Introduction
A number of advances in the understanding of cancer
on a biochemical, molecular, cellular and physiological level have
been made in recent years and used in clinical diagnosis and the
implementation of novel therapies. Despite this, there has been an
increase in the number of new clinical cases of cancer, which is
spurring the need for new medicines and treatment alternatives to
tackle what is now a leading cause of mortality worldwide (1).
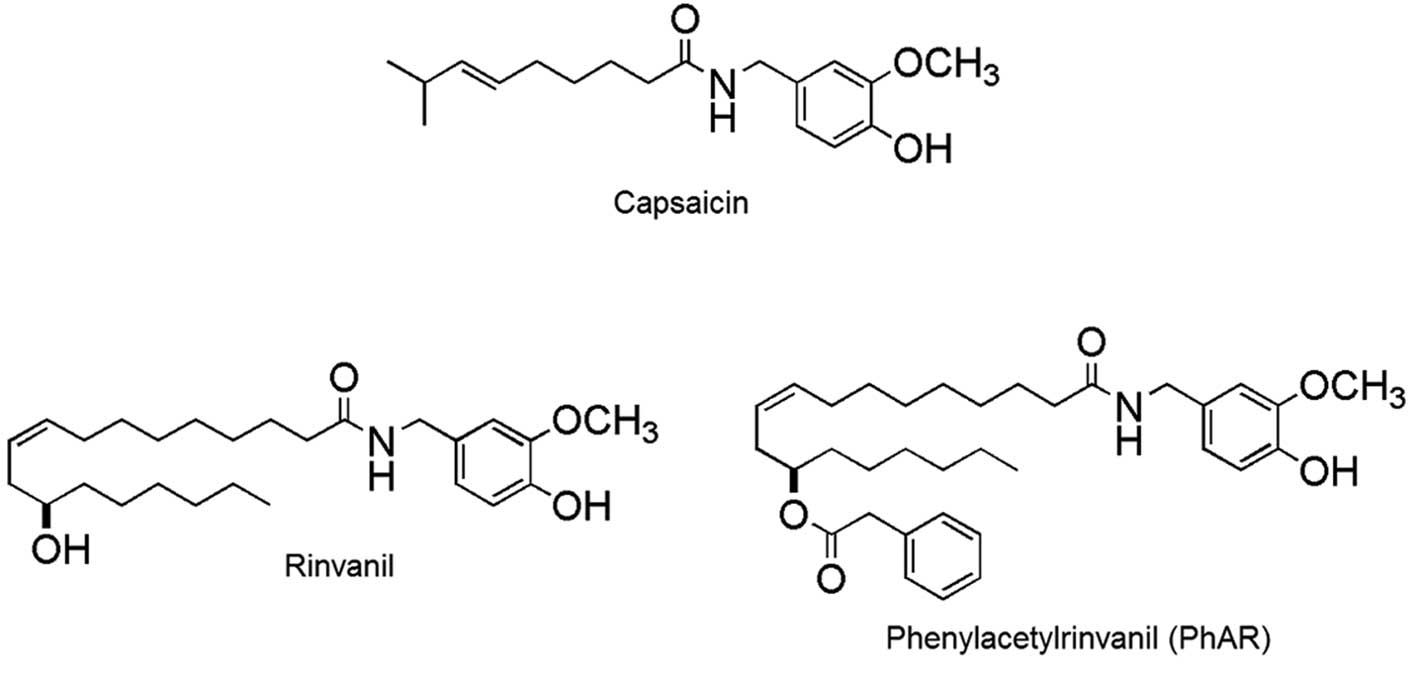
Capsaicin
(N-[(4-hydroxy-3-methoxyphenyl)methyl]-8-methyl-(6E)-6-nonenamide;
Fig. 1) is a naturally occurring
organic compound and the main member of the capsaicinoid family, a
group of compounds that give a characteristic pungency to the fruit
of the chili pepper plant (Capsicum spp.). This effect is
due to the agonist activity of the capsaicin receptor, transient
receptor potential vanilla subfamily 1 (TRPV1). Expressed in
sensory neurons, TRPV1 is a nonselective cation channel that is
activated by heat (≥43°C) or protons, and also plays an important
role in the transmission of pain impulses (2). Prolonged treatment with capsaicin
desensitises neurons and thus generates an analgesic effect
(3). Consequently, numerous studies
have been investigating the role of capsaicin and its interaction
with TRPV1 receptors in the treatment of a variety of pathologies
that present with hyperalgesia, including rheumatoid arthritis
(4,5), chronic neuropathic pain (6), diabetic neuropathy (7,8) and
other neuralgias (9). In the search
to identify new chemotherapeutic agents for cancer treatment, it
has been revealed that capsaicin has inhibitory effects on cell
proliferation and apoptosis induction in various types of cancer
cell lines, including HepG2 (human hepatoma); AGS (gastric cancer);
PC-3 (prostate cancer); MCF-7 (breast cancer); U373, U87, FC1 and
FLS (glioma); NB4, UF-1, Kasumi-1, HL-60, K562, KU812 and U937
(leukaemia); and HT-29 cells (colon cancer) among others (10–16).
Furthermore, several studies have demonstrated that capsaicin may
have chemoprotective properties against certain carcinogenic and
mutagenic agents (17,18) as well as the ability to induce
terminal differentiation in A172 human glioblastoma cells (19). The evidence from these studies
indicates the strong antitumour potential of capsaicin. The methods
of obtaining capsaicin from natural sources are often inefficient
and expensive, due to its low content in the fruits of the genus
Capsicum and the presence of other compounds with similar
polarity. In addition, due to its high pungency, it is difficult to
manage its production by synthetic methods (20), which is an obstacle to its
development as a potential chemotherapeutic agent. Two capsaicin
analogues, rinvanil and phenylacetylrinvanil (PhAR) have been
synthesised (Fig. 1). The latter is
the most potent TRPV1 receptor agonist synthesised to date and is
devoid of pungency (21,22); in fact, it is 1,000× more potent
than capsaicin in its affinity for TRPV1 receptors (23).
While the structural and physiological associations
between rinvanil, phenylacetylrinvanil (PhAR) and capsaicin are
known, their antineoplastic effects have not yet been described.
This study evaluates the antiproliferative and pro-apoptotic
effects of rinvanil and PhAR on P388 mouse leukaemia cell lines,
J774 and WEHI-3 leukaemia cell lines, and cultures of mononuclear
cells from normal mouse bone marrow.
Materials and methods
Capsaicin and capsaicin analogues
Capsaicin was purchased from Sigma-Aldrich (St.
Louis, MO, USA). Rinvanil and PhAR were synthesised and
characterised as described in the study by Castillo et al
(24).
Animals
In this study, 12-week-old female BALB/c were used
and maintained in pathogen-free conditions. Experiments were
performed in the animal facility of FES-Zaragoza, National
Autonomous University of Mexico (Iztapalapa, Mexico), in accordance
with institutional guidelines. The mice were provided with
autoclaved water and fed a standard powdered rodent diet ad
libitum. All experimental protocols were approved by the ethics
committee of FES-Zaragoza, National Autonomous University of Mexico
in accordance with national regulations for the care and use of
experimental animals.
Cell culture
Total bone marrow cells of the mice were obtained
from the femur by flushing with Iscove’s Modified Dulbecco’s Medium
(IMDM) supplemented with 10% FBS. Mononuclear cells (MNCs) were
obtained from the total cells via gradient separation with
Ficoll-Paque (Amersham Biosciences AB, Uppsala, Sweden) at a
density of 1.077 g/ml and washed twice with phosphate-buffered
saline (PBS). MNCs were cultured for 120 h in IMDM (Gibco-BRL,
Carlsbad, CA, USA) supplemented with 15% (v/v) FBS, 5% (v/v) horse
serum (Gibco-BRL,) and 5 ng/ml recombinant mouse interleukin-3
(rmIL-3) (R&D System, Minneapolis, MN, USA). The cells were
maintained in a humidified atmosphere containing 5% CO2
at 37°C and were maintained in a culture for a maximum of 120 h.
P388, J774, and WEHI-3 mouse myeloid leukaemia cells were obtained
from American Type Culture Collection (Manassas, VA, USA) and
cultured in Iscove’s Modified Dulbecco’s Medium (Gibco-BRL)
supplemented with 10% v/v foetal calf serum (Gibco-BRL) previously
heat inactivated and kept at 37°C with 5% CO2 and
saturating humidity.
Cell proliferation assays
For proliferation assays, cells were grown in
96-well plates (Corning, Tewksbury, MA, USA) in the presence or
absence of capsaicin, rinvanil and PhAR for 72 h under the culture
conditions described above; proliferation was assessed with the
sulforhodamine B (SRB) assay (Sigma-Aldrich) (25). Briefly, the cultured cells were
fixed by adding 50 μl/well of cold trichloroacetic acid (TCA;
Sigma-Aldrich) and incubated at 4°C for 1 h, followed by several
washings to remove the TCA before the cell culture plate was
allowed to dry at room temperature. The cells were then added to
100 μl/well of 0.4% SRB diluted in 1% acetic acid. After remaining
under the stain for 20 min, several washes were performed with 1%
acetic acid and the plate was allowed to dry at room temperature.
The dye was solubilised with 50 μl/well of 10 mM Tris base (pH
10.4) and the plate was read on a spectrophotometer (Tecan Spectra
A-5082; Tecan Austria GmbH, Grödig, Austria) at 570 nm.
Cell viability
P388, J774 and WEHI-3 cell lines and normal mouse
bone marrow cells were cultured in 96-well plates at cell densities
of 3×104, 5×103, 1.3×104 and
1×105, respectively. They were then treated with or
without IC50 concentrations of capsaicin, PhAR and
rinvanil (Table I) and incubated
for 72 h (leukaemia lines) or 120 h (mononuclear cells from normal
mouse bone marrow). Finally, the cell suspensions were mixed with
an equal volume of Trypan blue dye (Sigma-Aldrich) and counted
directly under a light microscope (Axio Vert.A1, Primo Star Carl
Zeiss, Göttingen, Germany). Unstained cells were considered as
viable and stained cells nonviable (26).
 | Table IIC50 values for the
different leukaemia cell lines and mononuclear cells of normal
mouse bone marrow. |
Table I
IC50 values for the
different leukaemia cell lines and mononuclear cells of normal
mouse bone marrow.
| IC50
(μg/ml)a |
|---|
|
|
|---|
| Treatment | P388 | J774 | WEHI-3 | Bone marrow |
|---|
| Capsaicin | 72.1±1.8 | 32.5±1.7 | 47.7±2.5 | 53.5±3.1 |
| Rinvanil | 49.2±2.2 | 10.2±3.0 | 31.2±3.6 | 72.6±2.4 |
| PhAR | 9.0±2.0 | 8.0±3.7 | 3.0±3.1 | 40.7±2.4 |
Apoptotic bodies
To assess the percentage of apoptotic bodies, the
P388, J774 and WEHI-3 cell lines and mouse bone marrow cells,
plated at a density of 1×105 cells/ml, were treated with
or without an IC50 concentration of capsaicin, rinvanil
and PhAR. After 24 h of incubation, a sample of cells was fixed
with methanol, stained with Giemsa dye and observed by light
microscopy using a 100X objective.
DNA fragmentation
To confirm the induction of apoptosis, DNA
fragmentation was analysed. Briefly, P388, J774 and WEHI-3 cell
lines (1×106 cells/5 ml) were cultured in the presence
or absence of IC50 concentrations of capsaicin, rinvanil
or PhAR for 24 h. Cells were collected by centrifugation and washed
twice with PBS. The cell pellet was resuspended in 0.3 ml lysis
buffer [100 mM NaCl, 10 mM Tris-HCl (pH 8.0), 25 mM EDTA (pH 8.0),
0.5% SDS, 100 μg/ml proteinase K; Promega, Madison, WI, USA] and
incubated while shaken at 37°C for 4 h. The lysate was treated with
100 μg/ml RNase (Promega) for 1 h at 37°C, followed by extraction
with phenol-chloroform-isoamyl alcohol (25:24:1); the upper phase
was collected and precipitated with absolute ethanol overnight at
−20°C. DNA was collected by centrifugation (14,000 × g; 4°C; 20
min) and washed with 75% ethanol. The DNA pellet was resuspended in
100 μl TE buffer [1 mM EDTA (pH 8.0), 10 mM Tris-HCl (pH 8.0)] and
incubated at 65°C for 1 h to facilitate solubilisation. Finally,
the DNA in 0.5 mg/ml ethidium bromide was subjected to
electrophoresis using a 2% agarose gel (Invitrogen Life
Technologies, Carlsbad, CA, USA) at 60 V for 2.5 h and visualised
under UV light (27).
The results are shown as the mean ± SD of three
independent experiments with three replicates. Statistical
significance was determined using one-way analysis of variance
followed by Dunnett’s contrast. P<0.05 was considered to
indicate a statistically significant difference.
Results
Rinvanil and PhAR inhibit the
proliferation of leukaemia cell lines and mouse bone marrow
cells
P388, J774 and WEHI-3 leukaemia cell lines and
normal mouse mononuclear bone marrow cells were cultured in 96-well
plates at cell densities of 3×104, 5×103,
1.3×104 and 1×105, respectively, to
demonstrate that rinvanil, PhAR and capsaicin (as a control),
inhibit the proliferation of leukaemia cell lines, as well as mouse
bone marrow cells, in a dose-dependent manner (Fig. 2). Based on the values of the average
doses of inhibition (IC50), the leukaemia cell lines,
except for P388 cells treated with capsaicin, were more sensitive
than the normal bone marrow cells (Table I). Even the IC50 value of
PhAR in P388 cells, 9.0 μg/ml, was increased to 40.7 μg/ml in bone
marrow, providing evidence that leukaemia cells are 4.5-fold more
sensitive compared with normal cells.
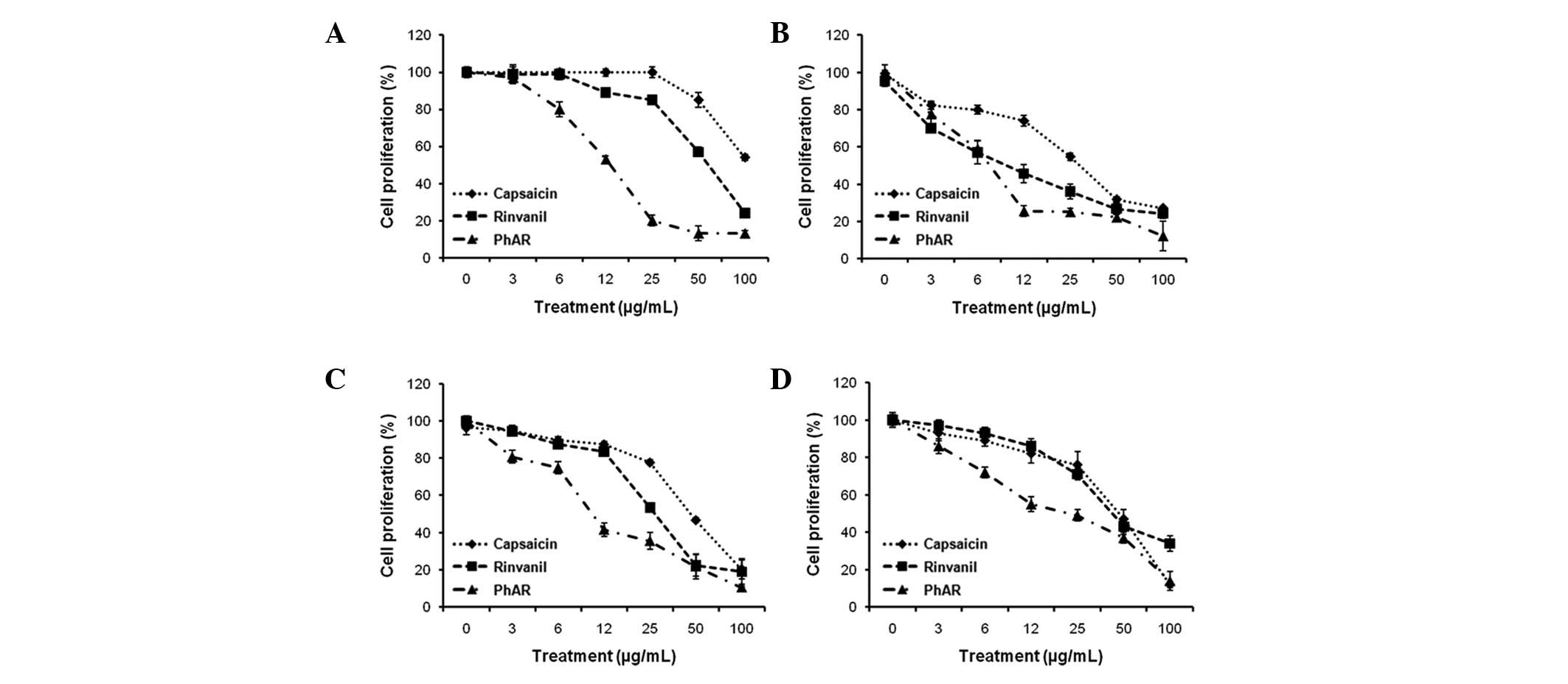 | Figure 2Antiproliferative effect of capsaicin,
PhAR and rinvanil in (A) P388, (B) J774 and (C) WEHI-3 leukaemia
cell lines and (D) mononuclear cells of normal mouse bone marrow.
The cells were grown in 96-well plates with cell densities of
3×104, 5×103, 1.3×104 and
1×105 cells/ml, respectively, and treated with
increasing concentrations of the compounds tested (0, 3, 6, 12, 25,
50 and 100 mg/ml). Cell proliferation was determined with the
sulforhodamine B assay after 72 h incubation for leukaemia cell
lines and 120 h for bone marrow cells. The results are shown as the
mean ± SD of three independent experiments with three replicates
each. PhAR, phenylacetylrinvanil. |
PhAR is selectively cytotoxic to P388,
J774 and WEHI-3 leukaemia cells
To determine if the decrease in proliferation was
due to cell death induced by capsaicin, rinvanil and PhAR, cell
viability was determined by evaluating membrane integrity with
trypan blue dye exclusion. The P388, J774 and WEHI-3 leukaemia
cells and normal mouse bone marrow cells were cultured with
IC50 concentrations of capsaicin, PhAR and rinvanil, and
incubated for 72 h (leukaemia lines) or 120 h (mononuclear mouse
bone marrow). Our results show that in the case of J774 and P388
leukaemia lines, the three compounds significantly reduced cell
viability with respect to the vehicle, although J774 cells were
more sensitive, with viability percentages of only 55, 76 and 60%
for cells treated with capsaicin, PhAR and rinvanil, respectively.
In the case of WEHI-3 cells, capsaicin was the only agent to
significantly reduce viability compared with cells treated with
vehicle alone. Cells from normal mouse bone marrow had viabilities
of 94 and 91% when treated with capsaicin and PhAR, respectively,
without significant difference from vehicle, while rinvanil reduced
viability to 80% (Table II). These
results confirm that PhAR is selectively cytotoxic to leukaemia and
similar to capsaicin (28,29).
 | Table IICell viability (%) of the leukaemia
cell lines and mononuclear cells of normal mouse bone marrow
treated with the IC50 concentrations of capsaicin,
rinvanil and PhAR. |
Table II
Cell viability (%) of the leukaemia
cell lines and mononuclear cells of normal mouse bone marrow
treated with the IC50 concentrations of capsaicin,
rinvanil and PhAR.
| Cell viability
(%) |
|---|
|
|
|---|
| Treatment | Capsaicin | Rinvanil | PhAR |
|---|
| (A) |
| Vehicle | 97±2.8 | 97±2.8 | 97±2.8 |
| IC50 | 80±9.0a | 93±6.2a | 93±3.8a |
| (B) |
| Vehicle | 98±1.9 | 98±1.9 | 98±1.9 |
|
IC50 | 55±6.2a | 76±6.9a | 60±8.0a |
| (C) |
| Vehicle | 98±4.2 | 98±4.2 | 98±4.2 |
|
IC50 | 86±5.0a | 91±7.4 | 91±7.7 |
| (D) |
| Vehicle | 97±3.3 | 99±3.3 | 99±3.3 |
|
IC50 | 94±2.2 | 80±6.3a | 91±3.7 |
Rinvanil and PhAR induce cell death by
apoptosis in P388, J774 and WEHI-3 cell lines, but not in mouse
bone marrow cells
One important characteristic of cancer cells is
their resistance to death by apoptosis (programmed cell death);
apoptosis induction is an important parameter in determining the
therapeutic antitumour potential of a compound (30). To assess whether PhAR and rinvanil
induce cell death by apoptosis, P388, J774 and WEHI-3 cell lines
and mouse bone marrow cells were cultured at a density of
1×105 cells/ml with or without IC50
concentrations of PhAR and rinvanil, using capsaicin as a positive
control for the induction of apoptosis. After 24 h of incubation
for leukaemia cell lines or 120 h for cultured bone marrow cells,
cells were stained with Giemsa and examined by light microscopy.
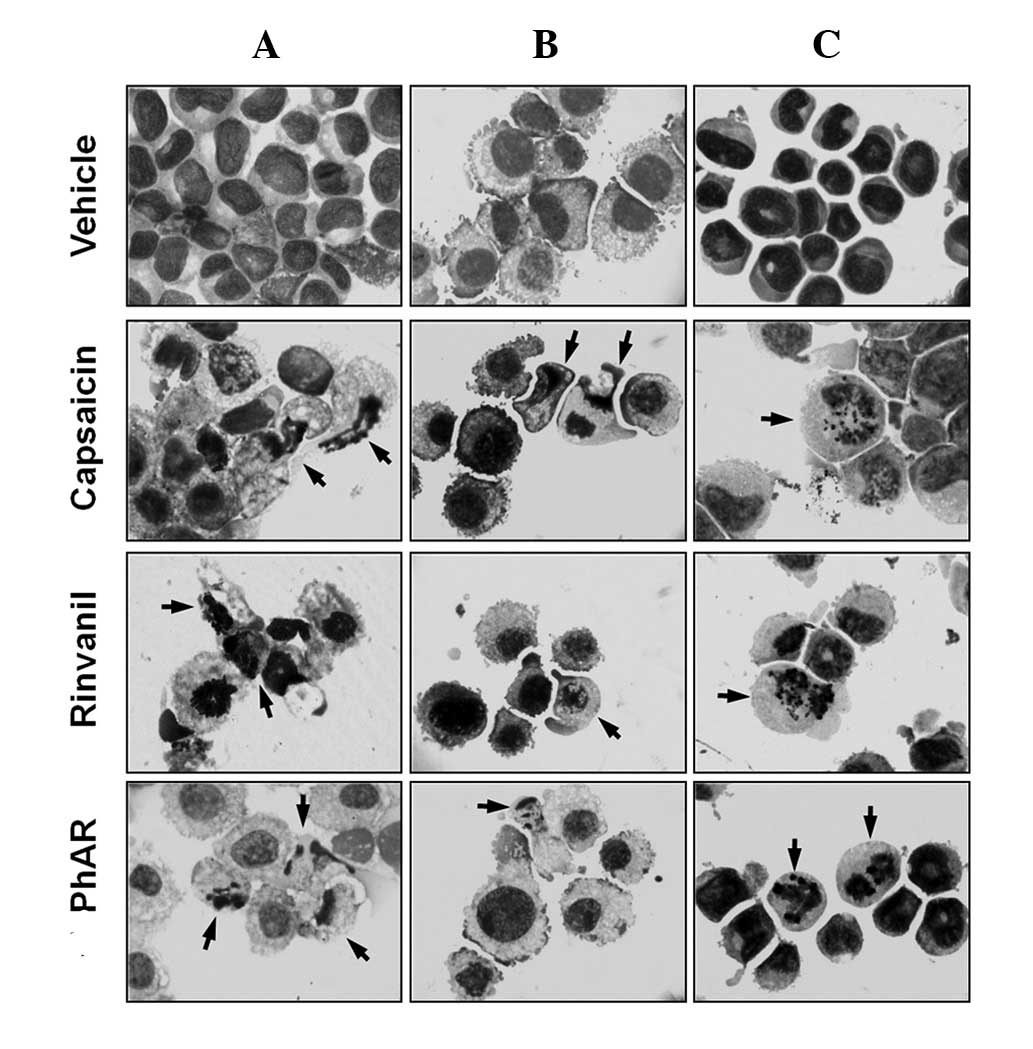
The cell lines demonstrated characteristics of apoptosis (31), including an increase in cell volume,
cytoplasmic modifications, change in nuclear size, nuclear
chromatin condensation and nuclear fragmentation, as well as an
intact cell membrane with the formation of apoptotic bodies
(Fig. 3). Cultured mouse bone
marrow mononuclear cells treated with these compounds showed no
significant changes in cell morphology with respect to the vehicle.
A quantification of the percentage of apoptotic bodies confirms
that the leukaemia cell lines undergo apoptosis in response to PhAR
and rinvanil treatment, while normal bone marrow cells do not
(Table III).
 | Table IIIPercentage of apoptotic bodies in
different leukaemia cell lines and mononuclear cells of normal
mouse bone marrow. |
Table III
Percentage of apoptotic bodies in
different leukaemia cell lines and mononuclear cells of normal
mouse bone marrow.
| Apoptotic bodies
(%) |
|---|
|
|
|---|
| Treatment | P388 | J774 | WEHI-3 | Bone marrow |
|---|
| Vehicle | 0 | 2±1 | 0 | 5±3 |
| Capsaicin | 41±2a | 31±3a | 17±3a | 6±4 |
| Rinvanil | 41±3a | 30±3a | 15±2a | 7±2 |
| PhAR | 42±5a | 37±2a | 16±2a | 6±4 |
As PhAR was demonstrated to have a lower
IC50 for apoptosis induction, as observed by the
formation of apoptotic bodies in cell lines, this molecule was used
to confirm the induction of apoptosis by DNA fragmentation
analysis.
For this, P388, J774 and WEHI-3 cells
(1×106 cells/5 ml) were cultured in the presence or
absence of IC50 concentrations of PhAR for a period of
24 h; as a control, P388 cells were treated with an IC50
concentration of capsaicin. DNA was then extracted and visualised
on an agarose gel under UV light (Fig.
4). These results show a typical pattern of internucleosomal
DNA fragmentation of 180–200 bp in leukaemia cells treated with
PhAR, as does capsaicin in P388 cells.
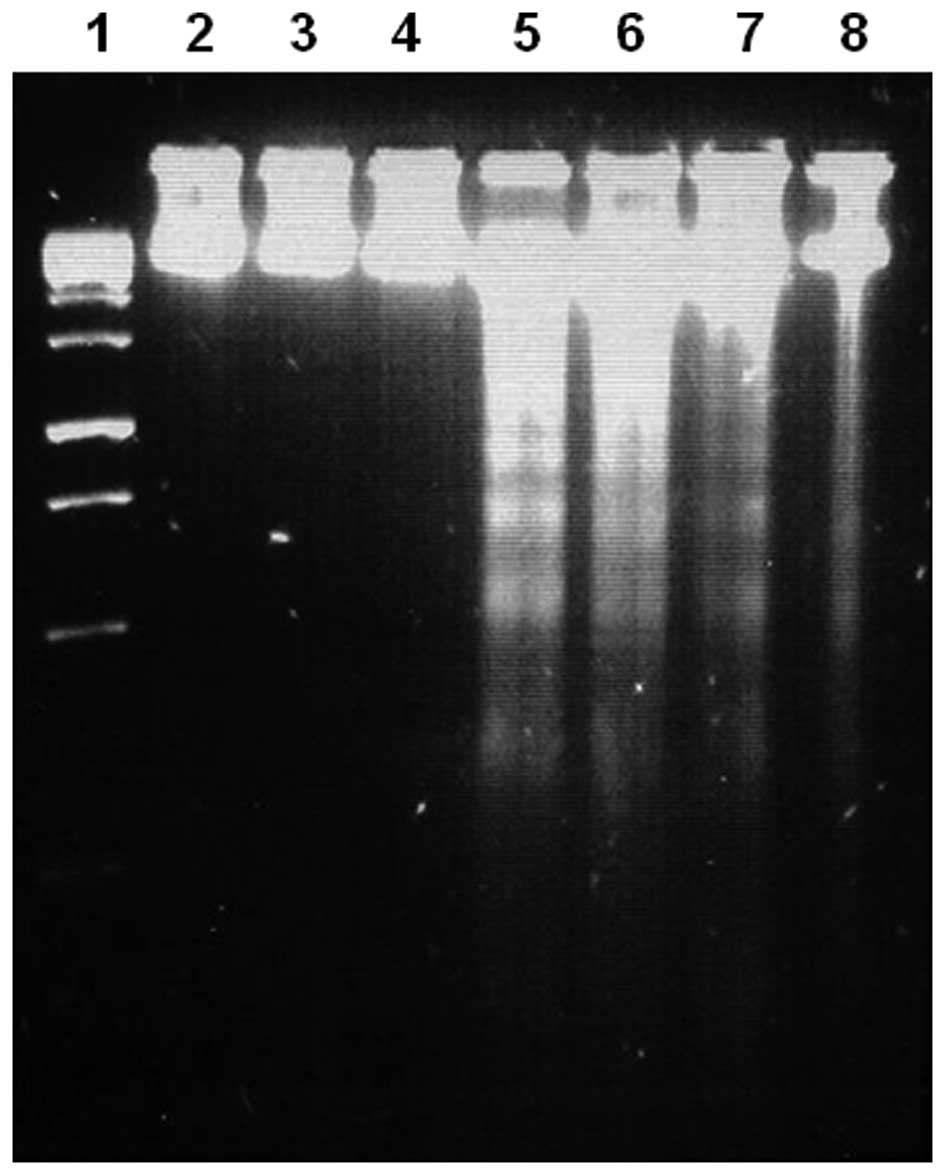 | Figure 4DNA fragmentation induced by PhAR in
P388, J774 and WEHI-3 leukaemia cell lines, or by capsaicin in P388
cells. Lane 1, molecular weight marker; lanes 2–4, P388, J774 and
WEHI-3 cells, respectively, treated with vehicle alone; lanes 5–7,
P388, J774 and WEHI-3 cells, respectively, treated with
IC50 concentrations of PhAR; lane 8, P388 cells treated
with an IC50 concentration of capsaicin. P388, J774 and
WEHI-3 cell lines (1×106 cells/5 ml) were cultured for
24 h in the presence or absence of IC50 concentrations
of PhAR, and P388 cells were treated with an IC50
concentration of capsaicin. At the end time of stimulation, DNA was
extracted and visualised on an 2% agarose gel under UV light. PhAR,
phenylacetylrinvanil. |
Discussion
It is known that capsaicin can inhibit the
proliferation of several human and mouse tumour cell lines without
affecting normal cell proliferation, and that this
antiproliferative effect is due to the induction of apoptosis
(28,29). As rinvanil and PhAR are synthetic
capsaicinoids, we evaluated the effect of these compounds on cell
proliferation using capsaicin as a control. Based on the values of
IC50, the leukaemia cell lines were more sensitive than
the normal bone marrow cells (except for P388 treated with
capsaicin). As the high potential for inhibition of proliferation
observed with PhAR for normal and leukaemia cells correlates with
its high affinity for vanilloid receptors, it would be of interest
in the future to assess whether the difference in proliferative
response is due to the density of receptors expressed in each cell
type.
To determine whether the decrease in proliferation
was due to cell death induced by capsaicin, rinvanil and PhAR, cell
viability was determined by evaluating membrane integrity with
trypan blue dye exclusion. The results confirm that PhAR is
selectively cytotoxic to leukaemia, similar to capsaicin (28,29).
Capsaicin, the pungent component present in chili
pepper, has potential anti-inflammatory, antioxidant,
antiproliferative and anticancer properties; it also has
chemopreventive effects against chronic inflammatory diseases,
including cancer (31). Rinvanil
and PhAR are synthetic capsaicinoids that have powerful cytotoxic
and apoptotic effects on leukaemia cells and as they lack pungency,
they may act as improved antineoplastic agents over the naturally
occurring capsaicin compound. PhAR, in particular, stands out for
its greater cytotoxic activity, which inhibits the proliferation of
leukaemia cells by promoting apoptosis. The majority of drugs
currently used for cancer chemotherapy, produce side effects such
as immunosuppression and anaemia which are in many cases permanent
or irreversible and lethal (33,34),
at this time the effect of PhaR in vivo is unknown, in the
future these events will be analyzed.
In conclusion, PhAR and rinvanil inhibit
proliferation of leukaemia lines and bone marrow mononuclear cells
in a dose-dependent manner, although the latter were less sensitive
to the cytotoxic effect of rinvanil and PhAR, as well as capsaicin.
Inhibition of cell proliferation was due to the induction of cell
death by apoptosis in leukaemia lines but not in normal cells. The
characteristics of low pungency and selective toxicity toward
leukaemia cells suggests that both rinvanil and particularly PhAR
may be biomedically relevant and promising anticancer agents.
Acknowledgements
The authors are grateful to Mrs. Ma de los Angeles
Peña, Mr. Javier Pérez-Flores and Mrs. Rocío Patiño-Maya from the
Institute of Chemisty, National Autonomous University of Mexico for
spectroscopic analysis. This study was supported in part by PAPIIT
(IN225610) of the Universidad Nacional Autónoma de México (UNAM),
CONACyT (101855) and ICyTDF (PICSA10156).
References
|
1
|
World Health Organization. World health
statistics. WHO Press; Switzerland: pp. 1–177. 2010
|
|
2
|
Caterina MJ, Schumacher MA, Tominaga M,
Rosen TA, Levine JD and Julius D: The capsaicin receptor: a
heat-activated ion channel in the pain pathway. Nature.
389:816–824. 1997. View
Article : Google Scholar
|
|
3
|
Winter J, Bevan S and Campbell EA:
Capsaicin and pain mechanisms. Br J Anaesth. 75:157–168. 1995.
View Article : Google Scholar
|
|
4
|
McCarthy GM and McCarty DJ: Effect of
topical capsaicin in the therapy of painful osteoarthritis of the
hands. J Rheumatol. 19:604–607. 1992.PubMed/NCBI
|
|
5
|
Matucci-Cerinic M, McCarthy G, Lombardi A,
Pignone A and Partsch G: Neurogenic influences in arthritis. J
Rheumatol. 22:1447–1449. 1995.PubMed/NCBI
|
|
6
|
Sindrup SH and Jensen TS: Efficacy of
pharmacological treatments of neuropathic pain: an update and
effect related to mechanism of drug action. Pain. 83:389–400. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Low PA, Opfer-Gehrking TL, Dyck PJ, Litchy
WJ and O’Brien PC: Double-blind, placebo-controlled study of the
application of capsaicin cream in chronic distal painful
polyneuropathy. Pain. 62:163–168. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ross DR and Varipapa RJ: Treatment of
painful diabetic neuropathy with topical capsaicin. N Engl J Med.
321:474–475. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Szallasi A and Blumberg PM: Vanilloid
(Capsaicin) receptors and mechanisms. Pharmacol Rev. 51:159–212.
1999.
|
|
10
|
Huang SP, Chen JC, Wu CC, et al:
Capsaicin-induced apoptosis in human hepatoma HepG2 cells.
Anticancer Res. 29:165–174. 2009.PubMed/NCBI
|
|
11
|
Chow J, Norng M, Zhang J and Chai J: TRPV6
mediates capsaicin-induced apoptosis in gastric cancer
cells-mechanisms behind a possible new ‘hot’ cancer treatment.
Biochim Biophys Acta. 1773:565–576. 2007.PubMed/NCBI
|
|
12
|
Sánchez AM, Malagarie-Cazenave S, Olea N,
Vara D, Chiloeches A and Díaz-Laviada I: Apoptosis induced by
capsaicin in prostate PC-3 cells involves ceramide accumulation,
neutral sphingomyelinase, and JNK activation. Apoptosis.
12:2013–2024. 2007.
|
|
13
|
Chou CC, Wu YC, Wang YF, Chou MJ, Kuo SJ
and Chen DR: Capsaicin-induced apoptosis in human breast cancer
MCF-7 cells through caspase independent pathway. Oncol Rep.
21:665–671. 2009.PubMed/NCBI
|
|
14
|
Amantini C, Mosca M, Nabissi M, et al:
Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1
vanilloid receptor and requires p38 MAPK activation. J Neurochem.
102:977–990. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ito K, Nakazato T, Yamato K, et al:
Induction of apoptosis in leukemic cells by homovainillic acid
derívate, capsaicin, through oxidative stress: implication of
phosphorylation of p53 at Ser-15 residue by reactive oxygen
species. Cancer Res. 64:1071–1078. 2004.
|
|
16
|
Kim YM, Hwang JT, Kwak DW, Lee YK and Park
OJ: Involvement of AMPK signaling cascade in capsaicin-induced
apoptosis of HT-29 colon cancer cells. Ann NY Acad Sci.
1095:496–503. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Surh YJ, Lee E and Lee JM: Chemoprotective
properties of some pungent ingredients present in red pepper and
ginger. Mutat Res. 402:259–267. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Surh YJ, Lee RC, Park KK, Mayne ST, Liem A
and Miller JA: Chemoprotective effects of capsaicin and diallyl
sulfide against mutagenesis or tumorigenesis by vinyl carbamate and
N-nitrosodimethylamine. Carcinogenesis. 16:2467–2471. 1995.
View Article : Google Scholar
|
|
19
|
Gil YG and Kang MK: Capsaicin induces
apoptosis and terminal differentiation in human glioma A172 cells.
Life Sci. 82:997–1003. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Appendino G: Capsaicin and capsaicinoids.
Modern Alkaloids: Structure, Isolation, Synthesis and Biology.
Fattorusso E and Taglialatela-Scafati O: Wiley-VCH; Weinheim,
Germany: pp. 73–109. 2008
|
|
21
|
Appendino G, De Petrocellis L, Trevisani
M, et al: Development of the first ultra-potent “capsaicinoid”
agonist at transient receptor potential vanilloid type 1 (TRPV1)
channels and its therapeutic potential. J Pharmacol Exp Ther.
312:561–570. 2005.
|
|
22
|
Appendino G, Minassi A, Morello AS, De
Petrocellis L and Di Marzo V: N-Acylvanillamides: development of an
expeditious synthesis and discovery of new acyl templates for
powerful activation of the vanilloid receptor. J Med Chem.
45:3739–3745. 2002. View Article : Google Scholar
|
|
23
|
Voets T, Droogmans G, Wissenbach U,
Janssens A, Flockerzi V and Nilius B: The principle of
temperature-dependent gating in cold- and heat-sensitive TRP
channels. Nature. 430:748–754. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Castillo E, Regla I, Demare P,
Luviano-Jardón A and López-Munguía A: Efficient chemoenzymatic
synthesis of phenylacetylrinvanil: an ultrapotent capsaicinoid.
Synlett. 18:2869–2873. 2008. View Article : Google Scholar
|
|
25
|
Vichai V and Kirtikara K: Sulforhodamine B
colorimetric assay for cytotoxicity screening. Nat Protoc.
1:1112–1116. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Strober W: Trypan blue exclusion test of
cell viability. Curr Protoc Immunol (Appendix 3). Appendix 3B.
2001. View Article : Google Scholar
|
|
27
|
Gross-Bellard M, Oudet P and Chambon P:
Isolation of high-molecular-weight DNA from mammalian cells. Eur J
Biochem. 36:32–38. 1973. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Morré DJ, Chueh PJ and Morré DM: Capsaicin
inhibits preferentially the NADH oxidase and growth of transformed
cells in culture. Proc Natl Acad Sci USA. 92:1831–1835.
1995.PubMed/NCBI
|
|
29
|
Morré DJ, Sun E, Geilen C, et al:
Capsaicin inhibits plasma membrane NADH oxidase and growth of human
and mouse melanoma lines. Eur J Cancer. 32A:1995–2003. 1996.
|
|
30
|
Call JA, Eckhardt SG and Camidge DR:
Targeted manipulation of apoptosis in cancer treatment. Lancet
Oncol. 9:1002–1011. 2008. View Article : Google Scholar
|
|
31
|
Fenech M: Cytokinesis-block micronucleus
cytome assay. Nat Protoc. 1084–1104. 2007. View Article : Google Scholar
|
|
32
|
Oyagbemi AA, Saba AB and Azeez OI:
Capsaicin: a novel chemopreventive molecule and its underlying
molecular mechanisms of action. Indian J Cancer. 47:53–58. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Griffin AM, Butow PN, Coates AS, et al: On
the receiving end V: Patient perceptions of the side effects of
cancer chemotherapy in 1993. Ann Oncol. 7:189–195. 1996. View Article : Google Scholar
|
|
34
|
Carey MP and Burish TG: Etiology and
treatment of the psychological side effects associated with cancer
chemotherapy: a critical review and discussion. Psychol Bull.
104:307–325. 1988. View Article : Google Scholar
|