Introduction
Apoptosis is an essential mechanism for the
preservation of the homeostasis and morphogenesis of human tissue.
Disturbance of this process by aberrantly extending cell viability
or favoring accumulation of the transforming mutation is considered
to contribute to carcinogenesis (1–3). The
two major classes of apoptosis inhibitors are the Bcl-2 family and
the inhibitor of apoptosis (IAP) protein family. The first IAP was
identified in baculovirus (4) and,
following this, a number of other IAPs have been identified in
various mammalian species, including humans (5). Survivin is one of eight IAP proteins
and has a number of distinct features which are not shared with
other IAP members (6). Survivin is
the shortest polypeptide, consisting of 142 amino acids, and its
expression is cell cycle-regulated and occurs in the G2/M phase. In
addition, survivin functions to inhibit apoptosis and regulate cell
division, and also enhances angiogenesis (7,8). In
general, IAP proteins are key in the negative regulation of
apoptosis, and act by directly binding to caspase-3 and -7,
inhibiting the process of cell death (9). The survivin protein is abundantly
expressed during fetal development in humans, but rarely presents
in adult tissues. However, expression of survivin has been reported
in the majority human tumors, which suggests that alterations in
survivin gene regulation commonly occur during tumorigenesis
(10). Due to this upregulation in
malignancy and its functional involvement in apoptosis as well as
proliferation, survivin is currently attracting considerable
interest as a potential cancer biomarker and a new target for
cancer treatment (11).
Targeting the apoptosis pathways for cancer
treatment is supported by several observations, which emphasize the
role of aberrant apoptosis in tumorigenesis and resistance to
anticancer treatment (12). Evasion
of apoptosis is critical for tumor growth and is a hallmark of
cancer cells (13). Specific
conventional antitumor therapies, including DNA-damaging and
antimicrotubule agents, exert their function by activating the
intrinsic apoptosis pathway (14).
Previously, the intracellular localization of
survivin in cancer cells has been reported to express biological
features of cancer behavior (15).
In addition, survivin mRNA levels or cytoplasmic expression of the
protein has been associated with a poor outcome in various types of
cancer (16–24). However, previous studies have
reported opposing conclusions with regard to the significance and
prognostic value of nuclear survivin expression (25–33).
These observations suggest that the differential localization of
survivin may indicate different protein functions and affect
patient prognosis (15).
The purpose of the present study was to investigate
the subcellular survivin expression levels in normal mucosa,
high-grade squamous intraepithelial lesions (HSILs) and squamous
cell carcinomas (SCCs) of the uterine cervix by
immunohistochemistry. In addition, the association between the
intracellular localization of survivin and histological diagnosis
of the uterine cervix were examined, and the biological
significance of the difference in intracellular localization of
survivin protein was evaluated.
Materials and methods
Samples
In total, 71 samples of cervical squamous tissue
were obtained, including 15 normal mucosa, 25 HSILs and 31 SCCs,
from cone biopsy and hysterectomy performed at the Department of
Obstetrics and Gynecology of the Chosun University Hospital
(Gwangju, South Korea) between January, 2005 and December 2011. The
Insititional Review Board of Chosun University Hospital waived the
requirement for written informed consent due to the nature of the
study (CHOSUN 2013-07-006-01).
Histopathological analysis
Each specimen was re-evaluated by retrospective
analysis of the medical records and the tissue slide files at the
Department of Pathology, College of Medicine, Chosun University
(Gwangju, South Korea). Age, human papilloma virus (HPV) infection
status and histological diagnosis were assessed. The examined
tissues were fixed in 10% neutral formalin and the prepared
paraffin-embedded tissues were sectioned (4–5 μm in thickness).
Hematoxylin and eosin staining was performed and the sections were
examined under a light microscope (Olympus BX51; Olympus
Corporation, Tokyo, Japan). A representative area of tumor suitable
for the study purpose was selected and slides were prepared for
immunohistochemical analysis.
Immunohistochemical staining
All the specimens were tested using a rabbit
anti-human survivin polyclonal antibody (1:1,000; NeoMarkers,
Fremont, CA, USA), according to the manufacturer’s instructions.
Immunolocalization was performed using the mouse ImmunoCruz
Staining System (sc-2050; Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), according to the manufacturer’s instructions. The
staining process was performed according to standard protocol.
Briefly, the 4-μm sections obtained following formalin fixation and
paraffin embedding were deparaffinized in xylene and then
rehydrated with distilled water through a graded series of ethanol
solutions. The sections were then placed in a glass jar with 10
mmol/l citrate buffer (pH 6.0) and were irradiated in a microwave
oven for 15 min. The sections were allowed to cool in the jar at
room temperature for 20 min. The slides were then rinsed with
Tris-buffered saline and, after quenching the endogenous peroxidase
activity in 0.3% hydrogen peroxide for 10 min, a blocking reagent
(sodium chloride-citrate; Ventana Medical Systems, Tucson, AZ, USA)
was added for 10 min. The slides were then washed as described
previously and subsequently subjected to the primary antibody
reaction. Immunohistochemistry was performed on the NexES
autoimmunostainer (Ventana Medical Systems) and slides were
incubated with the primary antibodies for 32 min. The ultraview
universial DAB detection kit (cat. no. 760–500; Ventana Medical
Systems) was used as the secondary detection method. This kit
includes biotinylated immunoglobulin secondary antibody, containing
affinity purified goat anti-mouse IgG and IgM (b200; l g/ml) and
goat anti-rabbit IgG (b200; l g/ml) in phosphate-buffered saline
with preservative. Incubation was performed for 8 min and was
followed by the addition of conjugated streptavidin horseradish
peroxidase for 8 min. Slides were then counterstained with
hematoxylin (cat. no. 760-2021; Ventana Medical Systems).
Analysis and interpretation of
staining
Representative histological sections of the lesions
were immunohistochemically stained with antibody against survivin
and analyzed for the expression of survivin. The immunostaining was
defined as positive when >20% of tumor cells were stained for
survivin in the nucleus or cytoplasm (15). The samples were subjectively
classified according to the staining intensity of the nucleus and
cytoplasm. Cases were classified as negative (score 0, 0–5%),
weakly positive (score 1, 5–20%), moderately positive (score 3,
20–50%) and strongly positive (score 4, >50%), according to the
intensity of the staining reaction (15). Next, the samples were reclassified
as low intensity (score 0–2) or high intensity (scores 3 and
4).
Statistical analysis
Statistical evaluation was performed using SPSS 12.0
(SPSS, Inc., Chicago, IL, USA). The χ2 test was used to
demonstrate the correlation between survivin expression and
histological diagnosis. P<0.05 was considered to indicate a
statistically significant difference.
Results
Immunohistochemistry results
The expression of survivin was examined in 71
cervical lesion samples. By immunohistochemistry, survivin
expression was observed in the nucleus and/or cytoplasm of cervical
squamous epithelial cells. The nuclear expression of survivin
without cytoplasmic expression was detected in 50.7% (36/71) of all
samples [normal, 100% (15/15); HSIL, 28.0% (7/25); and SCC, 45.2%
(14/31)], while the cytoplsmic expression of survivin without
nuclear expression was observed in 49.3% (35/71) of all samples
[normal, 0% (0/15); HSIL, 72.0% (18/25); and SCC, 54.8% (17/31)].
Furthermore, the nuclear and cytoplasmic dual reactivity of
survivin was detected in 40.8% (29/71) of all squamous epithelial
cell samples [normal, 0% (0/15); HSIL, 64.0% (16/25); and SCC,
41.9% (13/31)] (Table I).
 | Table IDifferences between the intracellular
localization of survivin in the normal mucosa, HSIL and SCC
(%). |
Table I
Differences between the intracellular
localization of survivin in the normal mucosa, HSIL and SCC
(%).
| Localization | Normal mucosa, n
(%) | HSIL, n (%) | SCC, n (%) | Total, n (%) | P-value |
|---|
| Nucleus | 15 (100) | 7 (28.0) | 14 (45.2) | 36 (50.7) | |
| Cytoplasm | 0 (0) | 18 (72.0) | 17 (54.8) | 35 (49.3) | |
| Duala | 0 (0) | 16 (64.0) | 13 (41.9) | 29 (40.8) | <0.001b |
| Total | 15 (21.1) | 25 (35.2) | 31 (43.7) | 71 (100.0) | |
Correlation between the intensity of
survivin expression and histological diagnosis
The correlation between the intensity of survivin
expression and histological diagnosis was examined. The intensity
of survivin expression tended to increase with tumor progression;
60.0% of normal mucosa, 76.0% of HSILs and 80.6% of SCCs revealed
high intensity of survivin expression. However, this correlation
was not found to be statistically significant (Table II).
 | Table IIDifferences between the intensity of
survivin expression in the normal mucosa, HSIL and SCC (%). |
Table II
Differences between the intensity of
survivin expression in the normal mucosa, HSIL and SCC (%).
| Intensity | Normal mucosa, n
(%) | HSIL, n (%) | SCC, n (%) | Total, n (%) | P-value |
|---|
| Lowa | 6 (40.0) | 6 (24.0) | 6 (19.4) | 18 (25.4) | |
| Highb | 9 (60.0) | 19 (76.0) | 25 (80.6) | 53 (74.6) | <0.1c |
| Total | 15 (21.1) | 25 (35.2) | 31 (43.7) | 71 (100.0) | |
Intracellular localization of survivin
among normal mucosa, HSIL and SCC samples
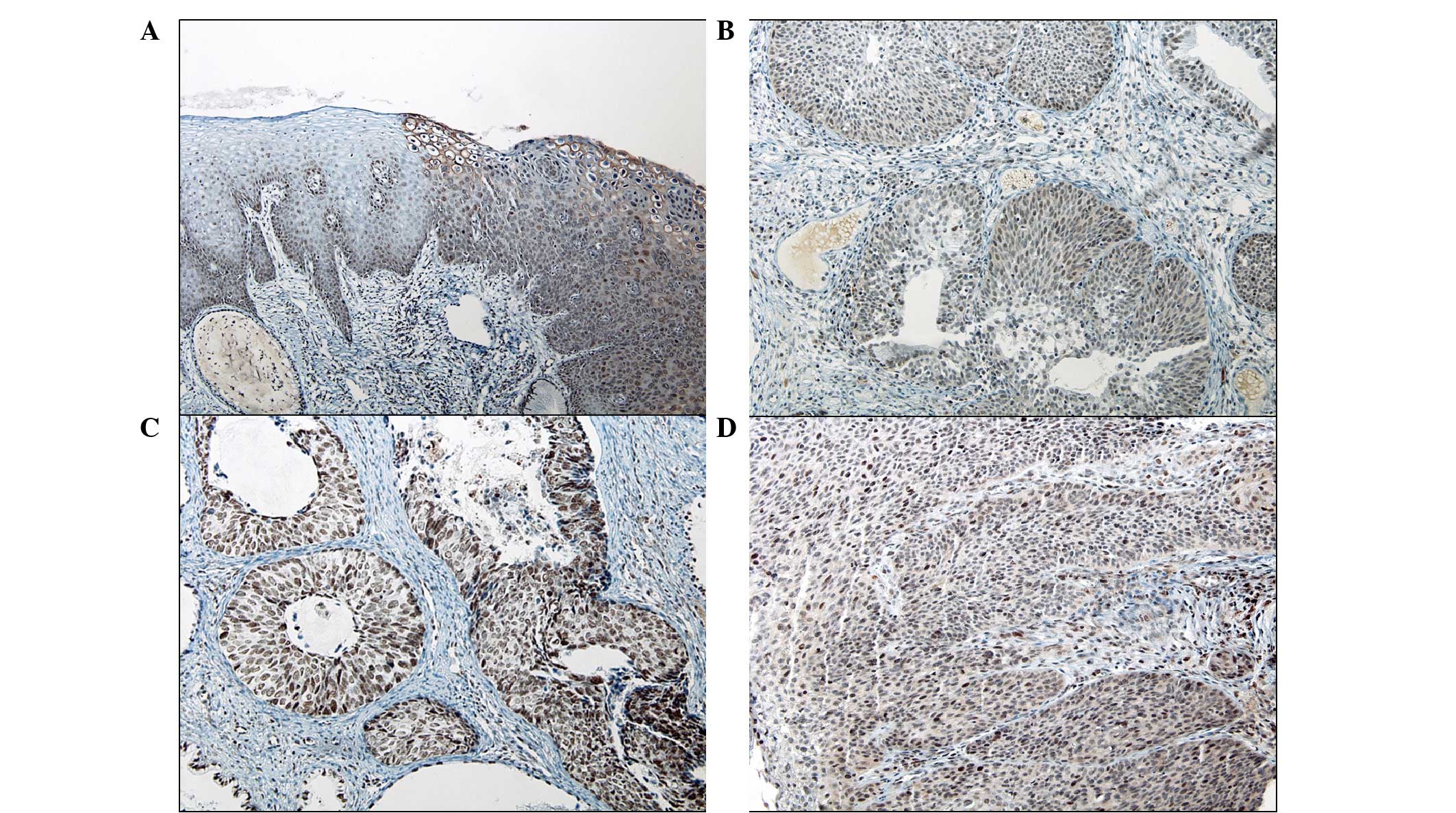
In viral cytopathic lesions (with HPV cytopathic
effects), the koilocytes showed cytoplasmic staining without
nuclear expression. A statistically significant difference was
identified in the intracellular localization of survivin among the
normal mucosa, HSILs and SCCs (P<0.001; Table I). In total, 72.0% (18/25) of HSIL
and 54.8% (17/31) of SCC cases expressed cytoplasmic
immunoreactivity, in contrast to the nuclear staining of all normal
mucosa samples. In addition, 64.0% (16/25) of HSIL and 41.9%
(13/31) of SCC cases showed coexpression in the nucleus and
cytoplasm (Fig. 1A–D). An inverse
correlation was identified between the decrement of nuclear
survivin expression and tumor progression, but this was not
statistically significant (P=0.08).
Discussion
In a number of developing countries, cervical cancer
is the most common fatal malignancy in females; however, there has
been a marked reduction in mortalities due to cervical cancer as a
result of the success of diagnostic cytopathology (34–36).
The majority of cervical cancers are SCC and most cervical SCC
cases are preceded by cervical intraepithelial lesions (CINs),
including low-grade squamous intraepithelial lesions (LSILs) and
HSILs (37,38). While HSIL more commonly appears to
progress to invasive cancer compared with LSIL, it is not always
possible to determine the risk of progression in individual SILs
(39). Molecular markers of
malignant potential may be important in the detection of lesions
that exhibit the greatest potential for progression to cancer and
may also be involved in increasing the sensitivity of current
diagnostic techniques (39).
Previously, survivin mRNA levels or cytoplasmic
protein expression have been associated with poor outcome in
various types of cancer, including breast cancer (16), lymphoma (17), non-small cell lung cancer (18), liver cancer (19), gastric carcinoma (20), ovarian carcinoma (24) and colorectal cancer (21–23).
In the present study, the cytoplasmic expression of survivin was
found to increase in dysplastic lesions (HSILs and SCCs) compared
with the normal mucosa. Certain previous contradictory studies have
shown that the nuclear staining of survivin is associated with a
favorable prognosis in gastric (25) and breast (26) cancer. In addition, the expression of
nuclear survivin in osteosarcoma (27), transitional cell carcinoma of the
urinary bladder (28), pancreatic
cancer (29) and non-small cell
lung cancer (30) has been found to
correlate with a good prognosis. However, in hepatocellular
carcinoma, esophageal SCC and epithelial ovarian tumors, the
expression of nuclear survivin has been found to correlate with an
unfavorable prognosis (31–33). In the present study, an inverse
correlation was identified between the decrement of nuclear
survivin expression and tumor progression, but was not
statistically significant (P=0.08). The reason for these different
prognostic results in the subcellular localization of survivin in
different cancers remains unclear (15). However, despite certain inconsistent
results, the bulk of data concerning numerous human cancer types
support the theory that the cytoplasmic expression of survivin is
associated with cancer progression and poor prognosis (9,40).
In the present study, survivin was detected in the
normal squamous epithelia, cervical dysplasia (HSILs and SCCs in
situ) and invasive SCC. Although there is an increasing
tendency of disease progression, the intensity of survivin
expression is unlikely to have a potential role as a diagnostic or
prognostic marker for cervical SCC. These results suggest that the
biological behavior of cervical dysplasia may differ according to
the intracellular localization of survivin. The function of
cytoplasmic survivin may be important for malignant progression.
Elucidation of the role of cytoplasmic survivin may define the
clinical significance of survivin expression in cervical dysplastic
lesions. In addition, we suggest that the inhibition of the
cytoplasmic localization of survivin may present a novel strategy
for cervical cancer treatment.
Acknowledgements
The current study was supported by research funds
from the Chosun Unversity, 2009.
References
|
1
|
Thompson CB: Apoptosis in the pathogenesis
and treatment of disease. Science. 267:1456–1462. 1995. View Article : Google Scholar
|
|
2
|
Barinaga M: Death by dozens of cuts.
Science. 280:32–34. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kiechle FL and Zhang X: Apoptosis:
biochemical aspects and clinical implications. Clin Chim Acta.
326:27–45. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Crook NE, Clem RJ and Miller LK: An
apoptosis-inhibiting baculovirus gene with a zinc finger-like
motif. J Virol. 67:2168–2174. 1993.PubMed/NCBI
|
|
5
|
LaCasse EC, Baird S, Korneluk RG and
MacKenzie AE: The inhibitors of apoptosis (IAPs) and their emerging
role in cancer. Oncogene. 17:3247–3259. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Adamkov M, Výbohová D, Horáček J, Kovalská
M and Furjelová M: Survivin expression in breast lobular carcinoma:
correlations with normal breast tissue and clinicomorphological
parameters. Acta Histochem. 115:412–417. 2013. View Article : Google Scholar
|
|
7
|
Li F, Yang J, Ramnath N, Javle MM and Tan
D: Nuclear or cytoplasmic expression of survivin: what is the
significance? Int J Cancer. 114:509–512. 2005. View Article : Google Scholar
|
|
8
|
Li F and Brattain MG: Role of the survivin
gene in pathophysiology. Am J Pathol. 169:1–11. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee WS, Cho SB, Rew JS, Lee JH, Park CS
and Joo YE: Expression of survivin in gastric carcinoma and its
relation to tumor cell proliferation and apoptosis. Kor J Pathol.
43:329–334. 2009. View Article : Google Scholar
|
|
10
|
Ambrosini G, Adida C and Altieri DC: A
novel anti-apoptotic gene, survivin, expressed in cancer and
lymphoma. Nat Med. 3:917–921. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Altieri DC: Survivin, cancer networks and
pathway-directed drug discovery. Nat Rev Cancer. 81:61–70. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Mita AC, Mita MM, Nawrocki ST and Giles
FJ: Survivin: key regulator of mitosis and apoptosis and novel
target for cancer therapeutics. Clin Cancer Res. 14:5000–5005.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hanahan D and Weinberg RA: The hallmarks
of cancer. Cell. 100:57–70. 2000. View Article : Google Scholar
|
|
14
|
Adams JM and Cory S: Life-or-death
decisions by the Bcl-2 protein family. Trends Biochem Sci.
26:61–66. 2001. View Article : Google Scholar
|
|
15
|
Qi G, Tuncel H, Aoki E, Tanaka S, Oka S,
Kaneko I, et al: Intracellular localization of survivin determines
biological behavior in colorectal cancer. Oncol Rep. 22:557–562.
2009.PubMed/NCBI
|
|
16
|
Tanaka K, Iwamoto S, Gon G, Nohara T,
Iwamoto M and Tanigawa N: Expression of survivin and its
relationship to loss of apoptosis in breast carcinomas. Clin Cancer
Res. 6:127–134. 2000.PubMed/NCBI
|
|
17
|
Adida C, Haioun C, Gaulard P, Lepage E,
Morel P, Briere J, et al: Prognostic significance of survivin
expression in diffuse large B-cell lymphomas. Blood. 96:1921–1925.
2000.PubMed/NCBI
|
|
18
|
Monzó M, Rosell R, Felip E, Astudillo J,
Sánchez JJ, Maestre J, et al: A novel anti-apoptosis gene:
Re-expression of survivin messenger RNA as a prognosis marker in
non-small-cell lung cancers. J Clin Onol. 17:2100–2104.
1999.PubMed/NCBI
|
|
19
|
Ikeguchi M, Ueta T, Yamane Y, Hirooka Y
and Kaibara N: Inducible nitric oxide synthase and survivin
messenger RNA expression in hepatocellular carcinoma. Clin Cancer
Res. 8:3131–3136. 2002.
|
|
20
|
Wakana Y, Kasuya K, Katayanagi S, Tsuchida
A, Aoki T, Koyanagi Y, et al: Effect of survivin on cell
proliferation and apoptosis in gastric cancer. Oncol Rep.
9:1213–1218. 2002.PubMed/NCBI
|
|
21
|
Kawasaki H, Altieri DC, Lu CD, Toyoda M,
Tenjo T and Tanigawa N: Inhibition of apoptosis by survivin
predicts shorter survival rates in colorectal cancer. Cancer Res.
58:5071–5074. 1998.PubMed/NCBI
|
|
22
|
Sarela AI, Macadam RC, Farmery SM, Markham
AF and Guillou PJ: Expression of the antiapoptosis gene, survivin,
predicts death from recurrent colorectal carcinoma. Gut.
46:645–650. 2000. View Article : Google Scholar
|
|
23
|
Sarela AI, Scott N, Ramsdale J, Markham AF
and Guillou PJ: Immunohistochemical detection of the anti-apoptosis
protein, survivin, predicts survival after curative resection of
stage II colorectal carcinomas. Ann Surg Oncol. 8:305–310. 2001.
View Article : Google Scholar
|
|
24
|
Sui L, Dong Y, Ohno M, Watanabe Y,
Sugimoto K and Tokuda M: Survivin expression and its correlation
with cell proliferation and prognosis in epithelial ovarian tumors.
Int J Oncol. 21:315–320. 2002.
|
|
25
|
Okada E, Murai Y, Matsui K, Isizawa S,
Cheng C, Masuda M and Takano Y: Survivin expression in tumor cell
nuclei is predictive of a favorable prognosis in gastric cancer
patients. Cancer Lett. 163:109–116. 2001. View Article : Google Scholar
|
|
26
|
Kennedy SM, O’Driscoll L, Purcell R,
Fitz-Simons N, McDermott EW, Hill AD, et al: Prognostic importance
of Survivin in breast cancer. Br J Cancer. 88:1077–1083. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Trieb K, Lehner R, Stulnig T, Sulzbacher I
and Shroyer KR: Survivin expression in human osteosarcoma is a
marker for survival. Eur J Surg Oncol. 29:379–382. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Lehner R, Lucia MS, Jarboe EA, Orlicky D,
Shroyer AL, McGregor JA and Shroyer KR: Immunohistochemical
localization of the IAP protein survivin in bladder mucosa and
transitional cell carcinoma. Appl Immunohistochem Mol Morphol.
10:134–138. 2002. View Article : Google Scholar
|
|
29
|
Tonini G, Vincenzi B, Santini D, et al:
Nuclear and cytoplasmic expression of survivin in 67 surgically
resected pancreatic cancer patients. Br J Cancer. 92:2225–2232.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Vischioni B, Van der Valk P, Span SW,
Kruyt FA, Rodriguez JA and Giaccone G: Nuclear localization of
survivin is a positive prognostic factor for survival in advanced
non-small-cell lungcancer. Ann Oncol. 15:1654–1660. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ito T, Shiraki K, Sugimoto K, Yamanaka T,
Fujikawa K, Ito M, et al: Survivin promotes cell proliferation in
human hepatocellular carcinoma. Hepatology. 31:1080–1085. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Grabowski P, Kühnel T, Mühr-Wilkenshoff F,
Heine B, Stein H, Höpfner M, et al: Prognostic value of nuclear
survivin expression in oesophageal squamous cell carcinoma. Br J
Cancer. 88:115–119. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tringler B, Lehner R, Shroyer AL and
Shroyer KR: Immunohistochemical localization of survivin in serous
tumors of the ovary. Appl Immunohistochem Mol Morphol. 12:40–43.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Birley HD: Human papillomaviruses,
cervical cancer and the developing world. Ann Trop Med Parasitol.
89:453–463. 1995.PubMed/NCBI
|
|
35
|
American Cancer Society. Cancer Facts and
Figures 1999. American Cancer Society; Atlanta, GA: 1999
|
|
36
|
Walboomers JM, Jacobs MV, Manos MM, Bosch
FX, Kummer JA, Shah KV, et al: Human papillomavirus is a necessary
cause of invasive cervical cancer worldwide. J Pathol. 189:12–19.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Richart RM: Cervical intraepithelial
neoplasia. Pathol Annu. 8:301–328. 1973.PubMed/NCBI
|
|
38
|
Wright TC, Kurman RJ and Ferenczy A:
Precancerous lesions of the cervix. Blaustein’s Pathology of the
Female Genital Tract. Kurman RJ: Springer-Verlag; New York, NY: pp.
229–232. 1994
|
|
39
|
Frost M, Jarboe EA, Orlicky D, Gianani R,
Thompson LC, Enomoto T and Shroyer KR: Immunohistochemical
localization of survivin in benign cervical mucosa, cervical
dysplasia, and invasive squamous cell carcinoma. Am J Clin Pathol.
117:738–744. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Xie D, Zeng YX, Wang HJ, Wen JM, Tao Y,
Sham JS and Guan XY: Expression of cytoplasmic and nuclear Survivin
in primary and secondary human glioblastoma. Br J Cancer.
16:108–114. 2006. View Article : Google Scholar : PubMed/NCBI
|