Introduction
Gastric cancer is a multi-step progression from
normal gastric mucosa to chronic gastritis, atrophy, intestinal
metaplasia, dysplasia and ultimately cancer (1). Three closely related trefoil factors
(TFFs) known in humans, pS2 (TFF1), spasmolytic polypeptide (SP or
TFF2) and intestinal TFF (ITF or TFF3) (2,3), have
been previously reported to be associated with the development of
various types of cancer (4). TFF1,
a tumor suppressor gene, exhibits decreased expression in
precancerous and gastric cancer tissues (5,6). TFF3
expression is significantly elevated in intestinal metaplasia
biopsy specimens compared with that in normal tissues, and the
samples with an elevated expression of TFF3 lack goblet cell
features (7). Furthermore, as TFF3
promotes tumorigenesis by increasing cell invasion and metastasis
(8), gastric carcinoma patients
with positive expression of TFF3 show invasive characteristics and
poor prognosis (9). TFF2 is a
principal cytoprotective TFF in the stomach and is highly expressed
in ulcer tissue (10). Certain
studies have previously reported that TFF2 expression is
upregulated in gastric cancer tissues and that the overexpression
is associated with cancer invasion, metastasis and a poor prognosis
(11,12). However, several studies have shown
that TFF2 expression is decreased significantly in gastric adenomas
compared with the associated normal tissue, suggesting that the
loss of TFF2 expression, as with the loss of TFF1, is an important
event in gastric carcinogenesis (13–15).
However, the correlation between the downregulation of TFF2
expression and clinicopathological data, as well as the detailed
molecular mechanism underlying TFF2 abnormal expression, remain
unclear.
The majority of gastric cancers are diagnosed in the
advanced stage (16), which is
generally resistant to radiotherapeutic or chemotherapeutic
treatments. Therefore, it is important to identify any early
regulatory molecules involved in gastric cancer progression, which
may aid the detection of gastric cancer at an early and curable
stage. Recently, it has been reported that the downregulated
expression of protease-activated receptor 4 (PAR4) in gastric
cancer tissues and the loss of PAR4 expression in gastric cancer
may result from hypermethylation of the gene promoter (17). In the current study, we aimed to
define the expression difference of TFF2 in gastric cancer and the
gene methylation level.
Materials and methods
Gastric tissue samples
Gastric specimens (n=28) were obtained from the
tumor and an adjacent non-cancerous area, ≥6 cm from the tumor
tissues of gastric carcinoma patients at the First Affiliated
Hospital of Kunming Medical College (Kunming, China). The mean age
of the patients at diagnosis was 56 years. The non-neoplastic
tissue was confirmed to lack tumor cell infiltration using
histological analysis. The tissues were immediately placed in
liquid nitrogen and stored at −80°C until use. A gastric cancer
tissue microarray representing 110 types of gastric cancer with
their non-neoplastic resection margins was constructed (18) at the Shanghai Outdo Biochip Center
(Shanghai, China). Human samples were used in accordance with the
requirements of the Ethical Committee of the Kunming Institute of
Zoology, the Chinese Academy of Sciences, under the guidelines of
the World Medical Assembly (Declaration of Helsinki). Written
informed consent was obtained from the patient’s families.
RNA extraction and polymerase chain
reaction (PCR)
RNA extraction and first-strand cDNA synthesis were
performed as previously described (19). For semi-quantitative reverse
transcription PCR (RT-PCR) and quantitative PCR (qPCR), the
following primers were used: Forward, 5′-CTGCTTCTCCAACTTCATCT-3′
and reverse, 5′-CTTAGTAATGGCAGTCTTCC-3′ for TFF2 (74-bp product);
and forward, 5′-ATGGGGAAGGTGAAGGTCG-3′ and reverse,
5′-GGGGTCATTGATGGCAACAATA-3′ for glyceraldehyde 3-phosphate
dehydrogenase (GAPDH; 107-bp product). GAPDH was used as an
internal control. Following RT-PCR, the amplicons were separated by
electrophoresis in a 2% agarose gel that was stained with ethidium
bromide and viewed under ultraviolet illumination. qPCR was
performed using a continuous fluorescence detector (Opticon
Monitor; Bio-Rad, Hercules, CA, USA) and PCR was performed using an
SYBR Green real-time PCR kit (Takara Bio, Inc., Dalian, China) with
the following reaction conditions: Initial denaturation at 95°C for
1 min followed by 40 cycles at 95°C for 15 sec, 60°C for 15 sec and
72°C for 20 sec. Each sample was run three times. No-template
controls (no cDNA in the PCR) were run to detect non-specific or
genomic amplification and primer dimerization. Fluorescence curve
analysis was performed using Opticon Monitor software. The relative
quantitative evaluation of TFF2 levels was performed using the
E-method (20) and expressed as a
ratio of the TFF2 to GAPDH transcripts in the tumor tissue divided
by that ratio in the non-neoplastic tissue of the same patient. The
identities of RT-PCR and qPCR products were confirmed by DNA
sequencing.
Cell culture
AGS and N87 human gastric cancer cells were obtained
from the American Type Culture Collection (Manassas, VA, USA). AGS
cells were cultured in a 1:1 mixture of Dulbecco’s modified Eagle’s
medium and Ham’s media. N87 cells were cultured in RPMI-1640 media
containing 10% fetal calf serum, 100 U/ml penicillin and 100 mg/ml
streptomycin. The cells were grown in a humidified atmosphere
containing 5% CO2 at 37°C. The cells were seeded at a
density of 1×106 cells/ml in a 60 mm dish and treated
with 10 mM 5-Aza-2′-deoxycytidine (5-Aza-2′-dC; Sigma-Aldrich, St.
Louis, MO, USA). DMSO was used as a control. The cells were
collected after 3 days and subjected to RT-PCR, qPCR and western
blot analysis.
Western blot analysis
Tissue and cell samples were homogenized in
radioimmunoprecipitation assay buffer containing a protease
inhibitor cocktail (Sigma-Aldrich). The protein concentration was
determined using a protein assay kit (Bio-Rad). Samples (containing
50 μg of protein) were loaded into a sodium dodecyl
sulfate-polyacrylamide gel electrophoresis gel, electrophoresed and
then electro-transferred onto a polyvinylidene fluoride membrane.
The membrane was subsequently blocked with 3% bovine serum albumin
and incubated with an anti-human TFF2 polyclonal antibody (Protein
Tech, Chicago, IL, USA) and a horseradish peroxidase-conjugated
secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA).
Protein bands were visualized using Super Signal reagents (Thermo
Fisher Scientific, Inc., Rockford, IL, USA).
Tissue immunohistochemistry (IHC)
Tissue IHC was performed as previously described
(21). Briefly, antigen retrieval
was performed by heating samples in an autoclave at 121°C for 5
min. Dewaxed sections were pre-incubated with blocking serum and
then incubated overnight with an anti-human TFF2 antibody (P-19;
Santa Cruz Biotechnology) at 4°C. Specific binding was detected
using a streptavidin-biotin-peroxidase assay kit (Maxim, Fujian,
China). The section was counterstained with Harris hematoxylin.
Direct microscopic micrographs were captured using a Leica DFC320
camera controlled using Leica IM50 software (Leica, Mannheim,
Germany). Sections incubated with normal goat IgG served as
negative controls, which were devoid of any detectable
immunolabeling. The specificity of the anti-TFF2 antibody was
confirmed using an overnight preincubation at 4°C with its antigen
in a 20-fold molar excess of antigen to antibody. The preincubation
with TFF2 antigens resulted in an absence of immunolabeling.
Immunohistochemical staining was semi-quantitatively assessed by
measuring the intensity of the staining (0, 1, 2 or 3) and the
extent of staining (0, 0%; 1, 1–10%; 2, 11–50%; and 3, 51–100%).
The scores for the intensity and extent of staining were multiplied
to yield a weighted score for each case (maximum possible, 9). For
the statistical analysis, the weighted scores were grouped into two
categories, in which scores of 0–3 and 4–9 were considered negative
and positive, respectively (22).
Bisulfite sequencing
Genomic DNA from carefully selected 20-μm sections
of gastric cancer, non-neoplastic tissues, and AGS and N87 cell
lines was isolated using the Universal Genomic DNA Extraction kit
(Takara, Bio, Inc.) and bisulfite-converted using the Clontech
EpiXplore™ Methyl Detection kit (Takara, Bio, Inc.). TFF2 promoter
sequences were amplified from the bisulfite-converted DNA by PCR,
purified from agarose gels and subcloned into the pBackZero T
Vector (Takara, Bio, Inc.). For each sample, 11 individual clones
were sequenced to identify methylated cytosine residues. The PCR
primer sequences used were forward, 5′-GGGATTTTTTTATGTTATTTGTTGG-3′
and reverse, 5′-ATAAAAAAACCCTCTCCTTCACTTACAAAA-3′.
Statistical analysis
All statistical results were analyzed using SPSS
11.0 software (SPSS, Inc., Chicago, IL, USA). Fisher’s exact and
χ2 tests were used to analyze the correlation between
TFF2 expression and clinicopathological parameters (Tables I and II). Differences in the numerical data
between the two paired groups were evaluated using the paired
Wilcoxon test (Fig. 1). P<0.05
was considered to indicate a statistically significant
difference.
 | Table ICorrelation between TFF2 mRNA
expression levels and clinicopathological data in gastric cancer
patients. |
Table I
Correlation between TFF2 mRNA
expression levels and clinicopathological data in gastric cancer
patients.
| | TFF2 mRNA levels | |
|---|
| |
| |
|---|
| | Not decreased
(n=2) | Decreased (n=26) | |
|---|
| |
|
| |
|---|
| Clinicopathological
parameters | Total (n=28) | n | % | n | % | P-value |
|---|
| Gender |
| Male | 18 | 0 | 0.0 | 18 | 100.0 | 0.119 |
| Female | 10 | 2 | 20.0 | 8 | 80.0 | |
| Age, years |
| ≤65 | 17 | 1 | 5.9 | 16 | 94.1 | 1.000 |
| >65 | 11 | 1 | 9.1 | 10 | 90.9 | |
| Lauren type |
| Intestinal | 8 | 1 | 12.5 | 7 | 87.5 | 0.497 |
| Diffuse | 20 | 1 | 18.2 | 19 | 81.8 | |
| TNM stages |
| T1 and T2 | 7 | 2 | 0.0 | 5 | 100.0 | 0.056 |
| T3 and T4 | 21 | 0 | 9.5 | 21 | 90.5 | |
| Differentiation |
| Poor | 22 | 0 | 0.0 | 22 | 100.0 | 0.040 |
| Well and
moderate | 6 | 2 | 33.3 | 4 | 66.6 | |
| Lymph node
metastasis |
| Negative | 5 | 2 | 40.0 | 3 | 60.0 | 0.026 |
| Positive | 23 | 0 | 0.0 | 23 | 100.0 | |
 | Table IICorrelation between TFF2 protein
expression levels and clinicopathological data in gastric cancer
patients. |
Table II
Correlation between TFF2 protein
expression levels and clinicopathological data in gastric cancer
patients.
| | TFF2 protein
levels | |
|---|
| |
| |
|---|
| | Not decreased
(n=20) | Decreased
(n=90) | |
|---|
| |
|
| |
|---|
| Clinicopathological
parameters | Total (n=110) | n | % | n | % | P-value |
|---|
| Gender |
| Male | 71 | 11 | 15.5 | 60 | 84.5 | 0.439 |
| Female | 39 | 9 | 23.1 | 30 | 76.9 | |
| Age, years |
| <65 | 63 | 15 | 23.8 | 48 | 76.2 | 0.086 |
| ≥65 | 47 | 5 | 10.6 | 42 | 89.4 | |
| TNM stages |
| T1 and T2 | 30 | 8 | 26.7 | 22 | 73.3 | 0.173 |
| T3 and T4 | 80 | 12 | 15.0 | 68 | 85.0 | |
| Lauren type |
| Intestinal | 81 | 17 | 21.0 | 64 | 79.0 | 0.268 |
| Diffuse | 29 | 3 | 10.3 | 26 | 89.7 | |
|
Differentiation |
| Poor | 84 | 11 | 13.1 | 73 | 86.9 | 0.020 |
| Well and
moderate | 26 | 9 | 34.6 | 17 | 65.4 | |
| Lymph node
invasion |
| Positive | 80 | 9 | 11.3 | 71 | 88.8 | 0.004 |
| Negative | 30 | 11 | 36.7 | 19 | 63.3 | |
Results
Downregulated expression of TFF2 mRNA in
types of gastric cancer and correlation with clinicopathological
parameters
TFF2 mRNA expression in gastric cancer tissues was
examined using RT-PCR. In total, four pairs of samples were
randomly selected from 28 patients and normalized to the GAPDH
level. As shown in Fig. 1A, TFF2
mRNA expression was significantly decreased in cancer tissues
compared with the associated normal mucosa. To quantify the
differences in the expression of TFF2 mRNA, qPCR was performed on
28 gastric tumor tissue samples. TFF2 expression was downregulated
in 93% (26 out of 28) of gastric cancer tissue samples compared
with the associated non-neoplastic tissues. In addition, the mRNA
levels of TFF2/GAPDH in gastric cancer tissues were significantly
lower than those in the corresponding non-neoplastic mucosal
tissues (mean ± SE, 3.4±2.7 vs. 9.6±5.4, respectively; P=0.046)
(Fig. 1B). The clinical
significance of the loss of TFF2 expression was further
investigated based on the clinical pathological data. As shown in
Table I, there were significant
differences in TFF2 mRNA expression in well- and moderately
differentiated tumors versus poorly differentiated tumors
(P=0.019), and in tumors with lymph node invasion versus
non-invasive tumors (P=0.026). In detail, TFF2 mRNA was reduced by
a fold-change of 15.0±4.1 (mean ± SE) in the 22 poorly
differentiated tumors compared with a fold-change of 1.9±0.7 in the
six well- and moderately differentiated tumors (P=0.009; paired
Wilcoxon test), and a fold-change of 15.6±3.7 in the 23 lymph node
invasive tumors compared with a fold-change of 1.1 ± 0.4 in the
five non-invasive tumors (P=0.002; paired Wilcoxon test) (Fig. 1C).
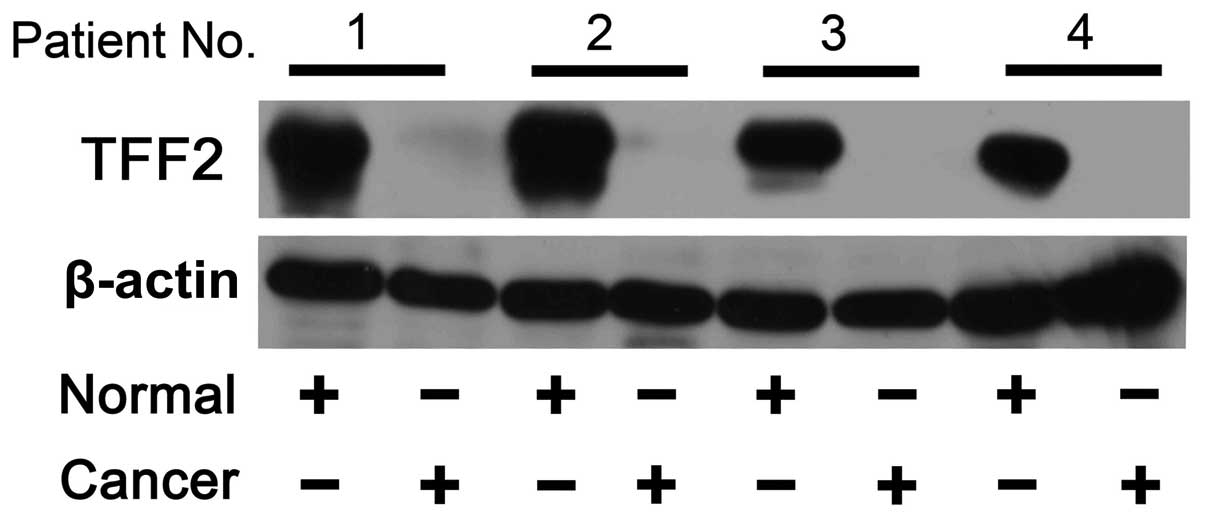
Downregulation of TFF2 protein expression
in gastric cancer tissues by western blot and tissue IHC
analyses
The protein expression levels of TFF2 in normal and
gastric cancer tissues were verified using western blot analysis.
After the samples were normalized to the β-actin level, a marked
reduction or loss of TFF2 protein was observed in four gastric
tumor tissue samples compared with the matched non-malignant
tissues (Fig. 2). TFF2 protein
levels in normal and malignant gastric mucosa were also assessed
using an IHC assay. In 110 gastric cancer tissue microarray assays,
TFF2 expression was downregulated in 82% (90 out of 110). TFF2 was
expressed at high levels in all investigated normal mucosa tissues
and staining was identified from the basal-to-middle portions of
gastric glands. Furthermore, TFF2 localization was in the cytoplasm
and the membrane of gastric normal epithelial cells (Fig. 3A). However, the expression was
significantly reduced in well- (Fig.
3B) and moderately (Fig. 3C)
differentiated intestinal gastric cancer tissues, while TFF2
expression was almost absent in the poorly differentiated
intestinal and diffuse types of gastric cancer (Fig. 3D). Sections incubated with normal
goat IgG served as negative controls (Fig. 3E). Analysis of the correlation
between TFF2 expression and clinicopathological data showed that
decreased TFF2 expression was closely associated with tumor cell
differentiation and lymph node invasion (Table II). In detail, TFF2 expression was
decreased in 86.9% of poorly differentiated cancers and 65.4% of
well- and moderately differentiated cancers (P=0.02; χ2
test). TFF2 expression was decreased in 88.8% of positive lymph
node invasion and 63.3% of negative lymph node invasion tumors
(P=0.004, χ2 test) (Table
II).
Treatment with 5-Aza-2′-dC increases TFF2
expression in the AGS gastric cancer cell line
To elucidate the potential molecular mechanisms
underlying the process of TFF2 downregulation in the progression of
gastric cancer, AGS cells were treated with 10 mM 5-Aza-2′-dC,
which is a demethylating agent. RT-PCR analysis showed that TFF2
expression in AGS cells was significantly increased following
5-Aza-2′-dC treatment for 3 days (Fig.
4A). qPCR indicated a 3.89-fold increase in the mRNA expression
levels of TFF2 following 5-Aza-2′-dC-treatment, while western blot
analysis also indicated that TFF2 protein expression increased in
AGS cells treated with 5-Aza-2′-dC (Fig. 4B). The results suggested that the
epigenetic alteration may be involved in the downregulation of TFF2
expression in the progression of gastric cancer.
Analysis of the promoter region
methylation of the TFF2 gene in gastric cancer tissues
Treatment with 5-Aza-2′-dC induced demethylation and
led to the upregulated expression of TFF2 in AGS cells. Therefore,
the methylation level of the TFF2 gene promoter was further
analyzed in three gastric cancer and non-neoplastic tissue samples,
as well as in AGS and N87 gastric cancer cell lines. Using the
genomic bisulfite sequencing method, 16 CpG sites were analyzed in
a 571-bp region containing part of the TFF2 promoter region. It
included six CpGs found after the transcription start site and 10
CpGs located in the ~300-bp 5′-flanking region. The average
promoter methylation level of three gastric cancer tissues was
70.4% and the control of non-neoplastic tissues was 41.0%, which
showed that gastric cancer tissues with a decreased expression of
TFF2 exhibited hypermethylation levels at the 16 CpG sites. In
addition, AGS and N87 gastric cancer lines exhibited 85.2 and 93.7%
methylation levels at the 16 CpG sites, respectively (Fig. 5). Therefore, these results indicated
that promoter hypermethylation may lead to the inhibition of TFF2
transcription in gastric cancer.
Discussion
TFFs are widely expressed in the mucosa of the
gastrointestinal tract and play a role in inflammation, injury and
repair. TFF2, a member of the TFFs, is expressed in the cytoplasm
of gastric mucosal neck cells and acts as a mitogen to promote cell
migration and suppress acid secretion (11). An SP-expressing metaplasia lineage
is markedly associated with early gastric cancer and may be an
important candidate for the development of metaplastic processes in
gastric adenocarcinoma (23,24).
In the present study, by RT-PCR, qPCR, western
blotting and immunohistochemical assays, the expression of TFF2 was
shown to be frequently downregulated in gastric cancer tissues
compared with the associated normal mucosa. In detail, TFF2 was
expressed in the neck cells and the deeper glands of the normal
gastric mucosa, but the expression was significantly decreased in
the cancer tissues. Furthermore, no TFF2 expression was detectable
in certain malignant tissues from poorly differentiated gastric
cancer patients or highly lymph node-invasive cancer patients. The
evidence that TFF2 expression was found to decrease is consistent
with the results of previous studies, and decreased TFF2 expression
is associated with the proliferation and malignant transformation
of gastric cancer mucosa (15).
However, the overexpression of TFF2 in gastric carcinoma tissues
has also been shown in additional previous studies (12). The contradictory results may be
attributed to the differences among cancer cell types. In the qPCR
and IHC analyses of the current study, the decreased expression of
TFF2 was 92.9% and 81.8%, respectively, and the reduced expression
was found to significantly correlate with tumor cell
differentiation and invasion. Therefore, there was reduced TFF2
expression in poorly differentiated tumor cells compared with well-
and moderately differentiated tumor cells, and reduced TFF2
expression in positive lymph node invasion tumors compared with
negative lymph node invasion tumors.
The dysregulation of TFF2 expression has been
associated with gastric cancer cell migration, invasion and
resistance to apoptosis. However, the underlying mechanisms
associated with aberrant TFF2 expression remain unclear.
Transcriptional silencing by promoter hypermethylation has emerged
as one of the important mechanisms of gastric cancer development
(25). TFF2 methylation has been
shown to inversely correlate with mRNA levels of TFF2 at the time
of Helicobacter pylori infection and to increase throughout
gastric tumor progression (26). In
the present study, a demethylating agent was found to increase the
expression of TFF2 in AGS cells. Therefore, the methylation status
of cytosines was further analyzed in sites of the TFF2 promoter
region of gastric cancer and non-neoplastic tissues, as well as in
AGS and N87 gastric cancer cell lines. Promoter hypermethylation
was confirmed in gastric cancer tissues compared with that in
non-neoplastic gastric mucosa. In addition, TFF2 promoter
hypermethylation was also found in AGS and N87 gastric cancer cell
lines. These results indicated that the TFF2 gene was
undermethylated in the normal mucosa, but overmethylated in gastric
cancer tissues, suggesting that promoter hypermethylation may lead
to the inhibition of TFF2 transcription in gastric cancer
tissues.
In conclusion, the current study showed that the
expression levels of TFF2 were downregulated in gastric cancer
tissues, particularly in poorly differentiated cancer cells and
lymph node-positive tumors. Notably, the aberrant DNA promoter
methylation is critical in the downregulation of TFF2 expression.
These results may be useful to elucidate the molecular role of TFF2
in carcinogenesis and the progression and metastasis of gastric
cancer.
Acknowledgements
The current study was supported by grants from the
Chinese National Natural Science Foundation (nos. 81160302,
31270835 and 31201720), NSFC Yunnan Union Funding (no. U113 2601),
the Science and Technology Project of Yunnan Province (no.
2011FZ109) and the Chinese Academy of Sciences ‘Western Light
Project’ (no. Y102291081).
Abbreviations:
|
TFFs
|
trefoil factor family
|
|
TFF2
|
trefoil factor 2
|
|
PCR
|
polymerase chain reaction
|
|
RT-PCR
|
reverse transcription PCR
|
|
IHC
|
immunohistochemistry
|
|
BSA
|
bovine serum albumin
|
|
GAPDH
|
glyceraldehyde 3-phosphate
dehydrogenase
|
|
DMEM
|
Dulbecco’s modified Eagle’s medium
|
|
FCS
|
fetal calf serum
|
|
5-Aza-2′-dC
|
5-Aza-2′-deoxycytidine
|
References
|
1
|
Correa P: Human gastric carcinogenesis: a
multistep and multifactorial process - First American Cancer
Society Award Lecture on Cancer Epidemiology and Prevention. Cancer
Res. 52:6735–6740. 1992.
|
|
2
|
Sands BE and Podolsky DK: The trefoil
peptide family. Annu Rev Physiol. 58:253–273. 1996. View Article : Google Scholar
|
|
3
|
Thim L: Trefoil peptides: from structure
to function. Cell Mol Life Sci. 53:888–903. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fox JG, Rogers AB, Whary MT, et al:
Accelerated progression of gastritis to dysplasia in the pyloric
antrum of TFF2 −/− C57BL6 x Sv129 Helicobacter
pylori-infected mice. Am J Pathol. 171:1520–1528.
2007.PubMed/NCBI
|
|
5
|
Fujimoto J, Yasui W, Tahara H and Tahara
E, Kudo Y, Yokozaki H and Tahara E: DNA hypermethylation at the pS2
promoter region is associated with early stage of stomach
carcinogenesis. Cancer Lett. 149:125–134. 2000. View Article : Google Scholar
|
|
6
|
Machado JC, Carneiro F, Ribeiro P, Blin N
and Sobrinho-Simões M: pS2 protein expression in gastric carcinoma.
An immunohistochemical and immunoradiometric study. Eur J Cancer.
32A:1585–1590. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Taupin D, Pedersen J, Familari M, Cook G,
Yeomans N and Giraud AS: Augmented intestinal trefoil factor (TFF3)
and loss of pS2 (TFF1) expression precedes metaplastic
differentiation of gastric epithelium. Lab Invest. 81:397–408.
2001. View Article : Google Scholar
|
|
8
|
Taupin DR, Kinoshita K and Podolsky DK:
Intestinal trefoil factor confers colonic epithelial resistance to
apoptosis. Proc Natl Acad Sci USA. 97:799–804. 2000. View Article : Google Scholar
|
|
9
|
Yamachika T, Werther JL, Bodian C, et al:
Intestinal trefoil factor: a marker of poor prognosis in gastric
carcinoma. Clin Cancer Res. 8:1092–1099. 2002.PubMed/NCBI
|
|
10
|
Wong WM, Poulsom R and Wright NA: Trefoil
peptides. Gut. 44:890–895. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Farrell JJ, Taupin D, Koh TJ, et al:
TFF2/SP-deficient mice show decreased gastric proliferation,
increased acid secretion, and increased susceptibility to NSAID
injury. J Clin Invest. 109:193–204. 2002. View Article : Google Scholar
|
|
12
|
Dhar DK, Wang TC, Maruyama R, et al:
Expression of cytoplasmic TFF2 is a marker of tumor metastasis and
negative prognostic factor in gastric cancer. Lab Invest.
83:1343–1352. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kim H, Eun JW, Lee H, Nam SW, Rhee H, Koh
KH and Kim KH: Gene expression changes in patient-matched gastric
normal mucosa, adenomas, and carcinomas. Exp Mol Pathol.
90:201–209. 2011. View Article : Google Scholar
|
|
14
|
Hu GY, Yu BP, Dong WG, et al: Expression
of TFF2 and Helicobacter pylori infection in carcinogenesis
of gastric mucosa. World J Gastroenterol. 9:910–914.
2003.PubMed/NCBI
|
|
15
|
Shi SQ, Cai JT and Yang JM: Expression of
trefoil factors 1 and 2 in precancerous condition and gastric
cancer. World J Gastroenterol. 12:3119–3122. 2006.PubMed/NCBI
|
|
16
|
Paoletti X, Oba K, Burzykowski T, et al:
Benefit of adjuvant chemotherapy for resectable gastric cancer: a
meta-analysis. JAMA. 303:1729–1737. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zhang Y, Yu G, Wang Y, et al: Activation
of protease-activated receptor (PAR) 1 by frog trefoil factor (TFF)
2 and PAR4 by human TFF2. Cell Mol Life Sci. 68:3771–3780. 2011.
View Article : Google Scholar
|
|
18
|
Kononen J, Bubendorf L, Kallioniemi A, et
al: Tissue microarrays for high-throughput molecular profiling of
tumor specimens. Nat Med. 4:844–847. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu SB, He YY, Zhang Y, et al: A novel
non-lens betagamma-crystallin and trefoil factor complex from
amphibian skin and its functional implications. PLoS One.
3:e17702008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
|
|
21
|
Gratio V, Walker F, Lehy T, Laburthe M and
Darmoul D: Aberrant expression of proteinase-activated receptor 4
promotes colon cancer cell proliferation through a persistent
signaling that involves Src and ErbB-2 kinase. Int J Cancer.
124:1517–1525. 2009. View Article : Google Scholar
|
|
22
|
Moss SF, Lee JW, Sabo E, et al: Decreased
expression of gastrokine 1 and the trefoil factor interacting
protein TFIZ1/GKN2 in gastric cancer: influence of tumor histology
and relationship to prognosis. Clin Cancer Res. 14:4161–4167. 2008.
View Article : Google Scholar
|
|
23
|
Halldórsdóttir AM, Sigurdardóttrir M,
Jónasson JG, et al: Spasmolytic polypeptide-expressing metaplasia
(SPEM) associated with gastric cancer in Iceland. Dig Dis Sci.
48:431–441. 2003.
|
|
24
|
Yamaguchi H, Goldenring JR, Kaminishi M
and Lee JR: Identification of spasmolytic polypeptide expressing
metaplasia (SPEM) in remnant gastric cancer and surveillance
postgastrectomy biopsies. Dig Dis Sci. 47:573–578. 2002. View Article : Google Scholar
|
|
25
|
Jones PA and Laird PW: Cancer epigenetics
comes of age. Nat Genet. 21:163–167. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Peterson AJ, Menheniott TR, O’Connor L, et
al: Helicobacter pylori infection promotes methylation and
silencing of trefoil factor 2, leading to gastric tumor development
in mice and humans. Gastroenterology. 139:2005–2017. 2010.
View Article : Google Scholar
|