Introduction
Invasion and metastasis are the main biological
characteristics of malignant tumors, which are considered lethal
factors in the majority of cancer patients. The degradation of the
basement membrane and the extracellular matrix (ECM) is a key step
in this process. Urokinase-type plasminogen activator (uPA) is an
essential protein that promotes invasion and metastasis. uPA is
important for the hydrolysis of the basement membrane and ECM. A
large number of previous studies have demonstrated higher
expression of uPA in malignant tumor tissue and blood circulation
components involved in tumor metastasis compared with normal
tissues (1,2). uPA has been regarded as a marker of
poor prognosis since the late 1980s. Multifactorial Cox model
analysis previously revealed that a high level of uPA expression
negatively correlates with disease-free and overall survival, but
positively correlates with the risk of recurrence in breast cancer
patients (2). High levels of uPA
and/or plasminogen activator inhibitor-1 antigens in cytosolic
extracts of human primary breast cancer tissue have been associated
with rapid disease progression and lower overall survival (3). Mani et al (4) demonstrated that the small-molecule
inhibition of the urokinase plasminogen activator receptor-uPA
complex blocks breast invasion by MDA-MB-231 cells and inhibits
matrix metalloproteinase-mediated ECM breakdown. The uPA system is
regarded as an independent factor for predicting the prognosis of
breast cancer and its significance is similar with that of the
armpit lymph node (5).
Artemisinin (ART) is a natural sesquiterpene lactone
from Artemisia annua with an endoperoxide group. ART and its
derivatives are widely used as antimalarial drugs without obvious
side effects. Previous reports of its antimalarial properties date
back to 300 B.C., when ART was used as a traditional Chinese
medicine for fever and chills. The anti-cancer properties of ART
were first assayed in vitro in the late 1980s. Efferth et
al (6) analyzed the anticancer
activity of ART against 55 cell lines. ART acquires a highly active
endoperoxide bridge once it encounters ferrous iron. Cancer cells
express higher amounts of the transferrin receptor and
consequently, have higher amounts of intracellular iron (7). Thus, these cells are prone to the
intracellular production of reactive oxygen. The anticancer
properties of ART have been extensively investigated and
characterized in various experimental settings, including oxidative
damage, apoptotic induction, cell cycle arrest, angiogenesis
inhibition, aborted lymphatic metastasis and enhanced
radiosensitivity (8–13). Dihydroartemisinin (DHA) is the main
active metabolite of ART and its antimalarial and antitumor
activities are stronger than those of the other ART derivatives.
The high activity of DHA has been attributed to its higher water
solubility, fine absorbency and excellent stability in clinical
applications. Its efficient selective anticancer effects and lower
toxicity have made DHA a novel research hotspot.
Cancer is a multifactorial ailment, thus, cancer
therapy must target the various aspects of the disease. Considering
the aforementioned factors, the classical concept of uPA and its
associated fibrinolytic system have highlighted novel insights into
the mechanisms of cancer progression. As described previously, DHA
has strong anticancer properties. However, its ability to decrease
the uPA levels in human breast cancer cell lines or weaken the
metastatic ability of these cells remain unknown. In the present
study, the potential mechanisms for the observed effects are
presented. In addition, the aim of the present study was to
investigate the antimetastatic effect induced by uPA and to
highlight a molecular basis for the clinical use of DHA in the
treatment of breast cancer.
Materials and methods
Cell culture
The highly metastatic MDA-MB-231 (purchased from
Shanghai Cell Bank of Chinese Academy of Sciences, Shanghai, China)
and the more common metastatic MCF-7 (courtesy of the Tumor
Pathology Laboratory of the Xi’an Jiaotong University, Xi’an,
China) breast cancer cell lines were used as the cancer cell
models. These lines were cultured in Dulbecco’s Modified Eagle
Medium (DMEM) supplemented with 10% fetal bovine serum and 1%
antibiotic mixture (100 U/ml penicillin and 100 μg/ml streptomycin)
in a CO2 incubator at 37°C with 5% CO2. This
study was approved by the ethics committee of The Second Affiliated
Hospital of Xi’an Jiaotong University (Xi’an, China).
MTT assay
The MDA-MB-231 and MCF-7 cells were treated with
various concentrations of DHA. Cell viability was measured using
the MTT assay, which is based on the conversion of MTT to form
crystals by mitochondrial dehydrogenase. Cells were plated at a
density of 1×104 cells/well in 96-well plates for 12 h
prior to treatment with DHA or dimethyl sulfoxide (control) for 24,
48 and 72 h. In total, 20 μl MTT (5 mg/ml in phosphate buffered
saline) was added to each well 4 h prior to the desired endpoint to
dissolve the formazan crystals. The absorbance, which represented
the optical density (OD), was measured at 570 nm in a 96-well plate
reader (model no. 550; Bio-Rad, Hercules, CA, USA).
Reverse transcription-polymerase chain
reaction (RT-PCR)
uPA and GAPDH gene transcription levels were
detected by RT-PCR using the following primer pairs: i) GAPDH
forward, 5′-ACCCAGAAGACTGTGGATGG-3′ and reverse,
5′-TTCTAGACGGCAGGTCAGGT-3′ (590 bp); and ii) uPA forward,
5′-AGAATTCACCACCATCGAGA-3′ and reverse, 5′-ATCAGCTTCACAACAGTCAT-3′
(474 bp). The primers were synthesized by Beijing Aoke
Biotechnology Co., Ltd. (Beijing, China).
Total RNA was isolated from cells using TRIzol
reagent, according to the manufacturer’s instructions. The 20 μl
PCR system contained 10X buffer (2 μl), cDNA (1 μl), 5 μmol/l
forward primer (0.8 μl), 5 μmol/l reverse primer (0.8 μl), 10
mmol/l dNTPs (2 μl) and Taq DNA polymerase (1 units). The
samples were first denatured at 94°C for 4 min prior to 30 PCR
cycles of 94°C for 30 sec, 55°C for 56 sec and 72°C for 1 min, with
an additional extension at 72°C for 10 min. The amplicons were
visualized on 1.5% agarose gels. The negative controls were run as
parallel experiments performed in the absence of cDNA. The GAPDH
PCR product (200 bp) was used as an internal reference standard to
compare the quantity of the cDNA template added to the PCR.
Statistical analysis
Statistical analysis was performed using SPSS
version 13.0 software (SPSS, Inc., Chicago, IL, USA). Data are
expressed as mean ± standard deviation and P<0.05 was considered
to indicate a statistically significant difference.
Results
Expression levels of uPA
Immunocytochemistry was used to detect the uPA
expression in the human breast cancer cell lines, MDA-MB-231 and
MCF-7. The various expression levels of uPA were observed using
light microscopy following the immunohistochemical staining of
samples from the two cell lines. Numerous deeply stained brown
particles were observed on the membrane and in the cytoplasm of
MDA-MB-231 cells (Fig. 1A).
Compared with the MDA-MB-231 cells, the weakly stained MCF-7 cells
exhibited sparse brown particles (Fig.
1B). In total, 10 fields-of-vision were randomly selected and
100 cells were counted in each field to calculate the positive cell
index using the following formula: Positive cell index = (number of
positive cells/1,000) × 100. The positive cell index was 63 and 24%
in the MDA-MB-231 and MCF-7 cells, respectively. The Student’s
t-test confirmed that the difference between the two values was
statistically significant (P<0.01).
Inhibition of cell growth by DHA
To assess the overall effect of DHA on the cellular
growth, five doses of DHA were administered and compared. The MTT
colorimetric assay demonstrated the viability of MDA-MB-231 cells
in the experimental and control groups. The experimental group was
treated with various concentrations of DHA for 24, 48 and 72 h and
the control group was treated with DMEM in the same manner parallel
to the intervention. The OD values were measured using MTT assay
and analyzed statistically to draw the growth inhibition curve
based on the half inhibition rate. The results showed that
MDA-MB-231 human breast cancer cells were inhibited by DHA in a
time- and dose-dependent manner. Specifically, as the DHA
concentration was increased and its time of activity was extended,
the inhibition rate was gradually increased, as detected by MTT in
the MDA-MB-231 cells (Fig. 2). The
inhibition rate was calculated based on half the IC50
value. The results showed that the inhibition rate of DHA on
MDA-MB-231 was 117.76±0.04 μmol/l at IC50 (24 h),
60.26±0.12 μmol/l at IC50 (48 h) and 52.96±0.07 μmol/l
at IC50 (72 h) (Table
I). These values were found to be statistically significant, as
compared with the control group (P<0.05).
 | Figure 2Inhibition of cell growth in
MDA-MB-231 cells by DHA. Control and experimental group treated
with 0, 6.25, 12.5, 25, 50 and 100 μmol/l DHA for 24, 48 and 72 h
were observed and photographed by an inverted system microscope.
(A) Control group, (B) experimental group. (C) Effects of DHA on
breast cancer MDA-MB-231 cells. Cell viability was measured with an
MTT assay. Y-axis, inhibition rate, was determined in assay and
repeated a minimum of three times in duplicate. X-axis, various
concertrations of DHA (0, 0 μmol/l; 1, 6.25 μmol/l; 2, 12.5 μmol/l;
3, 25 μmol/l ; 4, 50 μmol/l; 5, 100 μmol/l). DHA,
dihydroartemisinin. |
 | Table IOD of MDA-MA-231 cells interfered with
DHA. |
Table I
OD of MDA-MA-231 cells interfered with
DHA.
| OD values at various
time-points |
|---|
|
|
|---|
| DHA, μmol/l | 0 h | 24 h | 48 h | 72 h |
|---|
| 0.00 | 0.325±0.011 | 0.330±0.011 | 0.381±0.008 | 0.483±0.013 |
| 6.25 | 0.325±0.012 | 0.305±0.013 | 0.319±0.006 | 0.402±0.006 |
| 12.50 | 0.325±0.013 | 0.271±0.005 | 0.289±0.011 | 0.391±0.007 |
| 25.00 | 0.325±0.014 | 0.249±0.005 | 0.278±0.003 | 0.345±0.005 |
| 50.00 | 0.325±0.015 | 0.236±0.004 | 0.201±0.005 | 0.304±0.010 |
| 100.00 | 0.325±0.016 | 0.225±0.010 | 0.178±0.010 | 0.264±0.003 |
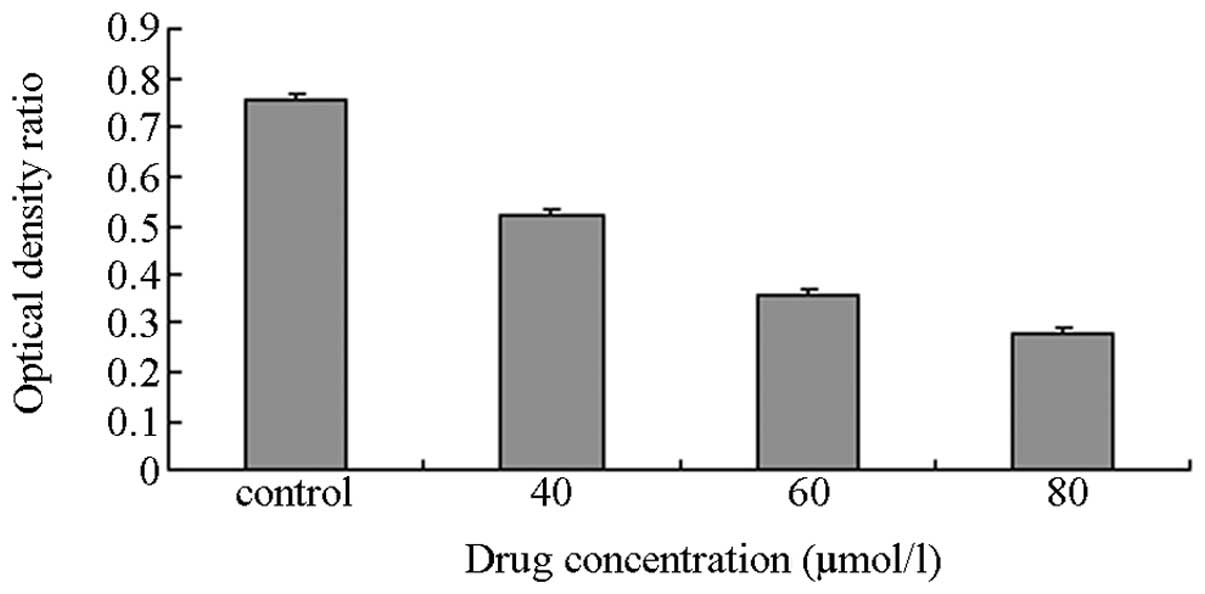
RT-PCR
The IC50 (48 h) value was 60.26±0.12
μmol/l and was taken as the baseline concentration. Thus, 40, 60
and 80 μmol/l DHA were selected as the concentration gradients for
the subsequent experiment. The MDA-MB-231 cells were incubated with
various concentrations of DHA as the experimental groups, whereas
an equivalent amount of DMEM was used for the control group. The
total intracellular RNA was extracted for RT-PCR. The OD ratio was
calculated between the target and reference genes. The results
showed that the OD ratio of the controls was 0.76 (Figs. 3–5).
The OD ratios of the experimental groups with DHA concentrations of
40, 60 and 80 μmol/l, were 0.52, 0.36 and 0.28, respectively. The
Student’s t-test of the experimental groups against the control
exhibited small P-values (P≈0.028), thereby, indicating that the
differences between groups were statistically significant.
Cell migration ability
The cell scratch migration assay evaluated the
migrating ability of MDA-MB-231 cells following treatment with a
40, 60 and 80 μmol/l concentration gradient of DHA in vitro.
At the selected times of 0, 24 and 48 h, the cell migration
distance was observed under an inverted microscope (magnification,
×100) and images of the cells were captured (Fig. 6). As the drug concentration
gradually increased, the migration distance of the MDA-MB-231 cells
was progressively shortened, thereby, indicating the gradual
decrease in the migrating ability of the MDA-MB-231 cells.
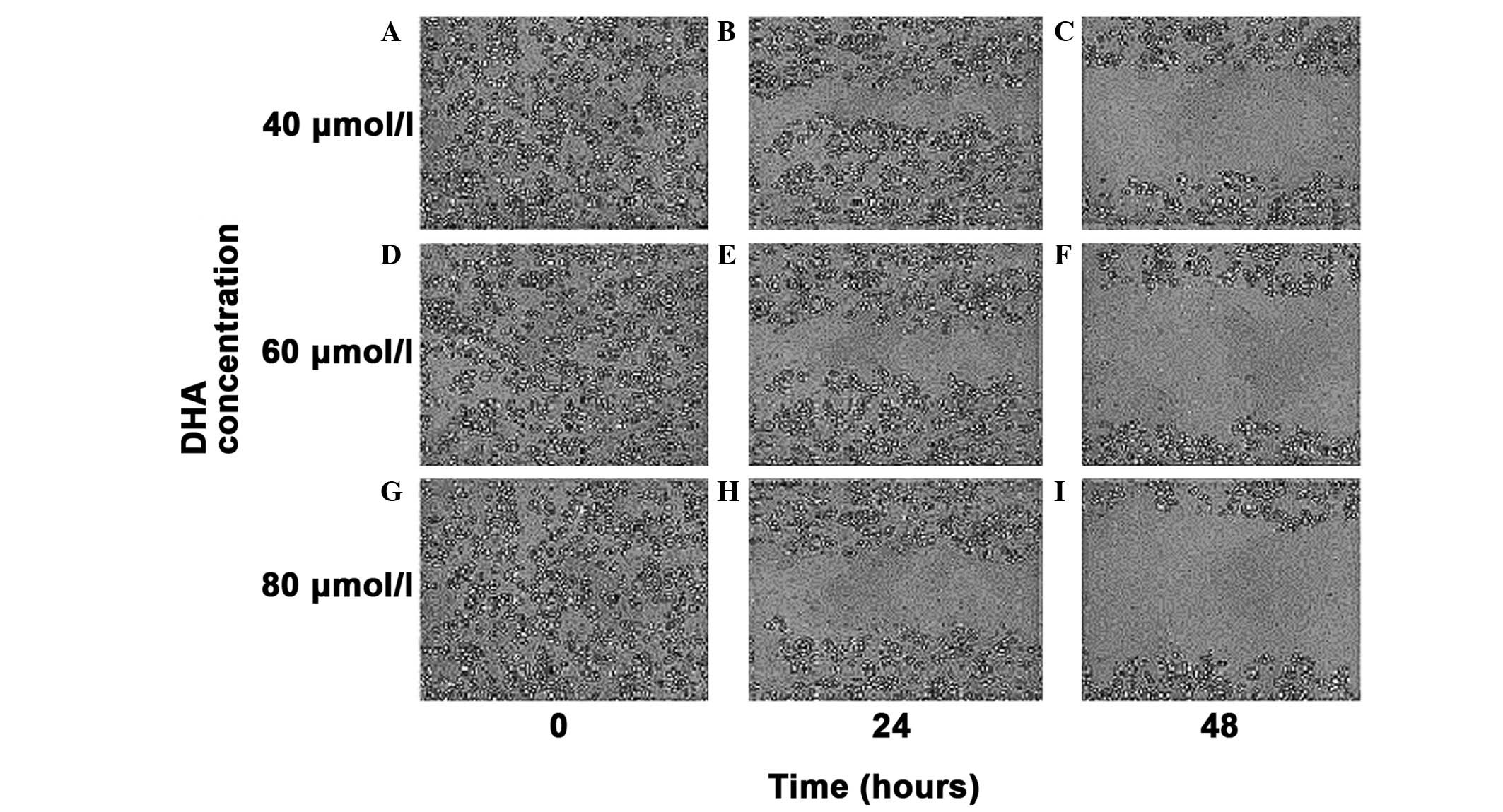 | Figure 6Cell scratch migration assay of the
migratory ability of MDA-MB-231 cells. (A,B,C) Migration distance
of the MDA MB 231 cells with a concentration of DHA of 40 μmol/l
for 0, 24 and 48 h in vitro. (D,E,F) Migration distance of
the MDA MB 231 cells with a concentration of DHA of 60 μmol/l for
0, 24 and 48 h in vitro. (G,H,I) Migration distance of the
MDA MB 231 cells with a concentration of DHA of 80 μmol/l for 0, 24
and 48 h in vitro. Magnification, ×100. |
Discussion
Breast cancer is one of the most common types of
malignancy in females worldwide. As a systemic disease, metastatic
breast cancer is considered incurable, with a median survival time
of 2–3 years. Thus, the development of novel drugs or treatments to
prolong survival or improve patient quality of life is urgently
required. Such developments are likely to make it possible to
maximize the efficacy but minimize the toxicity of these
treatments. The uPA-centered fibrinolytic degradation system is
important during the invasion and metastasis of breast cancer
cells. The elucidation of its specific mechanisms of action are
likely to allow for the possible development of effective drugs to
block this system. Singh and Lai (14) previously found that DHA exhibited no
evident cytotoxic effects on the normal breast HTB-125 cells, but
was extremely toxic to the human breast cancer cell line, HTB-27.
However, the toxicity of DHA may be improved by adding transferrin.
Lai and Singh (15) reported that
weekly oral intake of ART, at a dose of 10 mg/kg, was sufficient to
retard breast cancer development in DMBA-treated rats. Li et
al (16) confirmed that
artesunate delayed liver metastases in nude mice with transplanted
human breast cancer cells.
DHA is an ART analog that is well known for its
excellent antimalarial ability. DHA is a form of traditional
Chinese medicine, with the full intellectual property rights owned
by the Chinese government. The anticancer properties of DHA have
been gradually explored since its identification. However, the
mechanism of its antimetastatic activity in breast cancer remains
unclear. The current study assessed the effects of DHA on breast
cancer cells and on tumor growth and invasion. The results showed
that DHA reduces uPA expression and weakens the metastatic ability
of the breast cancer cell line, MDA-MB-231. To the best of our
knowledge, this is the first report of an ART derivative that may
decrease uPA levels in a human cancer cell line. However, the
associated mechanisms require further study. The following
mechanisms may explanation our results.
On a molecular level, the mitogen-activated protein
kinase (MAPK) signaling pathways and the nuclear transcription
factor, nuclear factor (NF)-κB, have important functions for
regulating uPA gene transcription and protein expression (17). The p38-MAPK signaling pathway
enhances the activity of the uPA promoter, which further
strengthens the uPA protein expression, thereby, improving
the invasion and metastatic ability of cells by activating the
NF-κB expression. De Cremoux et al (18) further analyzed this mechanism at the
gene level and found that the half-life of uPA mRNA was associated
with cell activation by p38-MAPK signaling (phosphorylated p38) in
the highly invasive MDA-MB-231 breast cancer cell lines. The
authors identified that uPA expression and cell invasion ability
were significantly reduced by transfecting SB203580 (a p38-MAPK
signaling inhibitor) into the MDA-MB-231 cells. In addition to the
urokinase system antagonist and inhibitor, novel drugs for
inhibiting MAPK or NF-κB may be useful for the treatment of
malignant tumors. Chen et al (19) previously found that DHA may decrease
the NF-κB content of the pancreatic cancer cell lines, BxPC-3 and
AsPC-1. In addition, Tan et al (20) detected MAPK-related protein
expression using western blot analysis of the ovarian cancer cell
lines, SKOV3 and OVCAR3, following treatment with DHA. The authors
results showed that DHA reduced the phosphorylation levels of
ERK1/2 and inhibited the p38-MAPK pathway. Hwang et al
(17) reported that DHA blocked
phosphorylation in the PKCα/Raf/MAPK pathway, downregulated NF-κB
in fibrosarcoma HT1080 cells and further affected the cell
migration ability.
Thse results indicate that DHA inhibits the
phosphorylation of the MAPK pathway and downregulates NF-κB
expression. However, the direct association between uPA and DHA has
not been confirmed. The results of the present study show that DHA
downregulates the expression of uPA mRNA in the MDA-MB-231 breast
cancer cell line. Similarly, the OD ratio between the target and
reference genes decreased gradually with the increasing
concentration and extended reaction time. The cell scratch
experiment further showed that DHA weakens the migration ability of
cells in vitro. Therefore, the attenuated migration ability
in cells pretreated with DHA indicates the involvement of the uPA
system and highlights novel clues for further investigation of the
use of DHA for breast cancer therapy.
Acknowledgements
The present study was supported by a grants from the
National Natural Science Foundation of China (nos. 81274136 and
81102711) and supported by the Ministry of Education Program for
New Century Excellent Talents of 2011.
References
|
1
|
Giannopoulou I, Mylona E, Kapranou A, et
al: The prognostic value of the topographic distribution of uPAR
expression in invasive breast carcinomas. Cancer Lett. 246:262–267.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Borstnar S, Sadikov A, Mozina B and Cufer
T: High levels of uPA and PAI-1 predict a good response to
anthracyclines. Breast Cancer Res Treat. 121:615–624. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jänicke F, Prechtl A, Thomssen C, et al;
German N0 Study Group. Randomized adjuvant chemotherapy trial in
high-risk, lymph node-negative breast cancer patients identified by
urokinase-type plasminogen activator and plasminogen activator
inhibitor type 1. J Natl Cancer Inst. 93:913–920. 2001.
|
|
4
|
Mani T, Wang F, Knabe WE, et al:
Small-molecule inhibition of the uPAR·uPA interaction: synthesis,
biochemical, cellular, in vivo pharmacokinetics and efficacy
studies in breast cancer metastasis. Bioorg Med Chem. 21:2145–2155.
2013.
|
|
5
|
Harbeck N, Kates RE, Gauger K, et al:
Urokinase-type plasminogen activator (uPA) and its inhibitor PAI-I:
novel tumor-derived factors with a high prognostic and predictive
impact in breast cancer. Thromb Haemost. 91:450–456.
2004.PubMed/NCBI
|
|
6
|
Efferth T, Dunstan H, Sauerbrey A, Miyachi
H and Chitambar CR: The anti-malarial artesunate is also active
against cancer. Int J Onco1. 18:767–773. 2001.
|
|
7
|
Gomme PT, McCann KB and Bertolini J:
Transferrin: structure, function and potential therapeutic actions.
Drug Discov Today. 10:267–273. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lu YY, Chen TS, Qu JL, Pan WL, Sun L and
Wei XB: Dihydroartemisinin (DHA) induces caspase-3-dependent
apoptosis in human lung adenocarcinoma ASTC-a-1 cells. J Biomed
Sci. 16:162009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nakase I, Gallis B, Takatani-Nakase T, et
al: Transferrin receptor-dependent cytotoxicity of
artemisinin-transferrin conjugates on prostate cancer cells and
induction of apoptosis. Cancer Lett. 274:290–298. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Nakase I, Lai H, Singh NP and Sasaki T:
Anticancer properties of artemisinin derivatives and their targeted
delivery by transferrin conjugation. Int J Pharm. 354:28–33. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Oh S, Kim BJ, Singh NP, Lai H and Sasaki
T: Synthesis and anti-cancer activity of covalent conjugates of
artemisinin and a transferrin-receptor targeting peptide. Cancer
Lett. 274:33–39. 2009. View Article : Google Scholar
|
|
12
|
Youns M, Efferth T, Reichling J,
Fellenberg K, Bauer A and Hoheisel JD: Gene expression profiling
identifies novel key players involved in the cytotoxic effect of
Artesunate on pancreatic cancer cells. Biochem Pharmacol.
78:273–283. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wang Z, Yu Y, Ma J, et al: LyP-1
modification to enhance delivery of artemisinin or fluorescent
probe loaded polymeric micelles to highly metastatic tumor and its
lymphatics. Mol Pharm. 9:2646–2657. 2012. View Article : Google Scholar
|
|
14
|
Singh NP and Lai H: Selective toxicity of
dihydroartemisinin and holotransferrin toward human breast cancer
cells. Life Sci. 70:49–56. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lai H and Singh NP: Oral artemisinin
prevents and delays the development of
7,12-dimethylbenz[a]anthracene (DMBA)-induced breast cancer in the
rat. Cancer Lett. 231:43–48. 2006.PubMed/NCBI
|
|
16
|
Li PC, Lam E, Roos WP, Zdzienicka MZ,
Kaina B and Efferth T: Artesunate derived from traditional Chinese
medicine induces DNA damage and repair. Cancer Res. 68:4347–4351.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hwang YP, Yun HJ, Kim HG, Han EH, Lee GW
and Jeong HG: Suppression of PMA-induced tumor cell invasion by
dihydroartemisinin via inhibition of PKCalpha/Raf/MAPKs and
NF-kappaB/AP-1-dependent mechanisms. Biochem Pharmacol.
79:1714–1726. 2010. View Article : Google Scholar
|
|
18
|
De Cremoux P, Grandin L, Diéras V, et al;
Breast Cancer Study Group of the Institut Curie. Urokinase-type
plasminogen activator and plasminogen-activator-inhibitor type 1
predict metastases in good prognosis breast cancer patients.
Anticancer Res. 29:1475–1482. 2009.PubMed/NCBI
|
|
19
|
Chen H, Sun B, Wang S, Pan S, Gao Y, Bai X
and Xue D: Growth inhibitory effects of dihydroartemisinin on
pancreatic cancer cells: involvement of cell cycle arrest and
inactivation of nuclear factor-kappaB. J Cancer Res Clin Oncol.
136:897–903. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Tan XJ, Plouet J, Lang JH, Wu M and Shen
K: Effects of dihydroartiminisin on proliferation and
phosphorylation of mitogen-activated protein kinase in epithelial
ovarian cancer cell lines. Zhonghua Fu Chan Ke Za Zhi. 43:662–665.
2008.(In Chinese).
|