a second layer of 1 ml 0.3% agar (in DMEM containing 10% FBS, 1 mM
glutamine, 100 units of penicillin and 100 μg/ml of streptomycin)
with a suspension of 1×104 cells/well. Fresh medium with
either BrMC or ChR was then added to the plates every 72 h. The
plates were incubated at 37°C for 3 weeks, and the tumor colonies
were then analyzed microscopically. Colonies with a diameter
>0.2 mm were counted.
Statistical analysis
The results are presented as the mean ± standard
deviation. All study data were analyzed using analysis of variance
followed by Dunnett’s test for pairwise comparison. An asterisk
indicates that the experimental values are significantly different
from those of the control (*P<0.05).
Results
BrMC preferentially inhibits cell
viability of HER-2/neu-overexpressing breast cancer cells
A previous study indicated that ChR and its analog
(NOC) preferentially inhibited growth in human
HER-2/neu-overexpression breast cancer cells (15); therefore, in the present study the
effects of BrMC on the growth of three breast cancer lines
(MDA-MB-453, BT-474 and MCF-7) and the immortalized noncancerous
MCF-10A breast cell line were investigated. The three breast cancer
cell lines examined were selected due to their varying levels of
HER-2/neu expression. In the present study, it was demonstrated
that MDA-MB-453 and BT-474 cell lines harbor HER-2/neu
overexpression, and MCF-7 and MCF-10A harbor low expression of
HER-2/neu (Fig. 1A). BrMC inhibited
cell viability in a dose-dependent manner (Fig. 1B); MDA-MB-453 and BT-474 cell lines
were most sensitive, the MCF-7 cell line was moderately sensitive
and the MCF-10A cell line was least sensitive to BrMC. These
results suggest that BrMC preferentially suppresses the viability
of HER-2/neu-overexpressing cell lines, MDA-MB-453 and BT-474.
 | Figure 1Effects of BrMC on the HER-2/neu
expression and proliferation of breast cancer lines (MDA-MB-453,
BT-474, MCF-7) and the human breast cell line (MCF-10A). (A)
Western blot analysis of the expression of HER-2/neu protein, where
β-actin was used as the loading control. (B) After incubation with
different concentrations of BrMC (0–10 μM) or chrysin (50 μM) for
24 h, the cell viability was examined by
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide
assay. Data are shown as the means ± SD, n=3.
*P<0.05, compared with respective controls;
**P<0.01, compared with respective controls; and
#P<0.05, compared with the other cell lines treated
with the same concentration of BrMC. BrMC,8-bromo-7-methoxychrysin;
HER-2, human epidermal growth factor receptor 2. |
BrMC inhibits HER-2/neu protein
expression through the regulation of proteasomal activity
Activation of the HER-2/neu network leads to
autophosphorylation of the receptor’s C-terminal tyrosine and,
subsequently, recruitment of cytoplasmic signal transducers to
these sites, which regulates cellular processes, such as
proliferation, inhibition of apoptosis and transformation (4). Therefore, the present study
investigated whether BrMC treatment could reduce such basal HER/neu
phosphorylation. MDA-MB-453 and BT-474 human breast cancer cells
were treated with BrMC for 24 h. The total cell lysates were
isolated and analyzed by western blot analysis using HER-2/neu and
phosphotyrosine-specific HER-2/neu antibodies. Fig. 2A shows that treatment of MDA-MB-453
and BT-474 cells with BrMC (at 2.5, 5.0 and 10.0 μM) and ChR (50
μM) for 24 h resulted in a substantial decrease in HER-2/neu
tyrosine phosphorylation. BrMC and ChR treatment similarly reduced
basal HER-2/neu levels in both cell lines (Fig. 2A). Overall, these findings indicate
that BrMC reduced the basal phosphorylation and constitutive
activation of HER-2/neu receptors in HER-2/neu-overexpressing
breast cancer cells.
To examine the role of proteolysis in BrMC-mediated
HER-2/neu downregulation, MG132, the proteasome inhibitor was used.
In the absence of MG132, BrMC treatment significantly reduced the
HER-2/neu levels (Fig. 2B).
Cotreatment with MG-132 resulted in accumulation of HER-2/neu
protein in MDA-MB-453 cells (Fig.
2B). These data suggest that proteasomal activity was
critically involved in BrMC-induced HER-2/neu degradation in
MDA-MB-453 cells.
BrMC inhibits the activation of Akt in
HER-2/neu-overexpressing breast cancer cells
A key mechanism by which HER-2/neu overexpression
stimulates tumor cell growth and renders cells chemoresistant
involves the HER-2/neu receptor. This mechanism also involves Akt
kinase; human breast cancer cells with overexpression and
amplification of HER-2/neu have been shown to increase Akt kinase
activity (4). The involvement of
HER-2/neu in the activation of the PI3K/Akt signaling pathway in
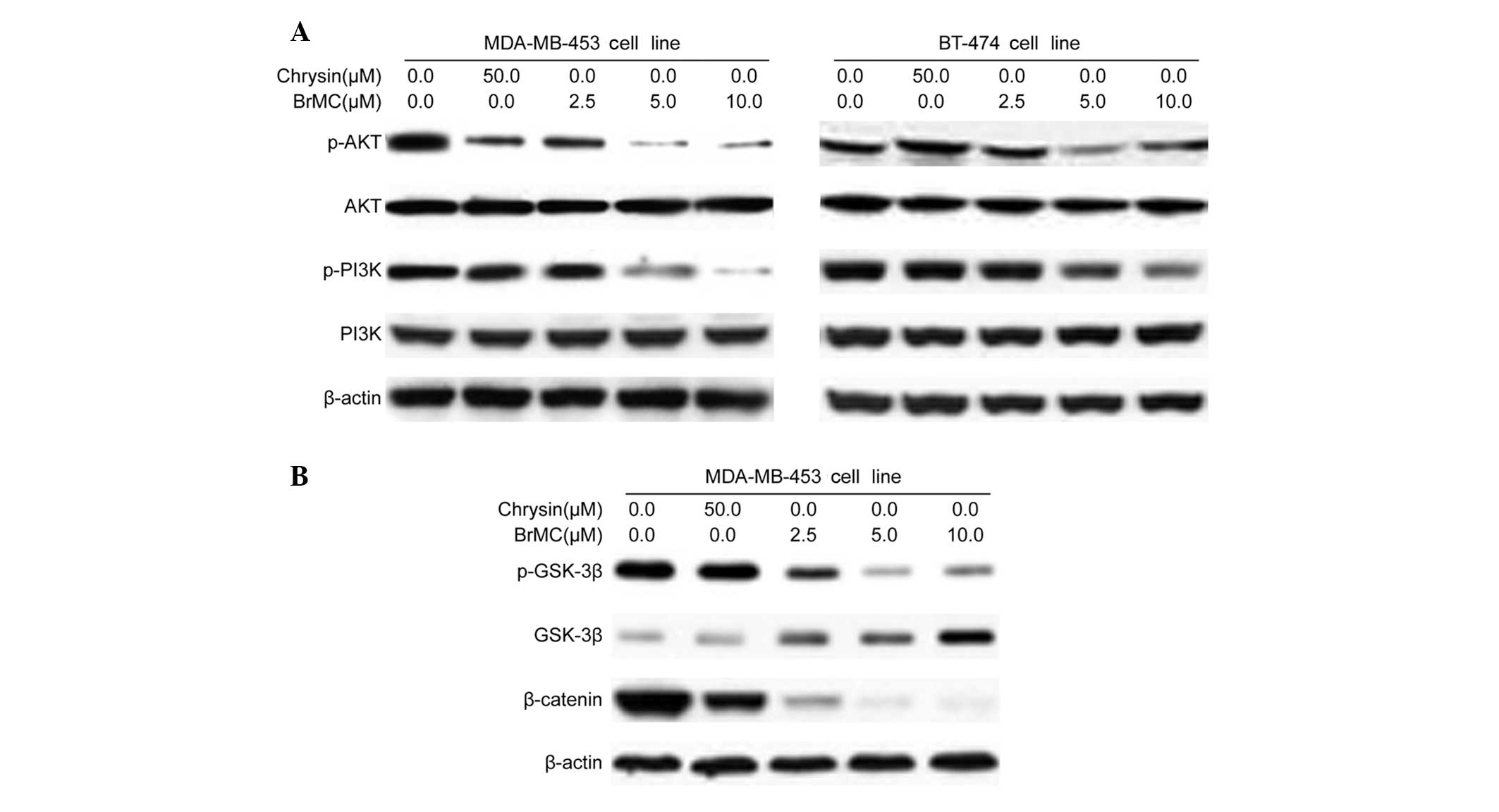
MDA-MB-453 and BT-474 cell lines was investigated. BrMC and ChR
treatment significantly inhibited the phosphorylation of Akt in
MDA-MB-453 HER-2/neu-overexpressing breast cancer cells in a
dose-dependent manner (Fig. 3A). In
addition, it was observed that BrMC treatment significantly
inhibited the expression of the Akt upstream kinase, PI3K; in
MDA-MB-453 and BT-474 cells; however, this effect did not occur
with ChR (Fig. 3A). These data
established that BrMC-induced HER-2/neu depletion and cell growth
inhibition may be mediated by the inactivation of PI3K/Akt activity
in HER-2/neu-overexpressing breast cancer cells.
When Akt is active, a number of substrates are
activated that are involved in apoptosis, cell cycle regulation and
protein synthesis (4). Akt may
potentially regulate cell cycle progression by phosphorylating and
inactivating GSK-3β, thereby stabilizing nuclear translocation of
β-catenin and increasing cyclin D1 and Cdk4 transcription (16). In BrMC-treated MDA-MD-453 cells,
phosphorylated GSK-3β levels decreased substantially, whereas total
GSK-3β levels increased (Fig. 3B).
This observation suggests that the treatment of cells with BrMC
augmented the activity of GSK-3β. Levels of β-catenin, a key
component of the Wnt signaling pathway, which is degraded via
polyubiquitination upon phosphorylation by GSK-3β, decreased
substantially following BrMC treatment (Fig. 3B). In conclusion, our data
demonstrated that BrMC may inhibit cell proliferation by
suppressing GSK-3β and the β-catenin pathway in
HER-2/neu-overexpressing breast cancer cells.
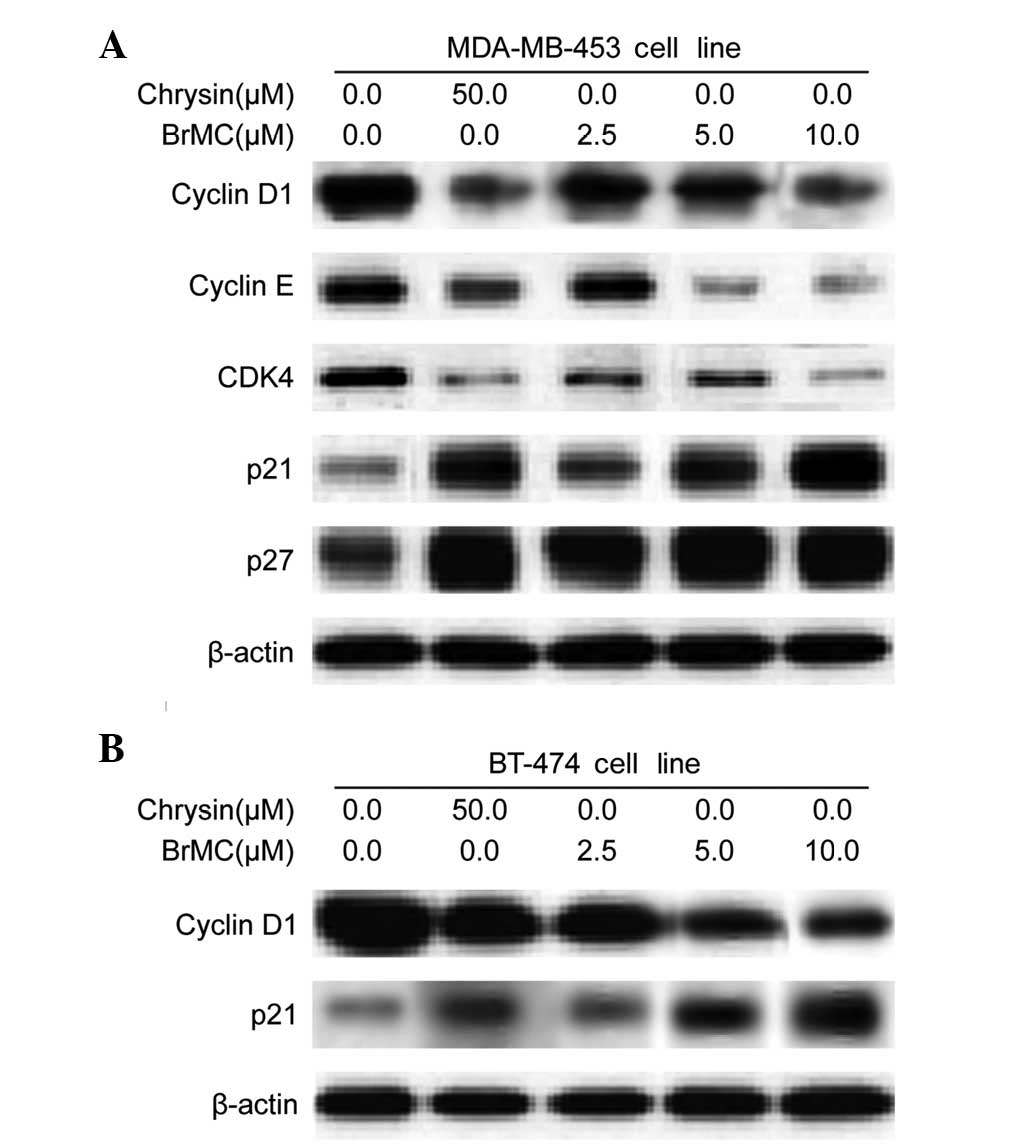
BrMC regulates cell cycle regulatory
proteins in HER-2/neu-overexpressing breast cancer cells
To examine the molecular mechanism(s) and underlying
changes in cell cycle patterns caused by BrMC treatment, the
effects of various cyclins and Cdks involved in cell cycle
regulation were investigated, in MDA-MB-453 cells. BrMC treatment
for 24 h caused a dose-dependent reduction of cyclin D1 and cyclin
E expression in HER-2/neu-overexpressing MDA-MB-453 cells (Fig. 4A). Cyclin D1 serves as the
regulatory subunit of Cdk4 and contributes to its stability.
Therefore, the effects of BrMC on Cdk expression were assessed;
treatment of MDA-MB-453 cells with BrMC resulted in a
dose-dependent decrease in Cdk4 expression (Fig. 4A). However, there was no change in
the Cdk1 and Cdk2 protein levels (data not shown). These results
imply that BrMC inhibits cell cycle progression by reducing the
levels of cyclin D1, cyclin E and Cdk4 in MDA-MB-453 cells. In
addition, Akt may contribute to the induction of cell cycle
progression by regulating the Cdk inhibitors, p27KIP and
p21CIP (17). Both
p27KIP and p21CIP protein levels
dose-dependently increased in response to BrMC treatment (Fig. 4A). A similar pattern of results was
also observed in BT-474 cells. BrMC treatment caused downregulation
of cyclin D1 and upregulation of p21CIP expression in a
dose-dependent manner in BT-474 cells (Fig. 4B).
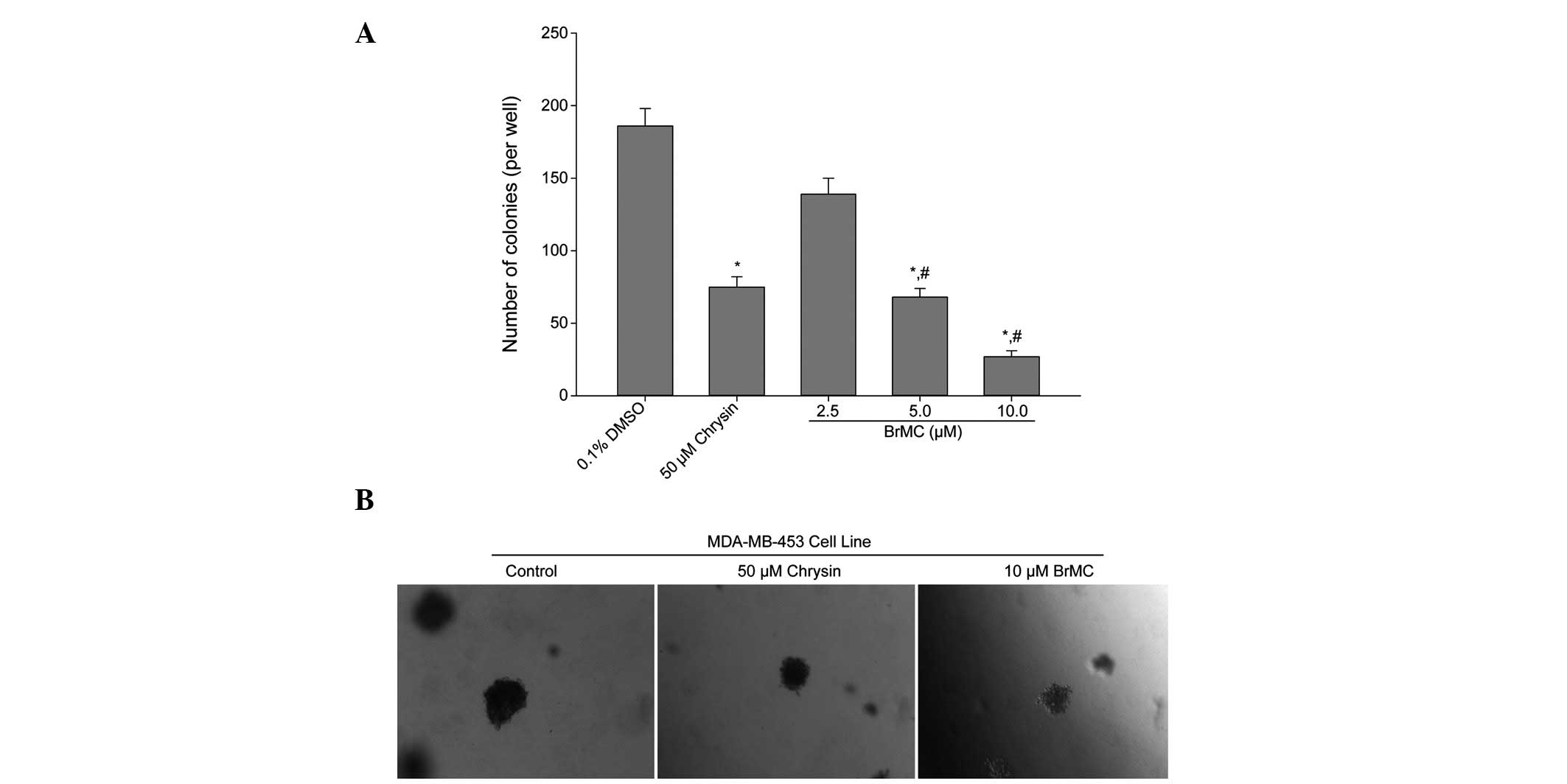
BrMC inhibits anchorage-independent
growth of HER-2/neu-overexpressing breast cancer cells
The effect of BrMC upon anchorage-independent colony
growth in soft agar was determined. Anchorage-independent growth is
a property of transformed and tumor cells, and is closely
correlated with tumorigenesis in vivo. Colony formation of
MDA-MB-453 cells, which are known to overexpress HER-2/neu, was
significantly (P<0.05) suppressed by BrMC treatment as compared
with that in the control group (Fig.
5A). Reductions in colony number were accompanied by a
reduction in colony size in MDA-MB-453 cells (Fig. 5B). Therefore, the data indicate that
BrMC treatment suppressed the transformation ability of
HER-2/neu-overexpressing breast cancer cells.
Discussion
Previous studies have shown that
5,7-dihydroxy-8-nitrochrysin, a novel synthetic ChR analog,
preferentially suppresses the viability of the MDA-MB-453 human
breast cancer cell line (ER-negative, HER-2-overexpressing) and
moderately suppresses the viability of the MCF-7 cell line
(ER-positive, HER-2-low), but has little effect on the immortalized
noncancerous HBL-100 breast cell line (ER-positive, HER-2-low)
(15). This finding indicated that
the novel synthetic ChR analog was differentially cytotoxic towards
different breast cancer cell lines without exerting harmful effects
on normal cells at higher concentrations.
In the present study, BrMC (another synthetic ChR
analog) was observed to mediate inhibition of cell proliferation in
HER-2/neu-overexpressing human breast cancer cells. It was
demonstrated that BrMC treatment efficiently inhibited the cell
viability of MDA-MB-453 and BT-474 cells with an IC50
value of 6.2 and 7.4 μmol/l, respectively. It was also revealed
that exposure of the HER-2/neu-overexpressing breast cancer cells
to BrMC resulted in HER-2/neu depletion and downregulation of
PI3K/Akt signaling cascades. Thus, such data indicate that BrMC may
be used as a possible chemopreventive or chemotherapeutic agent
against human breast cancers.
Overexpression of human epidermal growth factor
receptor-2 (HER-2/neu) has been frequently observed in breast
cancer cells and is known to indicate a poor clinical prognosis.
Therefore, drugs that reduce HER-2/neu activity may be a potential
target for breast cancer therapy. Depletion of HER-2/neu in
HER-2/neu-overexpressing human breast cancer cells has been
demonstrated to arrest cell proliferation (4). Trastuzumab (Herceptin), a humanized
antibody that targets the extracellular domain of HER-2/neu, has
become a commercialized medicine for the treatment of
HER-2/neu-overexpressing early-stage and metastatic breast cancers.
However, when used as a single agent, trastuzumab is beneficial in
only 15–30% of HER-2/neu breast cancer patients, which can be
significantly increased to 50–80% by the addition of
chemotherapeutic agents (18). In
the current study, observations showed that BrMC treatment
effectively downregulates HER-2/neu protein expression in
HER-2/neu-overexpressing MDA-MB-453 and BT-474 human breast cancer
cells. It has been previously reported that
5,7-dihydroxy-8-nitrochrysin blocks HER-2/neu expression by
inhibiting the phosphorylation of Akt in HER-2/neu-overexpressing
MDA-MB-453 breast cancer cells (5).
ChR inhibits the tyrosine kinase activity of HER-2/neu and induces
HER-2/neu degradation by the proteasome when inhibition of protein
degradation by MG-132 leads to the accumulation of the
NP-40-insoluble form of HER-2/neu. BrMC-induced growth inhibition
increases the susceptibility of HER-2/neu-overexpressing cancer
cells (5). These data indicate that
BrMC may be a promising anticancer agent for human breast
cancers.
Akt kinase and its downstream transcription factors
have been studied in detail to determine their role in cell
proliferation, survival, cell cycle control and other cellular
functions (19). In numerous cell
types, PI3K/Akt induces survival in response to a variety of
stimuli, including growth factor withdrawal and loss of cell
adhesion (20). BrMC was found to
have an inhibitory effect on the steady-state levels of total PI3K
protein in the present study. Additionally, the phosphorylation of
its downstream effector Akt, was inhibited, indicating that the
disruption of Akt signaling/Akt inactivation plays a functional
role in BrMC-mediated cytotoxicity in HER-2/neu-overexpressing
breast cancer cells. The present data also suggested that
BrMC-mediated inhibition of cyclin D1 is directly proportional to
the suppression of HER-2/neu and PI3K/Akt in human breast cancer
cells. Overall, these results suggest that HER-2/neu may regulate
cellular cyclin D1 via the PI3K/Akt pathway, implying that PI3K/Akt
signaling predominantly contributes to cell cycle progression.
In the present study, it was also demonstrated that
BrMC treatment downregulates β-catenin expression through
upregulation of its negative regulator, GSK-3β. The BrMC-induced
increase in GSK-3β may contribute to its effects on Wnt/β-catenin
pathway inhibition. Akt kinase has been shown to phosphorylate
several key substrates that regulate protein translation (21). In addition, the phosphorylation of
its substrate, GSK-3β, as well as nuclear β-catenin stabilization
and increased cyclin D1 transcription have all been demonstrated in
MDA-MB-453 cells (4). The
Wnt/β-catenin signaling pathway has been shown to play an important
role in the regulation of cyclin D1, which is crucial in cell cycle
regulation and progression in a variety of tumor cells (16). Recent studies clearly described that
the interactions between HER-2/neu and cyclin D1 appear to have
therapeutic relevance as several phytochemical or synthetic drugs
have been demonstrated to reduce cyclin D1 expression through the
inhibition of HER-2/neu, and the anti-HER-2/neu monoclonal antibody
trastuzumab (Herceptin) reduces cyclin D1 protein levels in human
breast cancer cells (22,23). In addition, the present study
results demonstrated that BrMC treatment significantly inhibited
MDA-M-453 proliferation, which was associated with the suppression
of GSK-3β and β-catenin expression and a decrease in expression of
their transcriptional targets, including cyclin D1 and Cdk4.
Anchorage-independent growth is a characteristic of
numerous types of tumor cells, which distinguishes them from their
normal counterparts (24).
Anchorage-dependent growth requires integrin-mediated signaling,
which is generated by cellular contact with extracellular matrix
ligands (25). Normal cells,
particularly epithelial cells, undergo apoptosis if they become
detached from their underlying or pericellular matrices, in a
process termed anoikis (24). By
contrast, a number of tumor and transformed cells have escaped this
requirement for survival and growth. Moreover, the ability of
HER-2/neu-overexpressing breast cancer cells to grow in an
anchorage-independent manner has been linked to elevation of the
PI3K/Akt cell survival pathway (26). In the current study, we found that
BrMC decreased MDA-MB-453 cell proliferation and markedly reduced
their capacity to form colonies in soft agar. The loss of
anchorage-independent growth of HER-2/neu-overexpressing breast
cancer cells treated with BrMC indicates that these cells may have
reverted to a less transformed phenotype. This inhibition may also
be mediated by the reduction of PI3K/Akt activation.
We hypothesized that BrMC induced cellular effects,
resulting from loss of HER-2/neu expression, which may cause
subsequent inactivation of PI3K and Akt in cells that are dependent
on this pathway for cell proliferation and inhibition of apoptosis.
Results from this study also highlight the importance of HER-2/neu
or PI3K/Akt components, including GSK-3β, β-catenin, cyclin D1 and
p21WAF1, which may serve as future targets for the
development of therapeutic strategies against
HER-2/neu-overexpressing breast cancer. The inhibition of cell
growth in HER-2/neu-overexpressing breast cancer cells following
BrMC administration provides a new strategy for breast cancer
therapy. However, in vivo studies are required to confirm
the pharmacological efficacy and safety of BrMC.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (General Program, grant no. 81172375),
the Construct Program of the Key Discipline of Basic Medicine in
Hunan Province and Research Fund for the Doctoral Program of Hunan
Normal University (grant no 110656).
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ao A, Morrison BJ, Wang H, López JA,
Reynolds BA and Lu J: Response of estrogen receptor-positive breast
cancer tumorspheres to antiestrogen treatments. PLoS One.
6:e188102011. View Article : Google Scholar
|
|
3
|
Shah S and Chen B: Testing for HER2 in
breast cancer: A continuing evolution. Patholog Res Int.
2011:9032022011.
|
|
4
|
Way TD, Kao MC and Lin JK: Apigenin
induces apoptosis through proteasomal degradation of HER2/neu in
HER2/neu-overexpressing breast cancer cells via the
phosphatidylinositol 3-kinase/Akt-dependent pathway. J Biol Chem.
279:4479–4489. 2004.
|
|
5
|
Jeong JH, An JY, Kwon YT, Li LY and Lee
YJ: Quercetin-induced ubiquitination and down-regulation of
Her-2/neu. J Cell Biochem. 105:585–595. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lee SJ, Yoon JH and Song KS: Chrysin
inhibited stem cell factor (SCF)/c-Kit complex-induced cell
proliferation in human myeloid leukemia cells. Biochem Pharmacol.
74:215–225. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Huang Q, Ong CN, Yang XF and Shen
HM: Chrysin sensitizes tumor necrosis factor-alpha-induced
apoptosis in human tumor cells via suppression of nuclear
factor-kappaB. Cancer Lett. 293:109–116. 2010. View Article : Google Scholar
|
|
8
|
Khoo BY, Chua SL and Balaram P: Apoptotic
effects of chrysin in human cancer cell lines. Int J Mol Sci.
11:2188–2199. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Walle T, Otake Y, Brubaker JA, Walle UK
and Halushka PV: Disposition and metabolism of the flavonoid
chrysin in normal volunteers. Br J Clin Pharmacol. 51:143–146.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Zheng X, Meng WD, Xu YY, Cao JG and Qing
FL: Synthesis and anticancer effect of chrysin derivatives. Bioorg
Med Chem Lett. 13:881–884. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ai XH, Zheng X, Tang XQ, Sun L, Zhang YQ,
Qin Y, Liu HQ, Xia H and Cao JG: Induction of apoptosis of human
gastric carcinoma SGC-7901 cell line by 5,
7-dihydroxy-8-nitrochrysin in vitro. World J Gastroenterol.
13:3824–3828. 2007.PubMed/NCBI
|
|
12
|
Yang XH, Zheng X, Cao JG, Xiang HL, Liu F
and Lv Y: 8-Bromo-7-methoxychrysin-induced apoptosis of
hepatocellular carcinoma cells involves ROS and JNK. World J
Gastroenterol. 16:3385–3393. 2010. View Article : Google Scholar
|
|
13
|
Xiao G, Tang X, Yao C and Wang C:
Potentiation of arsenic trioxide-induced apoptosis by
8-bromo-7-methoxychrysin in human leukemia cells involves depletion
of intracellular reduced glutathione. Acta Biochim Biophys Sin
(Shanghai). 43:712–721. 2011. View Article : Google Scholar
|
|
14
|
Koleske AJ, Baltimore D and Lisanti MP:
Reduction of caveolin and caveolae in oncogenically transformed
cells. Proc Natl Acad Sci USA. 92:1381–1385. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhao XC, Tian L, Cao JG and Liu F:
Induction of apoptosis by 5,7-dihydroxy-8-nitrochrysin in breast
cancer cells: the role of reactive oxygen species and Akt. Int J
Oncol. 37:1345–1352. 2010.PubMed/NCBI
|
|
16
|
Takahashi-Yanaga F and Sasaguri T:
GSK-3beta regulates cyclin D1 expression: a new target for
chemotherapy. Cell Signal. 20:581–589. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Steelman LS, Stadelman KM, Chappell WH, et
al: Akt as a therapeutic target in cancer. Expert Opin Ther
Targets. 12:1139–1165. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hahn T, Bradley-Dunlop DJ, Hurley LH,
Von-Hoff D, Gately S, Mary DL, Lu H, Penichet ML, Besselsen DG,
Cole BB, et al: The vitamin E analog, alpha-tocopheryloxyacetic
acid enhances the anti-tumor activity of trastuzumab against
HER2/neu-expressing breast cancer. BMC Cancer. 11:4712011.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Zhang X, Jin B and Huang C: The PI3K/Akt
pathway and its downstream transcriptional factors as targets for
chemoprevention. Curr Cancer Drug Targets. 7:305–316. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Datta SR, Brunet A and Greenberg ME:
Cellular survival: a play in three Akts. Genes Dev. 13:2905–2927.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Basso AD, Solit DB, Munster PN and Rosen
N: Ansamycin antibiotics inhibit Akt activation and cyclin D
expression in breast cancer cells that overexpress HER2. Oncogene.
21:1159–1166. 2002. View Article : Google Scholar
|
|
22
|
Priyadarsini RV and Nagini S: Cancer
chemoprevention by dietary phytochemicals: promises and pitfalls.
Curr Pharm Biotechnol. 13:125–136. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Xu J, Chen Y and Olopade OI: MYC and
breast cancer. Genes Cancer. 1:629–640. 2010. View Article : Google Scholar
|
|
24
|
Ghatak S, Misra S and Toole BP: Hyaluronan
oligosaccharides inhibit anchorage-independent growth of tumor
cells by suppressing the phosphoinositide 3-kinase/Akt cell
survival pathway. J Biol Chem. 277:38013–38020. 2002. View Article : Google Scholar
|
|
25
|
Millard M, Odde S and Neamati N: Integrin
targeted therapeutics. Theranostics. 1:154–188. 2011. View Article : Google Scholar
|
|
26
|
Menendez JA, Mehmi I, Verma VA, Teng PK
and Lupu R: Pharmacological inhibition of fatty acid synthase
(FAS): a novel therapeutic approach for breast cancer
chemoprevention through its ability to suppress Her-2/neu (erbB-2)
oncogene-induced malignant transformation. Mol Carcinog.
41:164–178. 2004. View
Article : Google Scholar
|