Introduction
Mesenchymal chondrosarcoma is a rare and aggressive
variant subtype of chondrosarcoma, which represents ~1% of all
chondrosarcomas. It commonly arises in the bone, however, in
~30–40% of cases, it occurs in an extraskeletal location (1).
Extraskeletal mesenchymal chondrosarcoma (ESMC) may
occur in any location that contains mesenchymal cells, however, the
majority arise in the lower extremities (particularly the thigh),
leptomeninges or the orbit (2).
Other sites, including the retroperitoneum, are extremely rare and
to the best of our knowledge, no cases of primary retroperitoneal
ESMC involving the vena cava have previously been reported. The
current study presents the case of a 61-year-old female with
primary retroperitoneal ESMC involving the vena cava. Patient
provided written informed consent.
Case report
On 27th June 2013, a 61-year-old female presented to
Zhongnan Hospital of Wuhan University (Wuhan, China) with a
six-month history of persistent abdominal pain and distension,
occasional reflux and nausea, as well as depression, fatigue and
weight loss of 10 kg. The patient had no history of any fever,
night sweats, chest pain, diarrhea or blood loss in the stools. The
patient’s general physical and chest examinations were
unremarkable, however, the abdominal examination revealed right
subabdominal tenderness and multiple hard lumps of different sizes.
The patient’s past medical and personal histories included cardiac
disease, hysteromyoma and peritoneal myxoma of 12 years. The
laboratory results revealed the patient’s hemoglobin levels to be
80 g/l with a red cell count of 2.61×1012 cells/l. In
addition, the patient’s serum lactate dehydrogenase levels were 487
U/l (normal range, 135–225 U/l) and mannosylated antigen CA125
levels were 206.5 U/ml (normal range, 0–35 U/ml).
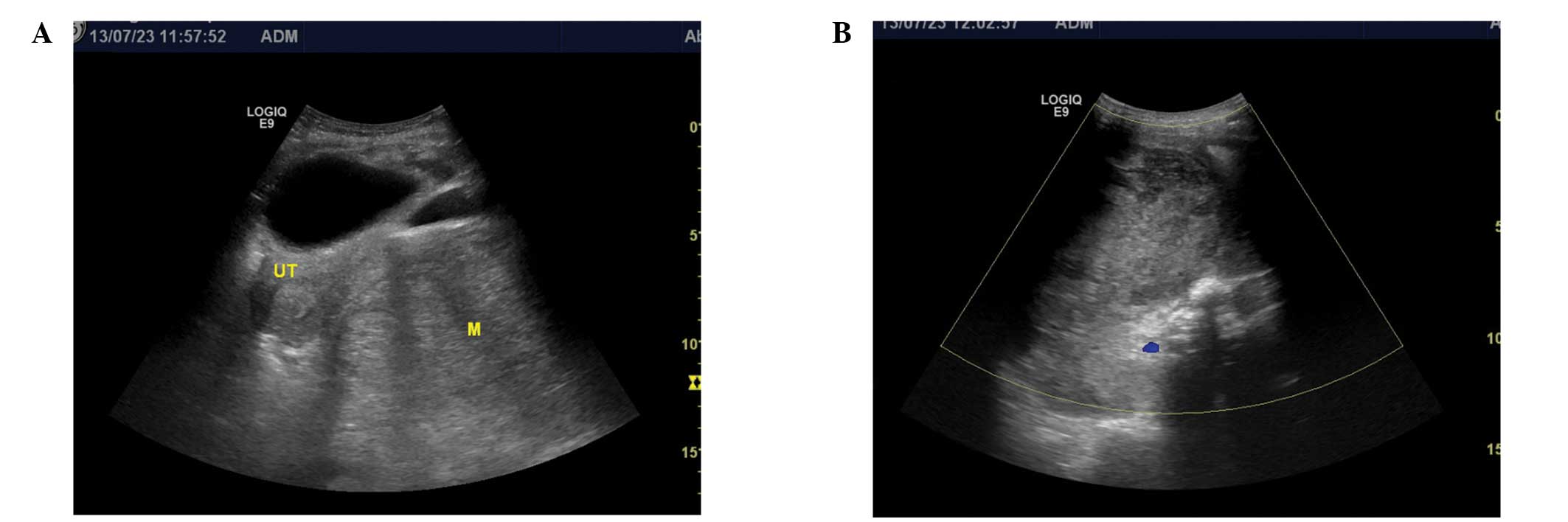
Abdominal and pelvic ultrasound examinations were
performed and revealed two huge, heterogeneous retroperitoneal
masses located on the rectouterine fossa adjacent to the inferior
vena cava, which measured between 126×103 and 95×78 mm in size
(Fig. 1A and B). The tumors
exhibited multiple scattered areas of increased echogenicity with
dense posterior shadowing that suggested foci of calcification.
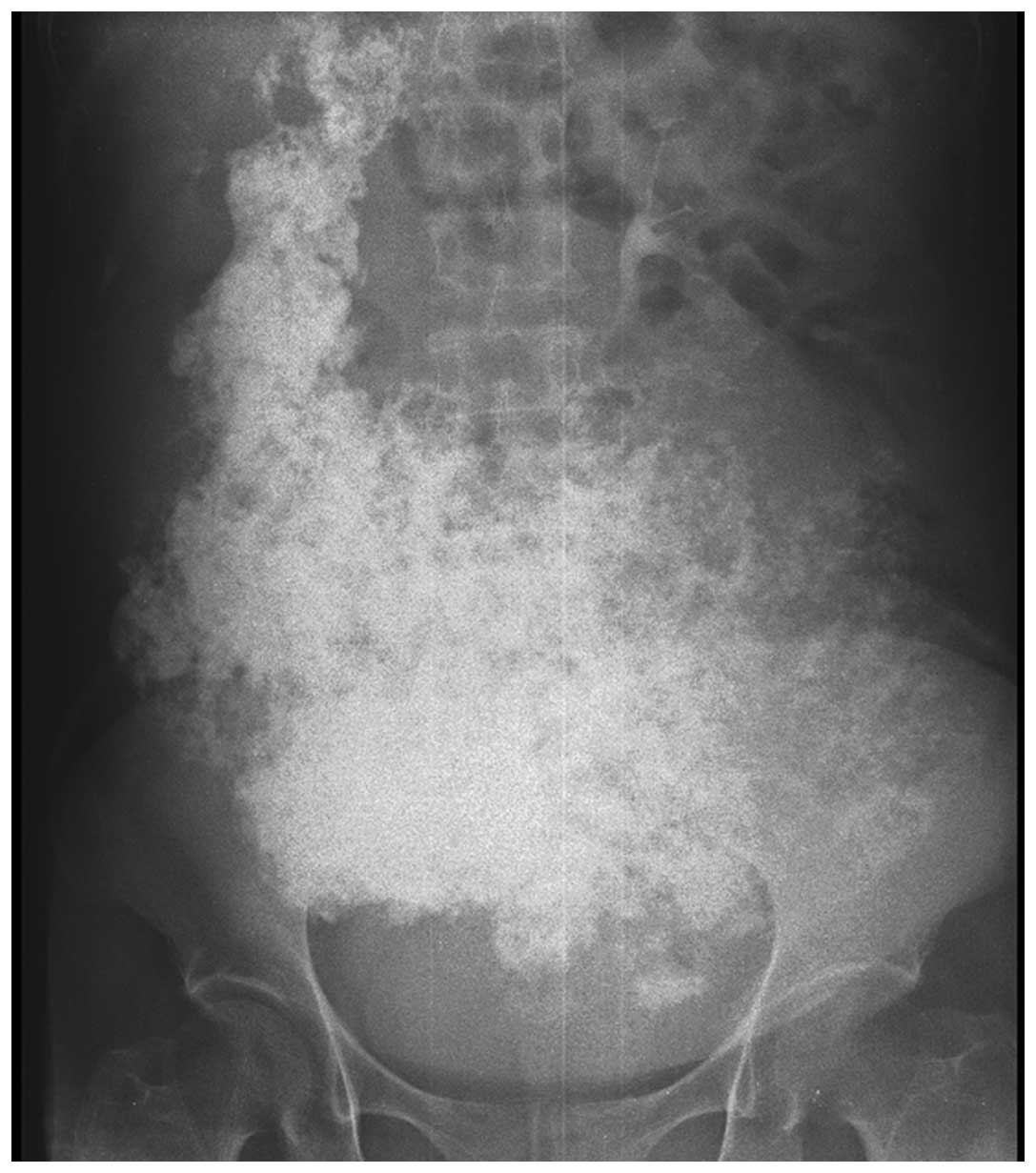
The plain radiography revealed extensive
calcifications in the abdomen (Fig.
2). Furthermore, unenhanced transverse computed tomography (CT)
scans of the abdomen and pelvis revealed multiple different sized
masses located in the right hepatorenal recess, retroperitoneum
(Fig. 3A), inferior vena cava
(Fig. 3B) and rectouterine fossa.
The masses appeared as heterogeneous masses with extensive and
dense, as well as ring- and arc-like calcifications.
Contrast-enhanced CT was performed following the mechanical
injection of 80 ml non-ionic iopromide (370 mg/ml of iodine) into
the antecubital vein at a rate of 3.0 ml/sec. Following the
initiation of the infusion of contrast material, images were
captured after a scanning delay of 45 sec. The contrast-enhanced
transverse CT scan revealed subtle heterogenous enhancement in the
periphery of the masses, however, the majority of masses were not
found to demonstrate enhancement (Fig.
3C). In addition, the mass in the inferior vena cava was
clearly identifiable in the coronal plane (Fig. 3D).
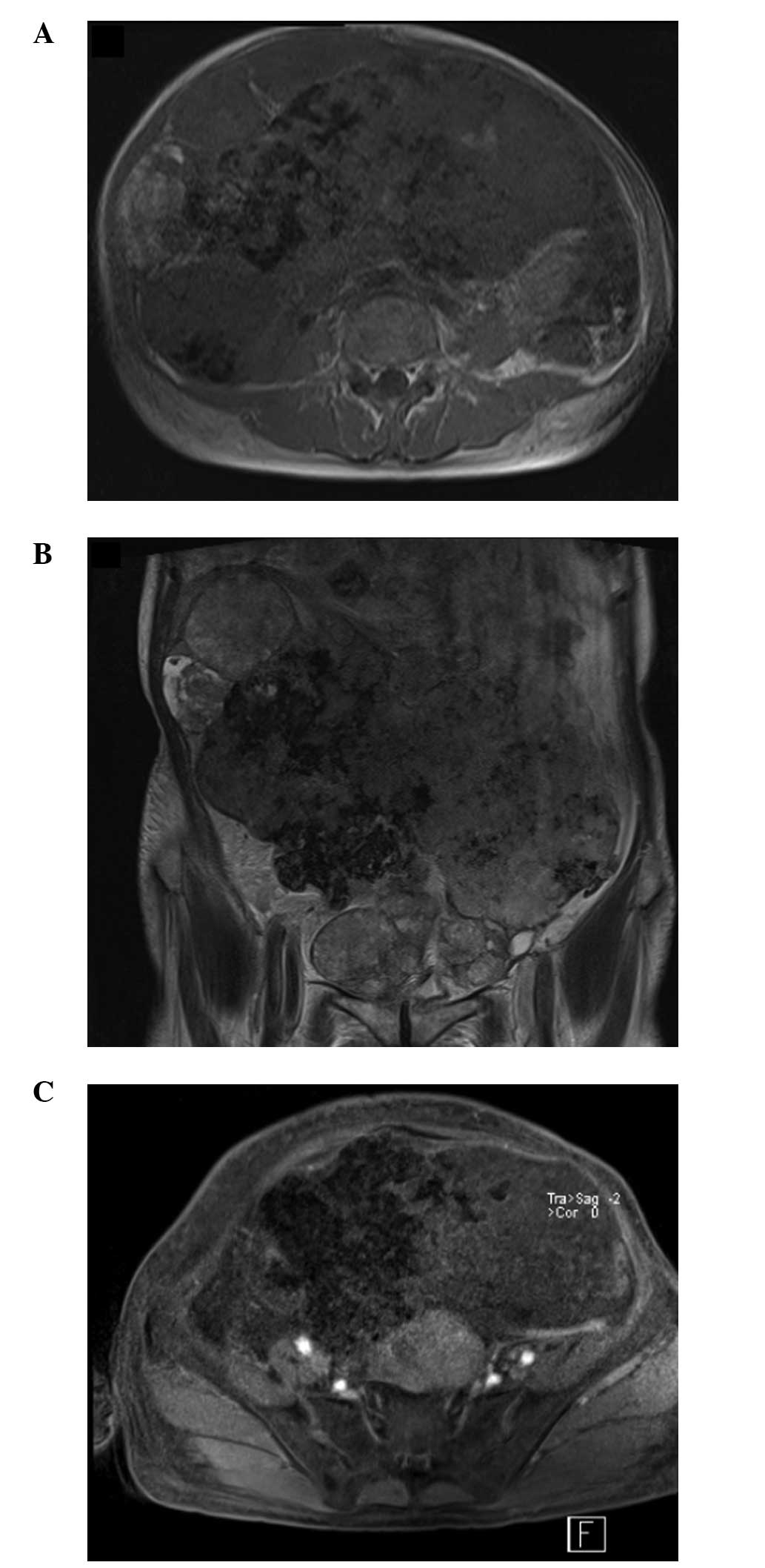
The patient also underwent abdominal and pelvic
magnetic resonance imaging (MRI) (Siemens Trio 3.0T; Siemens
Medical Solutions USA, Inc., Malvern, PA, USA) examinations and
corresponding gadolinium-based contrast enhancement. On T1-weighted
images (T1W1), all lesions showed low signal intensity and on
T2-weighted images (T2WI), all lesions predominantly showed
slightly high signal intensity, whereas the central area exhibited
low intensity in the two images (Fig.
4A and B). Furthermore, enhanced MRI revealed peripheral and
mild speculated enhancement around the lesions, which was
considered to be the result of calcification (Fig. 4C).
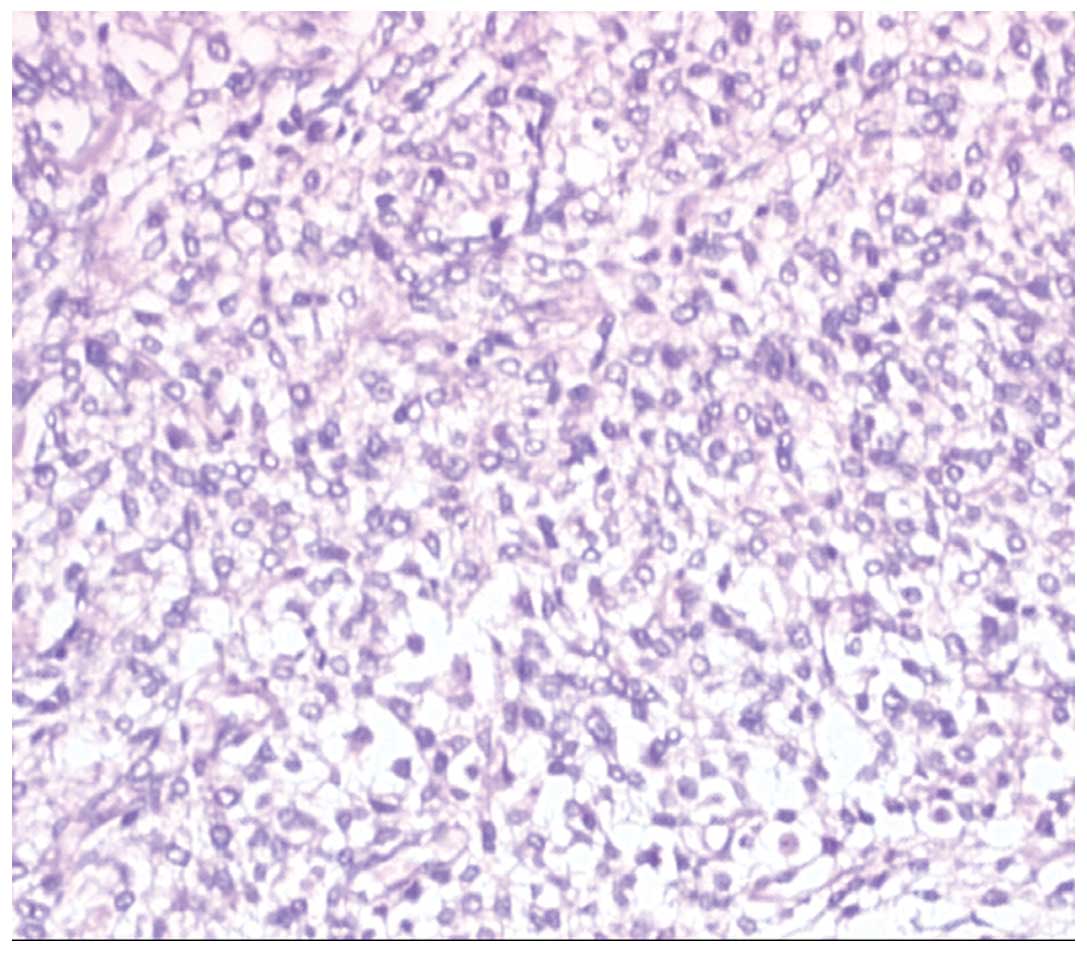
Subsequently, the patient underwent resection of the
masses and the histological examination of the masses revealed
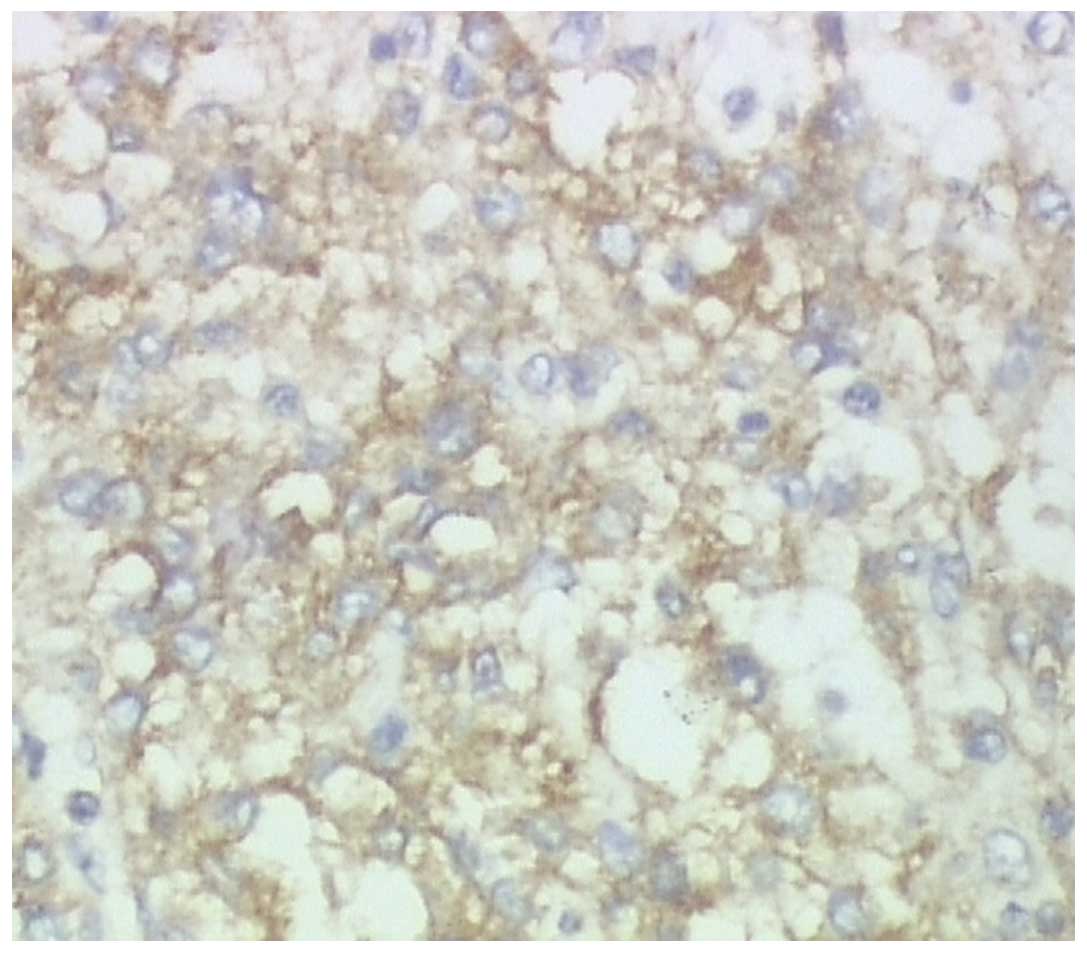
observations consistent with those of ESMC (Fig. 5). In addition, immunohistochemistry
of the cells showed reactivity for CD99 (Fig. 6), but was negative for CD117, Dog1,
CD34, S100, CD31, myogenic differentiation 1, CD138, CD1a, CD20,
CD3, synaptophysin, chromogranin A and Bcl-6. Additionally, the
Ki-67 index was 60%.
Discussion
Chondrosarcomas are a heterogeneous group of
malignant neoplasms that produce a cartilaginous matrix. Although
the majority of chondrosarcomas arise from cartilaginous or bony
structures, they may also develop in extraskeletal locations, such
as the soft tissues, where cartilage is not usually found. The
histological subtypes of extraskeletal chondrosarcoma include
myxoid, mesenchymal and well-differentiated, with the myxoid
subtype being the most common (3).
This highly malignant, cartilage-producing sarcoma was first
described in the bone by Lichtenstein and Bernstein in 1959
(4), and in the soft tissue by
Dowling in 1964 (5). Compared with
the myxoid subtype, ESMC is rare, more aggressive and exhibits a
poor prognosis (6). Furthermore,
ESMC accounts for <1% of all sarcomas, which are located mainly
in the orbit, the cranial and spinal meningeal coverings, the lower
limbs and particularly in the thigh (2). In rare instances, this type of tumor
has also been found to arise in the mediastinum, hand musculature,
retroperitoneum and kidney (1). A
literature search, limited to the English language, identified few
cases of ESMC of the retroperitoneum and no reported cases of
primary retroperitoneal ESMC involving the vena cava.
ESMC has a marginal female predominance and has two
peak ages of incidence in adults, depending on its location; 23.5
years old (range, 5–48 years old) for EMSC patients with central
nervous system involvement and 43.9 years old (range, 21–62 years
old) for ESMC patients with soft-tissue and/or muscular involvement
(7). The patient presented in the
current study was a 61-year-old female.
Ultrasonography is not a conventional diagnostic
tool for mesenchymal chondrosarcoma, however, it can be used as the
first imaging modality when these masses arise from soft tissue.
The images usually reveal a solid, heterogeneous mass, often
containing scattered areas of increased echogenicity and posterior
shadowing consistent with the features of multiple foci of
calcification (8,9).
Conventional radiography has less impact on the
diagnosis of ESMC due to a lower density resolution and the
overlapping of adjacent structures. CT depicts ESMC as a soft
tissue mass with ring- and arc-like, stippled and highly opaque
calcifications. Contrast-enhanced CT depicts EMSC as a soft tissue
mass, with a portion of enhancing viable tumor in the periphery and
central focal low attenuation areas that possibly present necrosis
(2). In the present case of EMSC,
the CT scan revealed extensive and dense, as well as ring- and
arc-like calcifications in the lobulated soft tissue mass. The
ring-and-arc mineralization was the most important of the imaging
observations associated with ESMC and, thus, is of important value
in the qualitative diagnosis. Calcification has also been reported
to be common (67%), but not extensive in mesenchymal chondrosarcoma
(10), however, in the present
study the patient exhibited wide and dense calcification.
MRI of ESMC patients often show equal or low signal
intensity on T1W1 and mixed high and low signal intensity on T2WI.
Since the intratumoral calcified and non-calcified components of
ESMC demonstrate low and high intensity on T2WI, respectively, they
are visible as areas of high signal intensity surrounding areas of
low signal intensity or as the ‘salt and pepper’ sign. Such
observations have previously been reported and are considered an
important characteristic of the disease (11). In addition, a diffuse heterogeneous
or nodular enhancement has been observed in calcified and
non-calcified areas following enhanced scanning, which are
important diagnostic signs for ESMC (11,12).
In the present study, the patient showed similar CT and MRI
observations.
However, the imaging results of the present study
did not enable the differentiation of ESMC from other neoplasms.
Thus, a definitive diagnosis was determined based on the
histological features. Microscopically, the most common feature of
mesenchymal chondrosarcoma is a biphasic pattern composed of sheets
of undifferentiated round, oval or spindle-shaped cells, as well as
small, usually well-defined, islets of well-differentiated,
benign-appearing cartilaginous tissue (13). Immunohistochemistry may further aid
the differentiation of these lesions from other mimicking lesions,
as ESMC is positive for CD99 and vimentin in the mesenchymal cells
and S-100 in the chondroid areas (14). S-100 protein was initially found in
the majority of ESMCs, however, studies have shown that <20% of
ESMCs are S-100-positive (15). In
addition, CD99 positivity is observed in Ewing’s sarcoma and has
also been reported as a potential positive marker for mesenchymal
chondrosarcoma (7). Currently, no
specific immunohistochemical markers have been determined for ESMC,
however, the present case was positive for CD99 expression.
Surgery is usually the primary treatment modality
for mesenchymal chondrosarcoma. However, if the tumor cannot be
completely resected or in cases of recurrent lesions, radiation
therapy and chemotherapy must be considered (14). Although, the benefits of
chemotherapy and/or radiation therapy remain unclear. The prognosis
of mesenchymal chondrosarcoma is poor due to the high probability
of metastases, which often occur several years following the
initial treatment. Furthermore, Nakashima et al (13) reported a five-year survival rate of
54% and a 10-year survival rate of 27%.
In conclusion, the current study presents the first
case of retroperitoneal extraskeletal myxoid chondrosarcoma
involving the vena cava. Although imaging features of ESMC are
non-specific, we speculate the following characteristics: i)
Calcification, particularly the ring- and arc-like calcifications;
ii) ‘salt and pepper’ sign on T2WI; iii) diffuse heterogeneous or
nodular enhancement; and iv) areas strengthened by calcification.
Therefore, typical dense and arc-like calcifications may confirm
the diagnosis of mesenchymal chondrosarcoma of extraskeletal
origin.
Acknowledgements
The authors would like to thank Zhi-gao Xu for
obtaining the pathological and immunohistochemical photomicrographs
presented in the current study.
References
|
1
|
González-Cámpora R, Otal Salaverri C,
Gomez Pascual A, Hevia Vazquez A and Galera Davidson H: Mesenchymal
chondrosarcoma of the retroperitoneum. Report of a case diagnosed
by fine needle aspiration biopsy with immunohistochemical, electron
microscopic demonstration of S-100 protein in undifferentiated
cells. Acta Cytol. 39:1237–1243. 1995.
|
|
2
|
Shapeero LG, Vanel D, Couanet D, Contesso
G and Ackerman LV: Extraskeletal mesenchymal chondrosarcoma.
Radiology. 186:819–826. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Taori K, Patil P, Attarde V, Chandanshive
S, Rangankar V and Rewatkar N: Primary retroperitoneal
extraskeletal mesenchymal chondrosarcoma: a computed tomography
diagnosis. Br J Radiol. 80:e268–e270. 2007. View Article : Google Scholar
|
|
4
|
Lichtenstein L and Bernstein D: Unusual
benign and malignant chondroid tumors of bone. A survey of some
mesenchymal cartilage tumors and malignant chondroblastic tumors,
including a few multicentric ones, as well as many atypical benign
chondroblastomas and chondromyxoid fibromas. Cancer. 12:1142–1157.
1959. View Article : Google Scholar
|
|
5
|
Dowling EA: Mesenchymal chondrosarcoma. J
Bone Joint Surg Am. 46:747–754. 1964.PubMed/NCBI
|
|
6
|
Bertoni F, Picci P, Bacchini P, et al:
Mesenchymal chondrosarcoma of bone and soft tissues. Cancer.
52:533–541. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Müller S, Söder S, Oliveira AM, Inwards CY
and Aigner T: Type II collagen as specific marker for mesenchymal
chondrosarcomas compared to other small cell sarcomas of the
skeleton. Mod Pathol. 18:1088–1094. 2005.PubMed/NCBI
|
|
8
|
Lange TA, Austin CW, Seibert JJ, Angtuaco
TL and Yandow DR: Ultrasound imaging as a screening study for
malignant soft tissue tumors. J Bone Joint Surg Am. 69:100–105.
1987.PubMed/NCBI
|
|
9
|
Johnson DB, Breidahl W, Newman JS, Devaney
K and Yahanda A: Extraskeletal mesenchymal chondrosarcoma of the
rectus sheath. Skeletal Radiol. 26:501–504. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Murphey MD, Walker EA, Wilson AJ,
Kransdorf MJ, Temple HT and Grannon FH: From the archives of the
AFIP: imaging of primary chondrosarcoma: radiologic-pathologic
correlation. Radiographics. 23:1245–1278. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hashimoto N, Ueda T, Joyama S, Araki N,
Beppu Y, Tatezaki S, Matsumoto S, et al: Extraskeletal mesenchymal
chondrosarcoma: an imaging review of ten new patients. Skeletal
Radiol. 34:785–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yang BT, Wang ZC, Liu S, Xian JF, Zhang
ZY, Liu ZL and Lan BS: CT and MRI diagnosis of chondrosarcoma in
sinonasal and orbital region. Zhonghua Fang She Xue Za Zhi.
6:572–576. 2006.(In Chinese).
|
|
13
|
Nakashima Y, Unni KK, Shives TC, Swee RG
and Dahlin DC: Mesenchymal chondrosarcoma of bone and soft tissue:
A review of 111 cases. Cancer. 57:2444–2453. 1986. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yang BT, Wang YZ, Wang XY and Wang ZC:
Mesenchymal chondrosarcoma of the orbit: CT and MRI findings. Clin
Radiol. 67:346–351. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Lucas DR, Fletcher CD, Adsay NV and
Zalupski MM: High-grade extraskeletal myxoid chondrosarcoma: a
high-grade epithelioid malignancy. Histopathology. 35:201–208.
1999. View Article : Google Scholar : PubMed/NCBI
|