Introduction
Adenoid cystic carcinomas (ACCs) are rare malignant
tumors of the breast. Although most frequently noted in the
salivary glands, ACCs are common in the uterine cervix, skin,
lungs, kidneys, esophagus and prostate. These tumors occur in
<0.1% of all patients diagnosed with breast cancer. The age
distribution is from 19–97 years, and the condition is more common
in the 50 to 60-year-old age group (1–3).
Typically, the tumors present as a subareolar mass or as pain in
the breast (4,5). The involvement of lymph nodes and
distant metastasis are extremely rare. ACC of the breast shares the
same histological characteristics with ACC of the salivary gland.
The prognosis of ACC of the breast is improved in comparison to
other pathological types of breast cancer and ACC of the salivary
gland (1,6). High survival rates following
mastectomy or breast protective surgery have been reported
previously (7).
ACC of the breast has a biphasic pattern.
Histologically, it consists of small basaloid cells with a solid
cribriform pattern or epithelial cells with a tubular growth
pattern. Although the exact origins remain unknown, it is estimated
that these tumors originate from the ductal epithelium or
myoepithelium. The presence of estrogen and progesterone receptors
tends to be negative in these tumors (1). ACC has an excellent prognosis, a low
local recurrence and a rare distant metastasis (8,9).
The aim of the present study was to report the case
of a patient who was admitted to the the Department of Radiation
Oncology (Karadeniz Technical University, Faculty of Medicine,
Trabzon, Turkey) with a diagnosis of ACC of the breast, and review
the clinical presentation in light of existing literature. Patient
provided written informed consent.
Case report
A 58-year-old postmenopausal patient was admitted to
the Department of General Surgery (Karadeniz Technical University,
Faculty of Medicine, Trabzon, Turkey) with complaints of pain in
the outer quadrant of the right breast. The patient has two
children and nothing particularly noteworthy in the personal
medical and family histories. The family history was negative for
breast cancer and the patient did not smoke or consume alcohol. The
breast examination revealed a lump under the upper outer quadrant
of the right breast, ~1 cm in diameter. The lump was tough and
mobile, however, there was no erythema, ecchymosis, skin ulceration
or dimpling identified. No axillary lymphadenopathy was detected
and there were no positive findings in the laboratory examinations.
On mammography, the patient was noted to have a dense mass with
spicular extensions in the upper outer quadrant of the right
breast. The mass was reported with a Breast Imaging-Reporting and
Data System score of 4, and the lesion was therefore removed by
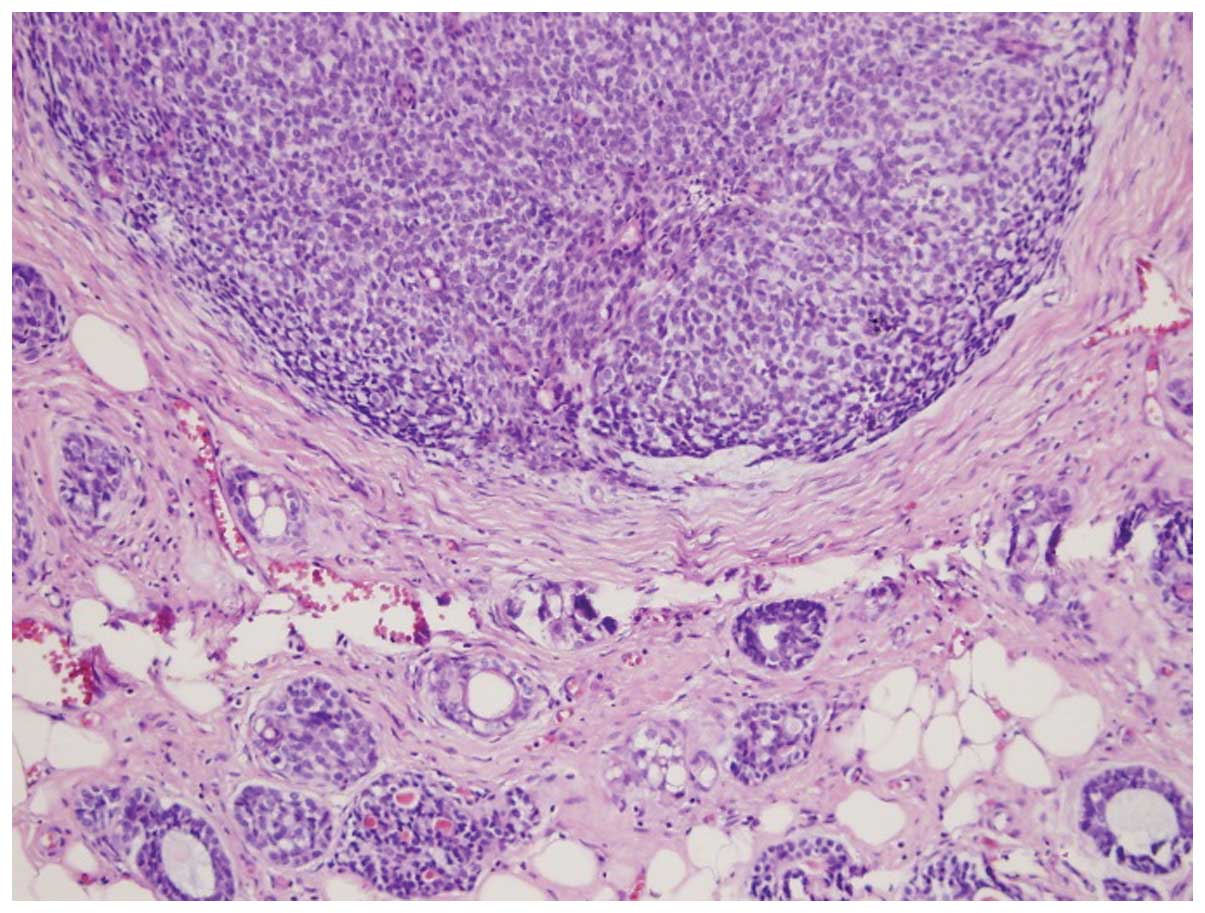
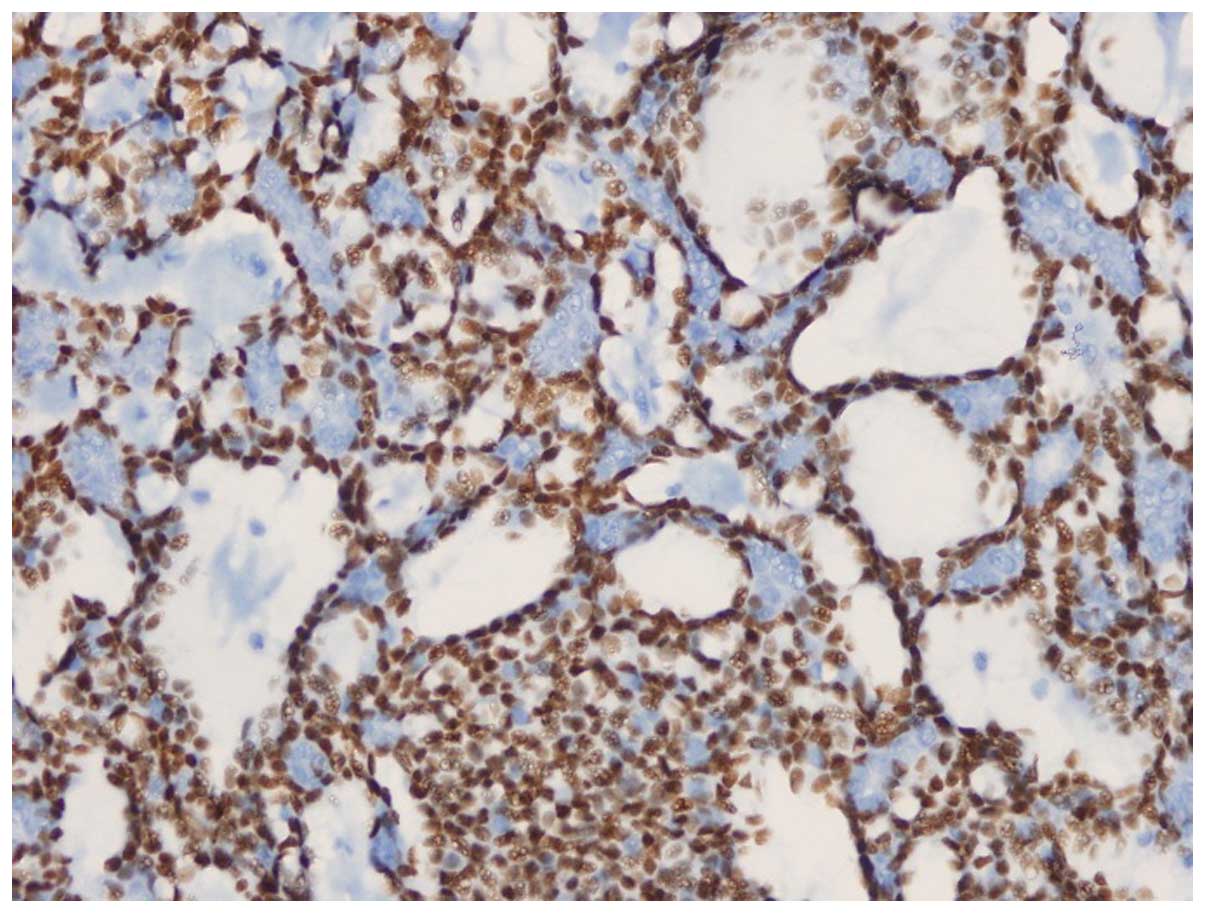
excisional biopsy. The pathology report demonstrated 1×1-cm and
1×0.5-cm ACCs in the form of two close foci, with <30% solid
pattern and positive results for cluster of differentiation 56
(CD56) focal immunoreactivity, smooth muscle actin, CD117, high
molecular weight keratin and estrogen receptor (2%). The ACCs also
had a Ki-67 score of 30%, diffuse nuclear p53 staining, perineural
invasion and positive surgical margins. The biopsy specimen was
negative for human epidermal growth factor receptor 2 (Her-2/neu),
chromogranine, lymphovascular invasion, synaptophysin and the
progesterone receptor (Figs. 1 and
2).
The pre-operative abdominal and thoracic tomography
showed no distant metastasis. Subsequently, the patient underwent
breast conservation surgery and sentinel lymph node dissection. The
pathology from the second surgery depicted ACC in the form of a
microscopic foci around the initial surgical cavity, with two
reactive sentinel lymph nodes and the closest negative margin at 2
mm. Therefore, the patient was treated with a dose of 50 Gy, with a
fraction of 2 Gy on a daily basis from two opposing parallel
tangential fields post-operatively, using 6-MV photon beams.
Following whole breast radiotherapy, a 10-Gy boost on the tumor bed
with 12-MeV electron beams was also delivered. Finally, 10 mg
tamoxifen was administered to the patient twice daily for 20 months
following radiotherapy. No recurrence and metastasis were
identified in the patient at month 20 of the follow-up period.
Discussion
ACC occurs in one in 1 million females every year
(7) and was initially described as
a cylindroma by Billroth in 1856 (10). Breast ACC was first described by
Geschickter in 1945 (11). ACC has
three varied growth patterns: Glandular, tubular and solid. Ro
et al (12) divided the
disease into three categories depending on the degree of solid
structure in the tumor. Based on this categorization, the grade of
the tumor increases with an increased rate of solid element: Grade
1, numerous glands and cystic components, without solid components;
grade 2, <30% solid components; and grade 3, >30% solid
components. According to this grading system, local excision is
recommended for grade 1, simple mastectomy is recommended for grade
2, and mastectomy and axillary dissection is recommended for grade
3. In the present case, the patient underwent breast conservation
surgery and sentinel lymph node dissection due to the presence of
high-grade disease.
ACCs are commonly detected in postmenopausal females
and in the geriatric population (1). However, there are isolated case
studies of ACCs occurring in males and children reported in the
literature (13). The left and
right breasts are equally affected and there is no tendency for the
occurrence to be bilateral. ACC is frequently localized in the
subareolar upper outer quadrant of the breast and is generally
multifocal. Although the tumor size is usually 2–3 cm, the existing
literature contains cases with a tumor size of 15 cm. The most
frequent symptoms at presentation include the finding of a
well-circumscribed palpable mass in the breast, pain in the breast
and nipple retraction (1). The
postmenopausal patient of the present study complained of breast
pain in concordance with the literature, and the tumor, again in
agreement with the literature, was localized in the upper outer
quadrant and was multifocal.
Perineural invasion is common in ACCs of the
salivary gland and this is believed to be the underlying cause for
the symptom of pain in these patients. However, perineural invasion
is extremely rare in ACCs of the breast (14). In the present case, perineural
invasion was present. By mammography, the tumor is characterized as
a well-circumscribed lobulated mass that presents with extremely
rare microcalcifications, and is associated with hypoechoic lesions
on ultrasonography. ACC is generally negative for the estrogen and
progesterone receptors and Cerb-B2. Notably, although it has a
triple-negative pattern, it does not clinically behave like a
triple-negative breast tumor (15).
This is explained by the downregulation of genes involved in
migration, proliferation and the immune response (1). In the present case, the progesterone
receptor and Cerb-B2 were negative, and the estrogen receptor was
positive at 2%, which is in agreement with the existing
literature.
There is no consensus regarding the optimal
treatment of ACCs of the breast due to the rare occurrence of these
tumors. Surgical approaches range from local excision to mastectomy
(14). Recurrence rates ranging
from 6–37% were reported following local excision (16–17).
Since extremely few recurrences were reported following mastectomy,
numerous clinicians recommend mastectomy for a diagnosis of ACC of
the breast. However, there is no randomized controlled trial
comparing breast conserving surgery to mastectomy. Recent studies,
however, have begun to report higher rates of lumpectomy (18), although, no information about
surgical margins has been reported in the majority of patients. The
role of radiotherapy following breast conservation surgery remains
unclear. Data for the role of radiotherapy in female breast ACCs
are limited. There are few studies containing a substantial number
of patients receiving adjuvant radiotherapy (12). Arpino et al (19) reported that 14 out of 182 patients
in a series experienced local recurrence and that 78% of them
developed recurrence following local excision. None of these cases
received radiotherapy. It was argued that no recurrence occurred in
any of the 22% of patients who received radiotherapy in the series,
therefore, mastectomy should be avoided. In a study by the Rare
Cancer Network (RCN), 66% of 61 patients received post-operative
radiotherapy, which was associated with an improvement in local
regional control by 12% in five years (95% for the group with
post-operative radiotherapy vs. 83% for the group without
radiotherapy). Based on the findings of the study, it was
recommended that radiotherapy should be performed following
lumpectomy irrespective of surgical margins (18). Previously, 376 patients with ACC of
the breast were identified by the Surveillance, Epidemiology, and
End Results database. In total, 60% of these patients were treated
with lumpectomy and 40% underwent mastectomy. Radiotherapy was
found to be a strong prognostic factor for overall and
cause-specific survival (20). In
the present case, breast conservation surgery was performed
followed by radiotherapy in the post-operative period.
Axillary lymph node involvement is extremely rare,
occurring in an average of 0–2% of ACCs of the breast (1). In a review containing 182 patients who
underwent axillary dissection, lymph node metastasis were reported
in only 4 patients (17). In a
study by the RCN, which monitored 61 patients, an axillary
dissection was performed in 41 patients (67%), while a sentinel
lymph node biopsy was carried out in 10 patients (16%), all of whom
revealed negative lymph nodes. An average 79-month follow-up of
these patients showed that there were no axillary supraclavicular
fossae or internal mammary chain metastases (18). Therefore, axillary dissection should
not be clinically performed except in the presence of nodal
metastasis. Additionally, sentinel lymph node sampling can be
performed if the tumor is >3 cm, has a high grade or contains
other invasive types of breast cancer (1). In the present case, a sentinel lymph
node dissection was performed, as the tumor was of a high grade and
a sentinel lymph node was found to be negative.
The role of adjuvant therapy and hormonal therapy is
controversial in patients with ACC of the breast. The presence of a
tumor with good biological characteristics and the absence of a
predisposition to distant metastasis increase the importance of
local disease control. Arpino et al (19) and McClenathan and de la Roza
(20) reported that treatments with
adjuvant chemotherapy and hormonal therapy do not increase the
survival rates of patients. Similarly, it was reported that
systemic therapy is a controversial contribution to survival
(7). Tamoxifen was administered
following radiotherapy and no chemotherapy was performed in the
present case since the estrogen receptor was 2% positive.
The 5-year survival rate for ACCs of the breast is
reported to be 85–90%, with a 100% disease-free survival rate
(2). Despite these results, ACC is
reported to present with local recurrences and distant metastases.
The most common site for distant metastasis is the lung followed by
the liver, kidneys and brain (21).
In conclusion, ACCs of the breast are extremely rare
neoplasms of the breast and have an extremely good prognosis.
Although no consensus exists regarding the optimal treatment,
breast conservative surgical and radiotherapy are recommended. The
affected patients require close follow-up due to the rare, but
possible, occurrence of distant metastasis.
References
|
1
|
Boujelbene N, Khabir A, Boujelbene N, et
al: Clinical review - breast adenoid cystic carcinoma. Breast.
21:124–127. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Veeratterapillay R, Veeratterapillay S,
Ward E, Khout H and Fasih T: Adenoid cystic carcinoma of the
breast: case report and review of literature. Ann R Coll Surg Engl.
94:e137–e138. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Fargahi S and Gu M: Adenoid cystic
carcinoma of the breast diagnosed by fine needle aspiration.
Cytopatology. 23:205–207. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Defaud-Hénon F, Tunon-de-Lara C, Fournier
M, et al: Adenoid cystic carcinoma of the breast: clinical,
histological and immonohistochemical characterization. Ann Pathol.
30:7–16. 2010.(In French).
|
|
5
|
Shin SJ and Rosen PP: Solid variant of
mammary adenoid cystic carcinoma with basaloid features: a study of
nine cases. Am J Surg Pathol. 26:413–420. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang S, Ji X, Wei Y, Yu Z and Li N:
Adenoid cystic carcinoma of the breast: Review of the literature
and report of two cases. Oncol Lett. 4:701–704. 2012.PubMed/NCBI
|
|
7
|
Millar BA, Kerba M, Youngson B, Lockwood
GA and Liu FF: The potential role of breast conservation surgery
and adjuvant breast radiation for adenoid cystic carcinoma of the
breast. Breast Cancer Res Treat. 87:225–232. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ghabach B, Anderson WF, Curtis RE, et al:
Adenoid cystic carcinoma of the breast in the United States (1977
to 2006): a population-based cohort study. Breast Cancer Res.
12:R542010. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bhosale SJ, Kshirsagar AY, Patil RK, et
al: Adenoid cystic carcinoma of female breast: A case report. Int J
Surg Case Rep. 4:480–482. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Billroth T: Die cylindergeschwalst.
Investigations on the development of the blood vessels Berlin: G.
Reimer; 1856, (In German).
|
|
11
|
Geschickter CF: Diseases of the Breast:
Diagnosis, Pathology and Treatment. 2nd edition. Lippincott;
Philadelphia, PA: 1945
|
|
12
|
Ro JY, Silva EG and Gallager HS: Adenoid
cystic carcinoma of the breast. Hum Pathol. 18:1276–1281. 1987.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hjorth S, Magnusson PH and Blomquist P:
Adenoid cystic carcinoma of the breast. Report of a case in a male
and review of the literature. Acta Chir Scand. 143:155–158.
1977.PubMed/NCBI
|
|
14
|
Thompson K, Grabowski J, Saltzstein SL,
Sadler GR and Blair SL: Adenoid cystic breast carcinoma: is
axillary staging necessary in all cases? Results from the
California Cancer Registry. Breast J. 17:485–489. 2011. View Article : Google Scholar
|
|
15
|
Vranic S, Bender R, Palazzo J and Gatalica
Z: A review of adenoid cystic carcinoma of the breast with emphasis
on its molecular and genetic characteristics. Hum Pathol.
44:301–309. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peters GN and Wolff M: Adenoid cystic
carcinoma of the breast. Report of 11 new cases: review of the
literature and discussion of biological behavior. Cancer.
52:680–686. 1983. View Article : Google Scholar
|
|
17
|
Sumpio BE, Jennings TA, Merino MJ and
Sullivan PD: Adenoid cystic carcinoma of the breast. Data from the
Connecticut Tumor Registry and a review of the literature. Ann
Surg. 205:295–301. 1987. View Article : Google Scholar
|
|
18
|
Khanfir K, Kallel A, Villette S, et al:
Management of adenoid cystic carcinoma of the breast: a Rare Cancer
Network study. Int J Radiat Oncol Biol Phys. 82:2118–2124. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Arpino G, Clark GM, Mohsin S, Bardou VJ
and Elledge RM: Adenoid cystic carcinoma of the breast: molecular
markers, treatment, and clinical outcome. Cancer. 94:2119–2127.
2002. View Article : Google Scholar
|
|
20
|
McClenathan JH and de la Roza G: Adenoid
cystic breast cancer. Am J Surg. 183:646–649. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Coates JM, Martinez SR, Bold RJ and Chen
SL: Adjuvant radiation therapy is associated with improved survival
for adenoid cystic carcinoma of the breast. J Surg Oncol.
102:342–347. 2010. View Article : Google Scholar
|