Introduction
Renal cell carcinoma (RCC) is one of the most common
types of malignant tumor of the human urinary system. To date, the
benefit of conventional therapies for RCC, including surgical,
radiological and chemotherapeutic approaches, is limited. Treatment
with interferon (IFN) and interleukin (IL)-2 remains the main
immunotherapy method for RCC, with the exception of surgery, but
only ~10% of advanced RCC patients respond to cytokine-based
immunotherapy (1,2). Therefore, more effective potential and
combined therapies must be found. New targeted therapy for RCC may
initiate a new avenue for cancer treatment, and targeted therapy
depends on the evaluation of target gene status.
B7-H4, also called B7x/B7s/VTCN1, is the newest B7
superfamily member identified as an inhibitory modulator of the
T-cell response. Combined with its receptor, B7-H4 may inhibit the
proliferation and cytokine production of CD4+ and
CD8+ T cells. The blocking of B7-H4/B7-H4 ligand
interactions may restore antitumor T-cell responses to ovarian
cancer cells (3). Previous studies
have reported that the B7-H4+ status is an independent
predictor of poor prognosis in multivariate analysis (4–9). To
date, few previous studies have analyzed the potential
contributions of B7-H4 to tumoral immune escape and therapeutic
targeting in RCC.
Herein, we present evidence for the potential
contributions of B7-H4 to tumoral immune escape in ccRCC, which
indicates that B7-H4 may be used as a new biological molecular
marker for select treatment options in patients with ccRCC.
Materials and methods
Cell culture, antibodies and
cytokines
The cell line, 786-0, was purchased from the Cell
Bank at the Chinese Academy of Sciences (Beijing, China) and was
cultured according to the manufacturer’s instructions. The
anti-B7-H4 antibody (Ab) was purchased from R&D Systems
(Minneapolis, MN, USA). Other Abs were purchased from Bioss
(Beijing, China) and the cytokines (IL-2, IFN-α and IFN-γ) were
purchased from Xiamen Amoytop Biotech Co., Ltd. (Xiamen,
China).
RCC tissue
A total of 154 specimens of RCC tissue were
collected from RCC patients undergoing radical nephrectomy in the
Department of Urology, Zhejiang Cancer Hospital (Hangzhou, China).
The final staging, grading and histological diagnosis were based on
the pathology report. Ethics approval was obtained from the local
Institution Review Board committee.
Immunohistochemistry (IHC) to tissue
microarray (TMA) and 786-0 cells
IHC was performed using a polyclonal B7-H4 Ab at a
dilution of 1:400. Antigen retrieval was performed by heating the
slides for 7 min in 10 mM citric acid buffer. The TMA consisted of
cores from 154 patients with clear cell RCC (ccRCC). IHC was
analyzed independently by two pathologists, and positive IHC was
determined when ≥5% of the cells showed B7-H4 staining. The 786-0
cells were fixed by 4% paraformaldehyde and directly incubated with
B7-H4 Ab at a dilution of 1:400. The remaining procedures were
performed as described for the TMA.
Cell proliferation assay
Cell proliferation was quantitated by a Cell
Counting Kit-8 (CCK-8) assay to generate a growth inhibition ratio
following stimulation with IL-2, IFN-α and IFN-γ for 24 h. 786-0
cells were seeded at 6,000 cells per well in a 96-well plate and
incubated for 24 h. The culture fluid, containing IL-2, IFN-α and
IFN-γ at the concentrations of 0, 100, 250, 500, 1,000, 2,000,
4,000 and 8,000 U/ml, was then added into each well and incubated
for 24 h. Next, 10 μl CCK-8 was added into each well and incubated
for 2 h. Each well was read at 450 nm using a spectrophotometer
(Eppendorf, Hamburg, Germany).
ELISA to cell culture supernatant
The protein of B7-H4 was diluted to 100, 50, 25, 10,
2, 0.5 and 0.1 ng/ml to be used as a standard. Samples were
collected following centrifugation at 1,200 × g. In total, 40 μl
sample, 10 μl anti-B7-H4 Ab and 50 μl streptomycin-horseradish
peroxidase were added into the ELISA kit and then incubated for 1 h
at 37°C. Following washing three times with phosphate-buffered
saline (PBS), 100 μl chromogenic agent was added to each well. In
addition, 50 μl stop buffer was added to each well following
incubation for 15 min at 37°C. The plates were then read at 450 nm
using a spectrophotometer. The minimum detectable concentration was
determined to be >0.1 ng/ml.
Reverse transcription (RT)-polymerase
chain reaction (PCR)
Total RNA was extracted from the 786-0 cells using
TRIzol reagent (Invitrogen Life Technologies, Carlsbad, CA, USA)
following stimulation with IL-2, IFN-α and IFN-γ (1,000 U/ml) for
24 h. A total of 2 μg RNA was reverse-transcribed using avian
myeloblastosis virus reverse transcription XL (Toyobo Co. Ltd,
Shanghai, China) for 90 min at 42°C in the presence of oligo(dT)
primer (Fermentas, Waltham, MA, USA). PCR was performed using Taq
polymerase. The primer sequences (Ying Wei Chuang, Guangzhou,
China) used were as follows: B7-H4 forward,
5′-CACTCATCATTGGCTTTGGTATTTCAG-3′ and reverse,
5′-CGACAGCTCATCTTTGCCTTCTTTG-3′; and actin forward,
5′-AGCGGGAAATCGTGCGTGAC-3′ and reverse,
5′-ACTCCTGCTTGCTGATCCATATC-3′. PCR was performed for 35 cycles,
which consisted of a pre-soak for 5 min at 94°C, denaturing for 30
sec at 94°C, annealing for 30 sec at 56°C and extension for 30 sec
at 72°C. Following completion of the cycle, the amplified products
were electrophoresed through a 1% agarose gel and stained with
ethidium bromide. Images were captured under an ultraviolet light
transilluminator (Syngene Co., Cambrisge, UK).
Flow cytometry
The surface expression of B7-H4 on the 786-0 cell
line following stimulation with IL-2, IFN-α and IFN-γ was
quantified by flow cytometry on a fluorescence-activated cell
sorter (FACs). For each analysis, 10,000 cells were evaluated. For
detecting intracellular B7-H4 expression, the 786-0 cells were
pre-permeabilized with permeabilization buffer for 10 min.
Following washing twice with PBS, the cells were further fixed by
fixation buffer (4% paraformaldehyde) and then B7-H4 monoclonal Abs
(mAbs) were added. Following extreme washing with PBS, B7-H4
expression was further detected by FACs. The FACs results were
analyzed using CELLQuest™ software (BD Biosciences, Franklin Lakes,
NJ, USA).
Functional cytotoxic assays with blocking
B7-H4 mAb
Lymphoblastoid cell lines were established from
mononuclear cells collected from the peripheral blood of healthy
donors by Ficoll-Hypaque centrifugation. All individuals provided
written informed consent. The cells were incubated with
concanavalin A (1, 2, 4 and 8 μg/ml) for 48 h, and CCK-8 was
performed to detect the lymphocyte proliferation rate of the T
cells. Functional assays were performed by incubation of the T
cells for 48 h, in the absence or presence of isotypic control
(purified mouse IgG1 κ) or B7-H4 blocking mAb. The cytotoxic effect
on the T cells was evaluated by CCK-8, which identifies apoptotic
cells.
Statistical analyses
SPSS version 13.0 (SPSS, Inc., Chicago, IL, USA) and
Microsoft Excel 2003 (Microsoft Corporation, Redmond, WA, USA) were
used for the statistical analyses. P<0.05 was considered to
indicate a statistically significant difference.
Results
Increased B7-H4 expression is associated
with adverse clinical features
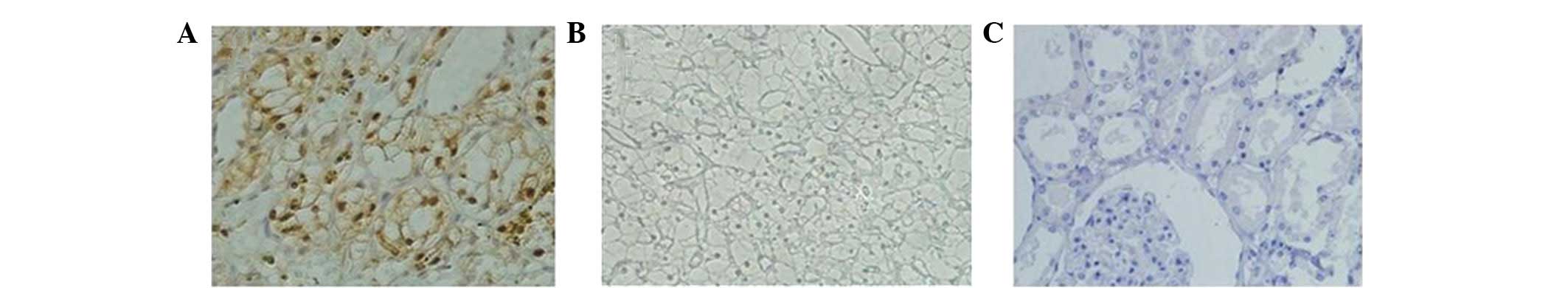
In total, 91 (59.09%) patient specimens exhibited
positive tumor B7-H4 staining (Fig.
1A). A comparison of clinical features by tumor B7-H4
expression is shown in Table I.
Positive tumor B7-H4 expression was associated with adverse
clinical features, including tumor-node-metastasis and clinical
stages.
 | Table IClinical features of tumor B7-H4
expression. |
Table I
Clinical features of tumor B7-H4
expression.
| Feature | B7-H4−
expression (n=63) | B7-H4+
expression (n=91) | χ2
(Fisher) | P-value |
|---|
| Gender |
| Male | 18 | 27 | 0.022 | 0.883 |
| Female | 45 | 64 | | |
| Age at surgery,
years |
| ≥65 | 20 | 28 | 0.017 | 0.898 |
| <65 | 43 | 63 | | |
| 2009 primary tumor
classification |
| T1 | 52 | 52 | 13.291 | 0.004 |
| T2 | 10 | 29 | | |
| T3 | 1 | 9 | | |
| T4 | 0 | 1 | | |
| Regional lymph node
involvement |
|
Nx/N0 | 63 | 87 | 4.282 | 0.039 |
|
N1/N2 | 0 | 4 | | |
| Distant metastases at
nephrectomy |
| M0 | 63 | 86 | 5.377 | 0.020 |
| M1 | 0 | 5 | | |
| 2009 TNM stage
groupings |
| I | 50 | 53 | 9.583 | 0.022 |
| II | 11 | 25 | | |
| III | 1 | 6 | | |
| IV | 1 | 7 | | |
IL-2, IFN-α and IFN-γ may upregulate
B7-H4 expression in 786-0 cells
The CCK-8 assay, a proliferation assay that is
directly proportional to the number of live cells in culture, was
used as an independent measure of the proliferation in the IL-2-,
IFN-α- and IFN-γ-treated 786-0 cells. These results supported the
fact that IL-2, IFN-α and IFN-γ may inhibit the proliferative
activity of 786-0 cells and exhibit a significant dose-effect
correlation. The maximum drug concentration of a 1% cellular
proliferation inhibition rate was 1,000 U/ml. IL-2, IFN-α and IFN-γ
were applied at this concentration to stimulate the 786-0 cells in
order to study the effect of IL-2, IFN-α and IFN-γ on B7-H4
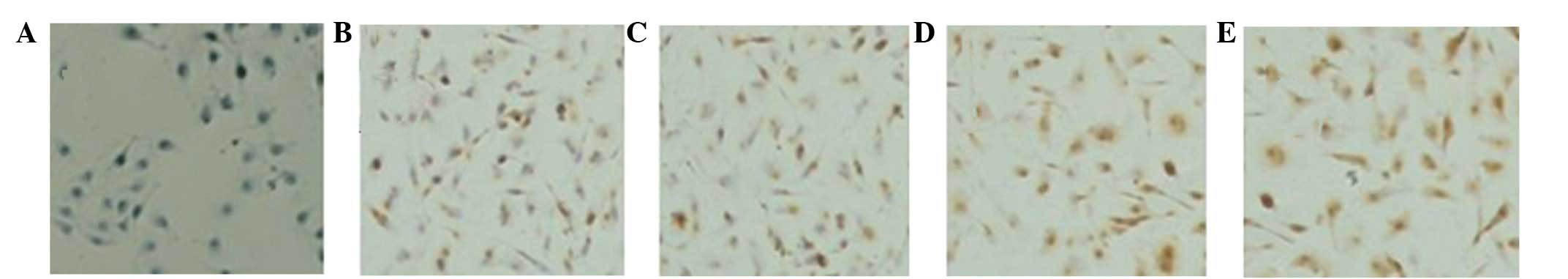
expression in ccRCC cells. The RT-PCR and IHC results showed that
the protein (Fig. 2)and mRNA
(Fig. 3) expression of B7-H4 may be
upregulated by IL-2, IFN-α and IFN-γ, of which, IFN-γ was the most
capable.
The ELISA assay was used to detect the quantitative
expression of soluble B7-H4 (sB7-H4) in the IL-2-, IFN-α- and
IFN-γ-treated 786-0 cells. The sB7-H4 expression was detected in
the unstimulated 786-0 cells at a concentration of 34.42±1.69
ng/ml. Following stimulation with IL-2, IFN-α and IFN-γ for 24 h,
the concentrations increased to 44.89±0.97 ng/ml, 46.74±2.25 ng/ml
and 47.31±1.12 ng/ml, respectively, in which the differences were
statistically significant (P<0.05). Flow cytometry (Fig. 4), which supplied quantified results
of the positive expression of B7-H4 in the 786-0 cells, revealed
similar results. The positive expression rate of B7-H4 in the
unstimulated 786-0 cells was 30.45±0.96%. Following stimulation
with IL-2, IFN-α and IFN-γ for 24h, the positive expression rates
became 44.89±0.94, 46.41±0.55 and 54.18±1.42%, respectively, which
were significantly different compared with the unstimulated cells
(P<0.05). The 786-0 cells stimulated by IFN-γ were the most
capable, and the positive expression rate was significantly
different compared with the other three groups (P<0.05).
However, no significant difference was identified between the cells
stimulated by IL-2 and IFN-α (P=0.235).
B7-H4 is a negative regulator of T-cell
cytotoxicity
To determine the role of B7-H4 in T-cell responses,
different ratios of effector to target cells (30:1, 20:1 and 10:1)
were applied. CCK-8 was used to detect the cytotoxicity of the T
cells. The results showed that masking B7-H4 with a specific
blocking Ab increased the T-cell killing of the 786-0 cells
(P<0.05). With the increase of the concentration of effector
cells, the cytotoxicity also increased significantly (P<0.05).
This identified the inhibitory role of B7-H4 in T cell-dependent
cytotoxicity, as in other tumor cell models (3).
Discussion
RCC is a typical immunogenic tumor frequently
harboring high levels of tumor-infiltrating T lymphocytes and
occasionally exhibiting spontaneous regression of metastases
following primary tumor removal (10–12).
As it is refractory to radiation and chemotherapy, immunotherapy,
including the use of IFN-α and IL-2, is the main treatment choice
for mRCC without surgery. However, in previous studies, the
efficacy of IL-2 and IFN-α has been extremely low in mRCC and it
has not been possible to observe the efficacy of IFN-γ (1,2).
The costimulatory B7 family members not only provide
positive signals to stimulate T-cell activation, but also deliver
negative signals to inhibit T-cell responses. Identified in 2003,
B7-H4 represents the newest member of the B7 family of
costimulatory ligands (13–15). Despite widespread B7-H4 mRNA
expression in various human tissues, the lack of
immunohistochemical staining of B7-H4 in the majority of normal
human tissues indicates that the expression of B7-H4 is relatively
restricted (6). B7-H4 is a type I
transmembrane protein, and expression may be detected in various
types of human cancer tissues, including breast (4), ovarian (5), pancreatic (6) and lung (7) cancer, melanoma (8) and RCC (9). The expression of B7-H4 has been found
to correlate with advanced stages, poor patient survival and tumor
infiltration by T regulatory cells (16), which made it a candidate of choice
for targeted therapy. Notably, in the present study, positive B7-H4
expression was associated with adverse clinical features in ccRCC,
which indicated that B7-H4 may be a feasible candidate of choice
for RCC-targeted therapy.
Tumor infiltrating lymphocytes may be a
manifestation of antitumor immunity, but a more abundant
infiltration of tumor tissue T cells has been associated with a
shorter survival of the RCC patients (11), which indicated that there is a
potential failure mechanism of T-cell immunity in RCC tissues. The
immunological mechanism mediated by T cells is also the main
therapeutic target in the IL-2 and IFN-α treatment of mRCC
patients. The results of the present study demonstrated that IL-2,
IFN-α and IFN-γ may upregulate the expression of B7-H4, which may
inhibit the proliferation and cytokine production of
CD4+ and CD8+ T cells. Additionally, IFN-γ
was found to be the most capable for this, which indicated that the
immune escape pathway induced by B7-H4 may be one of the most
important reasons for the low efficacy of IL-2 and IFN-α and the
inability to observe the efficacy of IFN-γ in mRCC treatment.
The current study further evaluated the functional
ability to reverse T-cell inhibition mediated by the B7-H4 protein,
which indicated that masking B7-H4 with a specific blocking Ab may
increase the cytotoxicity of T cells in ccRCC. These results
confirmed that B7-H4 is a regulatory molecule engaged in negative
signaling that impacts anti-responses mediated by T cells in ccRCC,
and also establishes a new paradigm for ccRCC cell eradication
using B7-H4-based targeting. The study indicates that the blocking
of B7-H4/B7-H4 ligand interactions may represent a feasible
therapeutic strategy for ccRCC.
Targeting immune checkpoint molecules, such as CTLA4
and B7-H1, has elicited marked clinical responses, particularly in
patients with pre-existing immune responses (17–19).
Based on our studies, we propose that the blocking of B7-H4/B7-H4
ligand interactions may be used as a potential treatment for RCC
patients. In addition, B7-H4 detection may be used to select the
appropriate treatment for RCC patients. RCC patients whose TMA
shows positive expression of B7-H4 must not select IFN-α/IL-2
treatment alone, since the T-cell-mediated antitumor responses must
have been repressed by the B7-H4 mediated immune escape pathway.
B7-H4 blocking treatment alone or combined with IFN-α/IL-2 may be
more suitable for B7-H4+ patients.
Acknowledgements
The authors would like to thank Shiyong Huang,
Jianhui Chen, Desheng Zhu, Yiming Su, Rongjin Fang, Cheng Zhao,
Zhao Hu, Youbiao Wang and Xiyuan Mu for participating in useful
discussions. The current study was funded by grants from project
support from the Appropriate Technical Transformation of Zhejiang
(no. 2013ZHB001) and the Outstanding Scientific Research and Talent
Cultivation of Zhejiang Cancer Hospital (no. 2012YC004).
References
|
1
|
Yang JC, Sherry RM, Steinberg SM, et al:
Randomized study of high-dose and low-dose interleukin-2 in
patients with metastatic renal cancer. J Clin Oncol. 21:3127–3132.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fyfe G, Fisher RI, Rosenberg SA, et al:
Results of treatment of 255 patients with metastatic renal cell
carcinoma who received high-dose recombinant interleukin-2 therapy.
J Clin Oncol. 13:688–696. 1995.
|
|
3
|
Dangaj D, Lanitis E, Zhao A, et al: Novel
recombinant human b7-h4 antibodies overcome tumoral immune escape
to potentiate T cell antitumor responses. Cancer Res. 73:4820–4829.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tringler B, Zhuo S, Pilkington G, et al:
B7-h4 is highly expressed in ductal and lobular breast cancer. Clin
Cancer Res. 11:1842–1848. 2005. View Article : Google Scholar
|
|
5
|
Kryczek I, Zou L, Rodriguez P, et al:
B7-H4 expression identifies a novel suppressive macrophage
population in human ovarian carcinoma. J Exp Med. 203:871–881.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Choi IH, Zhu G, Sica GL, et al: Genomic
organization and expression analysis of B7-H4, an immune inhibitory
molecule of the B7 family. J Immunol. 171:4650–4654. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Sun Y, Wang Y, Zhao J, et al: B7-H3 and
B7-H4 expression in non-small-cell lung cancer. Lung cancer.
53:143–151. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Quandt D, Fiedler E, Boettcher D, et al:
B7-h4 expression in human melanoma: its association with patients’
survival and antitumor immune response. Clin Cancer Res.
17:3100–3111. 2011.PubMed/NCBI
|
|
9
|
Krambeck AE, Thompson RH, Dong H, et al:
B7-H4 expression in renal cell carcinoma and tumor vasculature:
associations with cancer progression and survival. Proc Natl Acad
Sci USA. 103:10391–10396. 2006. View Article : Google Scholar
|
|
10
|
Bromwich EJ, McArdle PA, Canna K, et al:
The relationship between T-lymphocyte infiltration, stage, tumour
grade and survival in patients undergoing curative surgery for
renal cell cancer. Br J Cancer. 89:1906–1908. 2003. View Article : Google Scholar
|
|
11
|
Nakano O, Sato M, Naito Y, et al:
Proliferative activity of intratumoral CD8(+) T-lymphocytes as a
prognostic factor in human renal cell carcinoma: clinicopathologic
demonstration of antitumor immunity. Cancer Res. 61:5132–5136.
2001.
|
|
12
|
Couillard DR and DeVere-White RW: Surgery
of renal cell carcinoma. Urol Clin North Am. 20:263–275. 1993.
|
|
13
|
Zang X, Loke P, Kim J, et al: B7x: a
widely expressed B7 family member that inhibits T cell activation.
Proc Natl Acad Sci USA. 100:10388–10392. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Prasad DV, Richards S, Mai XM and Dong C:
B7S1, a novel B7 family member that negatively regulates T cell
activation. Immunity. 18:863–873. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Sica GL, Choi IH, Zhu G, et al: B7-H4, a
molecule of the B7 family member, negatively regulates T cell
immunity. Immunity. 18:849–861. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kryczek I, Wei S, Zhu G, et al:
Relationship between B7-H4, regulatory T cells, and patient outcome
in human ovarian carcinoma. Cancer Res. 67:8900–8905. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Brahmer JR, Tykodi SS, Chow LQ, et al:
Safety and activity of anti-PD-L1 antibody in patients with
advanced cancer. N Engl J Med. 366:2455–2465. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Topalian SL, Hodi FS, Brahmer JR, et al:
Safety, activity, and immune correlates of anti-PD-1 antibody in
cancer. N Engl J Med. 366:2443–2454. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ribas A: Tumor immunotherapy directed at
PD-1. N Eng J Med. 366:2517–2519. 2012. View Article : Google Scholar : PubMed/NCBI
|