Introduction
Low-frequency ultrasound (US) is usually referred to
as ultrasound with frequencies in the range of 20–100 kHz (1–2). The
relatively long wavelengths indicate that the low-frequency
ultrasonic wave is affected by larger obstacles than high-frequency
US in its passage through the propagation medium. This results in a
lower spatial resolution, and the low-frequency range is therefore
less useful for diagnostic medical applications. However, it has a
diverse set of industrial and medical applications (2). In fact, the industrial applications of
US mainly occupy this frequency range (3–4). The
bioeffects of low-frequency US include thermal and cavitational
effects and other ‘mechanical’ effects, including acoustic
micro-streaming and radiation forces, among which cavitation is
generally accepted as the most significant mechanism (5).
As a broad definition, acoustic cavitation is the
process by which any of the following occurs: i) The pulsation or
growth of small gas bubbles already present in a liquid; ii) the
formation of gas bubbles in the bulk or on nuclei as a result of
acoustic pressure variations; or iii) other types of growth,
splitting or interactions of gas bubbles in solution caused by
acoustic pressure oscillations (1).
Acoustic cavitation is further divided into stable and transient
types. The pulsation of cavitation bubbles over numerous acoustic
pressure cycles without collapse is known as stable cavitation
(6), whereas transient cavitation
is rapid and uncontrolled bubble growth over several pressure
cycles, leading to the eventual collapse into smaller bubbles
(1).
For inertial decavitation, bubbles have more time to
grow by rectified diffusion in the expansion half cycle when using
a lower frequency wave. Therefore, it can be hypothesized that at
lower US frequencies, transient cavitation will have a more
significant effect (2,7).
The permeability of individual cells for an improved
delivery of drugs and genes can be achieved by inertial cavitation
(collapsing bubbles). This process is known as sonoporation
(8); sound energy is used to create
pores and as a result enhance the permeability of plasma
membranes.
To further our understanding of cavitation-based
mechanisms, to optimize intracellular uptake, to control bioeffects
and to advance techniques for clinical applications, previous
US-enhanced delivery studies have focused on delivery into in
vitro cells in suspension (9–10) and
more recently into tissues of in vivo animal models. The
threshold for the onset of in vivo cavitation depends on the
presence of cavitation nuclei of appropriate size (11). The natural occurrence of bubbles has
only been observed in the digestive and respiratory tracts, but not
elsewhere for in vivo blood (12). Without cavitation nuclei in the
blood, the occurrence of cavitation at low acoustic pressures in
vivo is dependent on the injection of stabilized bubbles. The
objective of the present study was to associate the bioeffects of
tumor-bearing nude mice exposed by 21-kHz US and contrast agent
bubbles injected from the tail veins of mice.
Materials and methods
Animal protocol
In total, 25 male nude mice, aged 4 weeks old and
weighing 15–18 g, were purchased from the Animal Center of the
Shanghai Institute of Chinese Academy of Science (Shanghai, China).
All mice were treated and housed according to approved guidelines
(Guidelines for the Care and Use of Laboratory Animals). Following
anesthesia by intraperitoneal injection of 0.004 g ketamine, the
mice were secured to a superclean bench according to the principles
of aseptic procedures. Following local sterilization, each mouse
was then subcutaneously inoculated with 2×106 cells from
the DU145 cell line into the flank. The mice continued to be raised
under specified pathogen-free conditions subsequent to the
procedure, and were observed at 2-day intervals. Experiments were
initiated 2 weeks later, once the tumors had reached a size of 5–8
mm. This study was approved by the ethics committee of Shanghai
Jiao Tong University Affliated Sixth Hospital, (Shanghai,
China).
Experimental groupings for tumor therapy
and experimental protocol
In total, 15 nude mice, each with a subcutaneous
tumor of 6 mm in size, were randomly divided into three groups,
with five mice in each group. These groups were as follows: The A
group, negative control (sham treatment); the B group,
low-frequency US; and the C group, US+microbubbles (MB). The MBs
used were from a US contrast agent (SonoVue, Bracco Imaging SpA,
Milan, Italy). The mice were anesthetized by intraperitoneal
injection of 0.3 ml 1% pentobarbital sodium. Following successful
anesthesia, the tumor xenografts were subsequently sonicated using
a transducer (Fig. 1), manufactured
in the Shanghai Institute of Ultrasound in Medicine at Shanghai
Jiaotong University (Shanghai, China), and placed on the skin with
contact gel (Aquasonic 100; Parker Laboratories, Inc., Fairfield,
NJ, USA). The diameter of the therapeutic US transducer was ~13 mm,
which covered the entire tumor. Low-frequency US parameters were
set at 21 kHz, 26 mW/cm2, a duty cycle of 40% (2 sec on,
3 sec off) and a duration of 3 min once every other day for two
weeks, which was the same as our previous study (13).
Color Doppler flow imaging (CDFI)
CDFI is a useful tool for assessing tumor
neovascularity and also for monitoring anti-angiogenic therapies.
At the initiation (0 weeks) and completion (2 weeks) of the present
experiment, the subcutaneous prostate cancer of the nude mice was
examined by CDFI. CDFI images of the tumors were obtained using a
Mylab90 instrument (Esaote, Genoa, Italy) handled by an experienced
examiner. The frequency of the probe used was 15 MHz. For the
present study, the sensitivity of the instrument was set at a low
velocity in order to display a low blood flow signal. Only the
intratumoral blood signal was evaluated.
Histological examination
At the end of the experiment, the tumor size of each
mouse was calculated using a calibrator, and then the mice were
euthanized and the tumors collected, fixed and embedded in
paraffin. Sections were taken from the middle region of each tumor,
followed by hematoxylin and eosin (HE) staining and subsequent
light microscopy. A histopathologist blinded to the study evaluated
the microscopy findings.
Western blotting assays
The mice were sacrificed at the end of the
experiment and the tumors were collected. The detection of the
protein expression of cyclooxygenase (COX)-2 and vascular
endothelial growth factor (VEGF) was assessed using the western
blot assay. The following primary antibodies were used: goat
polyclonal anti-COX-2 and goat polyclonal anti-VEGFB antibodies
(1:500 dilution; Santa Cruz Biotechnology, Inc., Santa Cruz, CA,
USA). The cancerous tissues were lysed in radioimmunoprecipitation
assay buffer (150 mM NaCl, 100 mM Tris-HCl, 1% Tween-20, 1% sodium
deoxycholate and 0.1% SDS) with 0.5 mM EDTA, 1 mM
phenylmethanesulfonyl fluoride, 10 μg/ml aprotinin and 1 μg/ml
pepstatin. Proteins were subjected to SDS-PAGE and transferred to
polyvinylidene fluoride membranes, which were then treated with the
primary and secondary antibodies (goat anti-mouse IgG horseradish
peroxidase-conjugated, 1:500 dilution, Santa Cruz Biotechnology,
Inc.). Visualization was carried out using an enhanced
chemiluminescence method (Amersham Bioscience, Boston, MA, USA).
Subsequent to being stripped, the membranes were reprobed with
β-actin (Oncogene, CN Biosciences, Inc., Darmastadt, Germany). The
proteins were quantified using an Image Acquisition and Analysis
System (Ultra-Violet Products, Upland, CA, USA).
Transmission electron microscopy
(TEM)
For the TEM analysis, each tumor sample (~1
mm3) was fixed in 2% glutaraldehyde and
phosphate-buffered saline (PBS) for 2 h at 4°C, and then washed
twice with PBS buffer for 10 min. Following treatment with 1%
osmium tetroxide in PBS, the specimens were fixed in 4°C for 2 h
and dehydrated with 30%, 50% and then 70% ethanol three times each
for a duration of 10 min. The samples were then embedded in
propylene oxide for 2 h and stained with lead citrate E. Finally,
subsequent to sectioning, the specimens were examined using TEM
(Philips CM-120; Philips, Eindhoven, The Netherlands).
Statistical analysis
The statistical analysis was performed using SPSS,
version 11.0 (SPSS Inc., Chicago, IL, USA). Student’s t-test was
used to make a statistical comparison between groups. All testing
was carried out using Prism 3.0 (GraphPad, San Diego, CA, USA).
Error bars represent the standard error above the mean. P<0.05
was considered to indicate a statistically significant
difference.
Results
CDFI
Prior to the treatment, CDFI demonstrated a blood
flow signal within all the tumors of the three groups. In the US+MB
group only, the blood flow signal disappeared following 2 weeks of
treatment, while in the control and US group, the flow signal in
the tumors remained (Fig. 2).
Tumor size calculation
The tumor size of the three groups is manifested in
Figs. 2 and 3. There were significant differences in
tumor size among the three groups, as determined using the ANOVA
test; F=8.418 and P=0.0052. There was a significant difference
between the US+MB group and the control and US groups, with t=3.804
and P=0.0052, and t=3.117 and P=0.0143, respectively (Figs. 2 and 3).
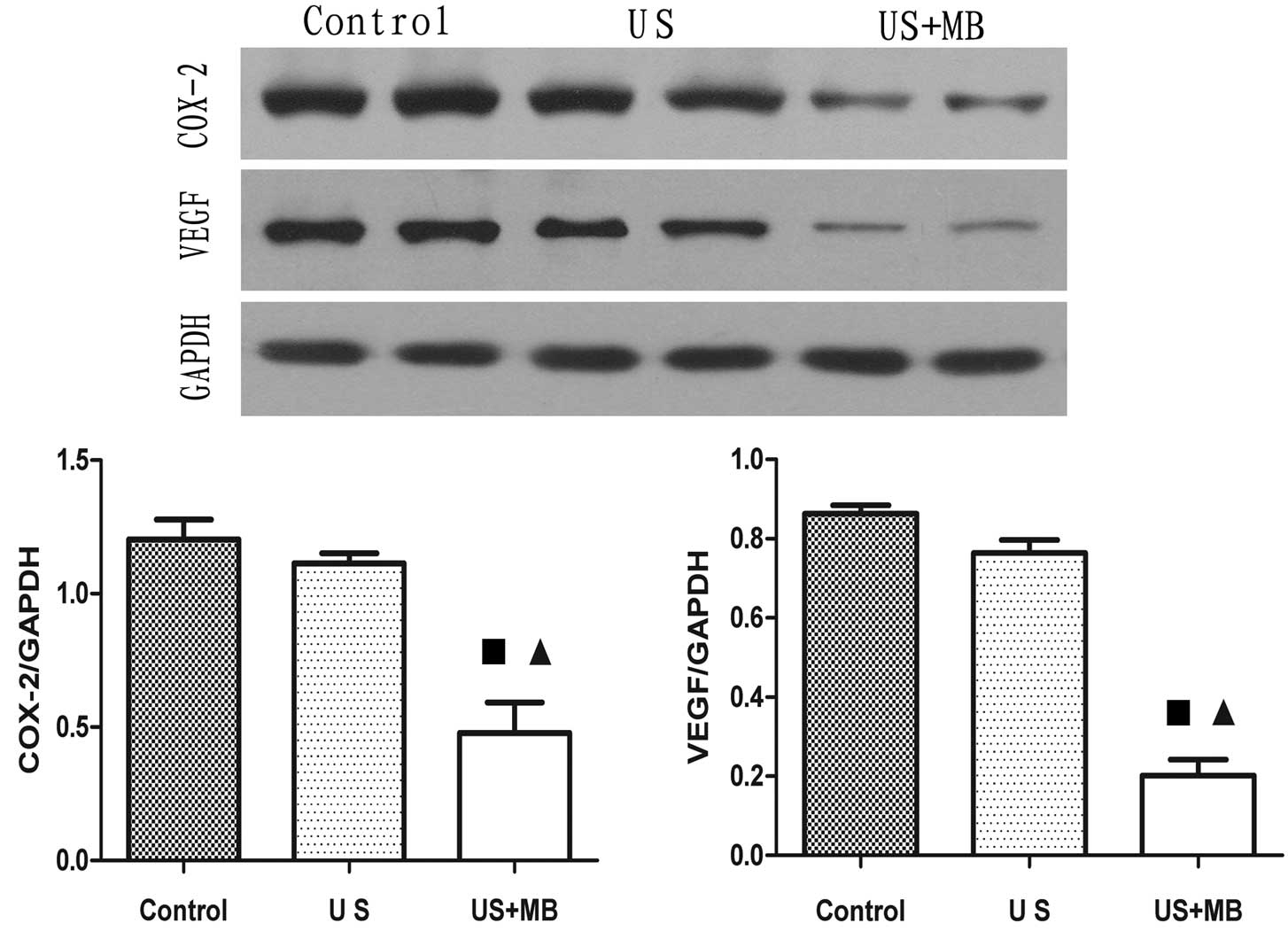
Western blotting assays results
The mean intensity values for COX-2 in the vascular
endothelial cells and cytoplasm in the control, US and US+MB groups
were 1.203±0.074, 1.114±0.036 and 0.4783±0.114, respectively. There
was a significant difference between the US+MB group and the
control and US groups, with t=5.338 and P=0.0007, and t=5.303 and
P=0.0007, respectively.
The mean intensity values for VEGF in the vascular
endothelial cells and cytoplasm in the control, US and US+MB groups
were recorded as 0.863±0.021, 0.764±0.033 and 0.202±0.041,
respectively. There was a significant difference between the US+MB
group and the control and US groups, with t=14.59 and P<0.0001,
and t=10.8 and P<0.0001, respectively (Fig. 4).
HE staining results
In the control and US groups, the tumor cells were
intact, with nuclei that were abnormal, large and deeply stained.
However, in the US+MB group, the tumor cells in the exposed area
presented with coagulative necrosis and the nuclei disappeared
(Fig. 5).
TEM results
TEM revealed apparent cytoplasmic vacuolation and
dilatation of perinuclear cisternae in the tumor cells, and
vascular lumen occlusion in the US+MB group. The majority of tumor
cells were identified as normal in the other two groups. Intact
vascular lumens and normal erythrocytes in the tumor vessels were
also found in the control and US groups (Fig. 5).
Discussion
In this era of US research, several novel
applications of US for therapy are undergoing intensive
investigation and development. MB-based therapeutic strategies are
under study for US-directed and targeted therapy. In the present
study, low-frequency, low-energy US aided by stabilized MBs was
used for the treatment of nude mouse tumors.
This procedure caused substantial destructive
effects on the tumor cells, which was evidenced by coagulation
necrosis (Fig. 5) and apparent
tumor shrinkage (Figs. 2 and
3) compared with the two other
groups. In this strategy, the external US exposure activates MBs as
cavitation nuclei in the circulation at a desired site of
treatment. The addition of US contrast agents, as a source of
cavitation nuclei during exposures, renders cavitation activity
more predictable and also lowers the intensity threshold for its
onset (14). US fields produce a
varying local pressure, which causes gas-filled bubbles to expand
and contract due to their high compressibility, and these
volumetric oscillations are significant in their effectiveness in
therapeutic applications (11). In
the present study, tumor destruction was apparently due to inertial
cavitation. US combined with MB caused a decrease in tumor growth.
It is worth noting that the same exposures without MBs did not
cause significant tumor size shrinkage compared with the sham
control. Tumor growth inhibition may be the result of US-mediated
bioeffects by low-frequency US sonication with MBs.
Angiogenesis is the development of new blood
vessels. For the growth of tumors and the formation of metastases,
new blood vessels are required. Neovascularization can be
identified inside the tumor and in the peritumoral tissue (15). Visualization of tumor vascularity
(16) can be demonstrated by CDFI,
and it is an established technique for the evaluation of
anti-neovascular effects (17). In
animal models, CDFI can track the response of tumors to
chemotherapy and radiotherapy (17). Prior to the treatment in the present
study, the blood flow signals were all identified within the tumors
in the control, US and US+MB groups. However, following treatment,
the intertumoral flow signal disappeared in the US+MB group, while
it remained in the control and the US groups (Fig. 2). It is known that anti-angiogenic
agents can inhibit angiogenesis (18). Vascular targeting agents, including
drugs and vascular disrupting agents, aim to inhibit new
vasculature growth or destroy existing vasculature, respectively.
It is possible that a low frequency ultrasound combined with MBs
may have specific anti-angiogenic effects.
In order to evaluate the results of the treatment,
western blotting assays were used to detect angiogenesis-associated
gene proteins, including VEGF and COX-2, in the tumor tissue. VEGF
and COX-2 are associated with carcinogenesis due to the stimulation
of cell proliferation, the inhibition of apoptosis and the
enhancement of angiogenesis (19).
The inhibition of VEGF and COX-2 is conceivably an attractive
therapeutic target in the treatment of cancer. The results of the
present study showed that following treatment, VEGF and COX-2 gene
expression in the US+MB group was lower compared with the control
and US groups, which was consistent with our previous study
(13). Tumor sonication following
intravenous injection of MBs could downregulate
angiogenesis-associated gene proteins in nude mouse tissues.
Using TEM in the present study, intact vascular
lumens and normal erythrocytes were observed in the blood vessels
following treatment in the control and US groups (Fig. 5, arrowhead). However, following US
treatment in the presence of MBs, degeneration of the endothelial
cells and lumen occlusion were observed in these vessels, which
indicates that the effect of US+MB is different from that of the
other two groups. The tumor cell changes that were observed
included cytoplasmic vacuolation and dilatation of the perinuclear
cisternae in the US+MB group. US in combination with the contrast
agent resulted in apparent damage to the blood vessels and tumor
cells in the cancer of the nude mice. The results of the present
study, indicate that further study is required into the underlying
mechanism responsible for these effects.
MBs, which are artificially augmented cavitation
nuclei, play a significant role in the treatment of murine tumors
during anti-vasculature therapy. A significant factor that will
determine whether cavitation will or will not occur is the
available physical space for bubbles to form and grow. The
induction of cavitation within intact cells and in the
extracellular matrix is difficult (8). However, when a high enough in
vitro US pressure fields exists, the vasculature possesses
injected cavitation nuclei and the required dimensions for the
initiation of cavitation. In the US group of the present study, the
reason that tumor growth inhibition was not obvious is due to the
dearth of cavitation nuclei and a lack of physical space for bubble
oscillation and expansion in the tumoral interstitium. The addition
of MBs has also been found to decrease the intensity threshold for
producing damage in treated vessels (20).
Insonified by low-frequency US pressure, bubbles
become unstable, grow, oscillate and collapse in blood vessel
fields, and this phenomenon is referred to as the acoustic
cavitation bioeffect. Damage to nearby biological cells and
structures, including vascular endothelia and vessel lumens, can
occur due to the concentration of acoustical energy and its
conversion into local mechanical perturbation during cavitation.
When US exposes the tumors following an injection of MBs, the
interaction of the US beam with the MBs in the blood vessels
results in the expansion, oscillation and collapse of the bubbles,
followed by vicinal blood vessel distention, invagination and
deformation (21–22). Bubble expansion significantly
distends the vessel to ~2.7 times its original diameter (21), followed by bubble collapse at the
msec phase leading to almost axially symmetric vessel invagination.
During invagination, the notch-like shape on the sides of the
vessel wall indicate high strains on the vessel wall. Invagination,
which generates higher strains on the vessel wall than distention,
was commonly observed in the present study when bubbles collapsed
near the vessel wall, which pulled the vessel inward toward the
lumen. We hypothesize that the cumulative effects of vessel
invagination produced by substantial MB fragmentation irradiated by
21-kHz US had a tendency to lead to vascular stenosis, and that the
long-term effects of this will eventually lead to vascular
occlusion. More in-depth studies are required to prove that this
proposed mechanism is conclusive. In the present study, it was
observed that the lumen occlusion of a vessel, leading to a
decreased blood supply, may be the major reason for the
disappearance of the blood flow signal on CDFI, for the decline in
COX-2 and VEGF expression in western blotting assays and for the
inhibition of tumor growth after a 14-day treatment.
There were certain limitations to the present study.
First, there was a lack of a MB-only group. However, it was assumed
that the diameter of the bubbles was ~2.5 μm, which was smaller
than the red blood cells, but larger than the vascular endothelial
gap. Thus, the bubbles seldom penetrated into the tissue spaces.
Therefore, in the MB only group, these bubbles had little effect on
the vascular endothelia and tumor tissues. Second, potential
adverse effects on blood vessels in normal tissues were not
investigated in the US+MB group and thus require further
exploration in the future.
Synergistic effects were found when combining the
low-frequency US exposures with the agents with regard to apparent
lumen occlusion of the micro-vessel walls, decreased regulation of
VEGF and COX-2 and increased tumor regression of targeted tumors.
The mechanisms of the anti-tumor effects are complex and may be
mediated by acoustic cavitation. However, in vivo cavitation
bioeffects were determined by several experimental acoustic
parameters, including pressure, exposure time, frequency and MB
concentration. Elucidation of the mechanism by which the
interactions between the bubbles, low-frequency US wave and blood
vessels create these bioeffects is required. This will ultimately
be achieved by continuing to collect in vivo experimental
data, along with continuing to develop appropriate experimental
apparatus, which together will enable more efficient optimization
of the treatments with regard to the multiple exposure parameters
that may be selected. In addition, by having a comprehensive
understanding of the way in which the acoustic and physical
characteristics of the tissues are involved in these mechanisms,
more challenges remain prior to the combination of low-frequency US
and MBs becoming a realistic clinical therapy.
Acknowledgements
This study was supported in part by the National
Natural Science Foundation of China (81271597) and the Key Basic
Research Project of Shanghai Science and Technology Commission
(10JC1412600). The authors would like to thank Dr Mao Xin and Dr
Xie Guo Ming for helping with the tumor cell culture and tumor
inoculation.
References
|
1
|
Polat BE, Hart D, Langer R and
Blankschtein D: Ultrasound-mediated transdermal drug delivery:
mechanisms, scope, and emerging trends. J Control Release.
152:330–348. 2011. View Article : Google Scholar
|
|
2
|
Ahmadi F, McLoughlin IV, Chauhan S and
ter-Haar G: Bio-effects and safety of low-intensity, low-frequency
ultrasonic exposure. Prog Biophys Mol Biol. 108:119–138. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
de La Rochebrochard S, Naffrechoux E,
Drogui P, Mercier G and Blais JF: Low frequency ultrasound-assisted
leaching of sewage sludge for toxic metal removal, dewatering and
fertilizing properties preservation. Ultrason Sonochem. 20:109–117.
2013.
|
|
4
|
Golmohamadi A, Möller G, Powers J and
Nindo C: Effect of ultrasound frequency on antioxidant activity,
total phenolic and anthocyanin content of red raspberry puree.
Ultrason Sonochem. 20:1316–1323. 2013. View Article : Google Scholar
|
|
5
|
Gogate PR and Kabadi AM: A review of
applications of cavitation in biochemical
engineering/biotechnology. Biochem Eng J. 44:60–72. 2009.
View Article : Google Scholar
|
|
6
|
Carvell KJ and Bigelow TA: Dependence of
optimal seed bubble size on pressure amplitude at therapeutic
pressure levels. Ultrasonics. 51:115–122. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ueda H, Mutoh M, Seki T, Kobayashi D and
Morimoto Y: Acoustic cavitation as an enhancing mechanism of
low-frequency sonophoresis for transdermal drug delivery. Biol
Pharm Bull. 32:916–920. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Frenkel V: Ultrasound mediated delivery of
drugs and genes to solid tumors. Adv Drug Deliv Rev. 60:1193–1208.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Delalande A, Kotopoulis S, Postema M,
Midoux P and Pichon C: Sonoporation: mechanistic insights and
ongoing challenges for gene transfer. Gene. 525:191–199. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Delalande A, Postema M, Mignet N, Midoux P
and Pichon C: Ultrasound and microbubble-assisted gene delivery:
recent advances and ongoing challenges. Ther Deliv. 3:1199–1215.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Stride EP and Coussios CC: Cavitation and
contrast: the use of bubbles in ultrasound imaging and therapy.
Proc Inst Mech Eng H. 224:171–191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hitchcock KE: Ultrasound-enhanced drug
delivery in a perfused ex vivo artery model. PhD dissertation.
University of Cincinnati. ProQuest, Publication no. AAI3419966.
Cincinnati, OH: 2010
|
|
13
|
Shen ZY, Shen E, Zhang JZ, et al: Effects
of low-frequency ultrasound and microbubbles on
angiogenesis-associated proteins in subcutaneous tumors of nude
mice. Oncol Rep. 30:842–850. 2013.PubMed/NCBI
|
|
14
|
Bhadane S: High intensity focused
ultrasound and microbubble induced tissue ablation: Effect of
treatment parameters on thermal lesion volume and temperature
(unpublished MSc thesis). Ryerson University; Toronto, ON: 2012
|
|
15
|
Shen ZY, Hu B and Wu MF: Correlation
between blood flow signal of color flow imaging and nottingham
prognostic index in patients with breast carcinoma. Breast Care
(Basel). 7:126–130. 2012. View Article : Google Scholar
|
|
16
|
Sehgal CM, Weinstein SP, Arger PH and
Conant EF: A review of breast ultrasound. J Mammary Gland Biol
Neoplasia. 11:113–123. 2006. View Article : Google Scholar
|
|
17
|
Cosgrove D: Angiogenesis imaging -
ultrasound. Br J Radiol. 76:S43–S49. 2003. View Article : Google Scholar
|
|
18
|
Kerbel R and Folkman J: Clinical
translation of angiogenesis inhibitors. Nat Rev Cancer. 2:727–739.
2002. View
Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghosh N, Chaki R, Mandal V and Mandal SC:
COX-2 as a target for cancer chemotherapy. Pharmacol Rep.
62:233–244. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Huber PE, Mann MJ, Melo LG, et al: Focused
ultrasound (HIFU) induces localized enhancement of reporter gene
expression in rabbit carotid artery. Gene Ther. 10:1600–1607. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chen H, Brayman AA, Bailey MR and Matula
TJ: Blood vessel rupture by cavitation. Urol Res. 38:321–326. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Chen H, Brayman AA and Matula TJ: Imaging
targeted microbubble interactions with microvessels. In:
Ultrasonics Symposium (IUS), IEEE; San Diego, CA. pp. 1117–1120.
2010
|