Introduction
Proinflammatory cytokines, including chemokines,
attack inflammatory cells and regulate hematopoietic cell migration
to bone marrow or lymph nodes and neuronal migration (1,2).
Abnormal expression of chemokines or their receptors is positively
correlated with the development, progression and metastasis of
tumor cells (3,4). CXC chemokine receptor-4 (CXCR4) is
highly expressed on the surface of several different types of
tumors (5). CXCL12 (CXC motif
chemokine 12) [also known as stromal cell-derived factor (SDF-1)]
has been identified as the specific ligand of CXCR4 and it is
likely that the CXCR4/CXCL12 axis is involved in the development,
progression and metastasis of tumors (6,7).
Forkhead box 3 (Foxp3) is a transcription factor that is required
for the differentiation of regulatory T cells (Tregs) and is
associated with T-cell tolerance and immune suppression (8). Emerging evidence indicates that Foxp3
is expressed in tumor cells and may play a role in the tumor
evasion of cancers (9–11).
Neuroblastoma is one of the most common types of
solid tumor found in children worldwide. As metastasis of
neuroblastoma occurs at a high frequency (12), metastasis is the ultimate step in
the progression of tumor cells toward autonomy from the host, it is
required to identify the mechanisms underlying tumor cell
metastasis. Although the abnormal expression of CXCR4 and Foxp3 may
be involved in the metastasis and immune evasion of other types of
tumors (13–16), their role in neuroblastoma and their
response to chemotherapy remain largely unclear. Thus, the present
study aimed to examine the expression of CXCR4 and Foxp3 in
neuroblastoma cell lines LAN-5 and SK-N-SH. The effects of
chemotherapy drugs, such as cyclophosphamide (CTX) and pirarubicin
(THP), on the expression of CXCR4 and Foxp3 were also
investigated.
Materials and methods
Cell lines and culture condition
The LAN-5 neuroblastoma cell line was kindly
provided by Dr Stuart Elliott Siegel (Children’s Hospital Los
Angeles, Los Angeles, CA, USA) and the origin has been described
previously (17,18); the SK-N-SH cell line was purchased
from American Type Culture Collection (Cambridge, MA, USA). The
cells were maintained in RPMI-1640 (Gibco, Paisley, UK)
supplemented with 10% fetal calf serum (FCS; Gibco) in an
atmosphere of 5% CO2 at 37°C.
Reagents
CTX was purchased from Shanxi Pude Pharmaceuticals
Co., Ltd. (Shanxi, China) and THP was purchased from Shenzhen Wanle
Pharmaceuticals Co., Ltd. (Shenzhen, China). The polyclonal mouse
anti-human CXCR4 antibody was purchased from BioLegend (San Diego,
CA, USA). TRIzol was purchased from Invitrogen Life Technologies
(Carlsbad, CA, USA). The reverse transcription kit (ReverTra Ace-α)
and SYBR Green Real-time PCR kit were purchased from Toyobo Co.,
Ltd. (Osaka, Japan).
Quantitative polymerase chain reaction
(qPCR)
A total of 5×106 cells were collected.
Total RNA was isolated using TRIzol reagent and transcribed into
cDNA using a reverse transcription kit (Toyobo Co., Ltd.). qPCR was
performed using 2× SYBR Green Real-time PCR Master Mix according to
the manufacturer’s instruction (Toyobo, Co., Ltd). For
amplification of CXCR4, the sense and antisense primers were
5′-CGTGCCCTCCTGCTGACTATT-3′ and 5′-GCCAACCATGATGTGCTGAA-3′,
respectively. The forward and reverse primers for Foxp3 were
5′-GTTCACACGCATGTTTGCCTTC-3′ and 5′-GCACAAAGCACTTGTGCAGACTC-3′,
respectively. The forward and reverse primers for the control gene,
human GAPDH, were 5′-AATGGAAATCCCATCACCATCT-3′ and
5′-CGCCCCACTTGATTTTGG-3′, respectively. PCR was performed in a
MX3000 machine (Eppendorf, Hamburg, Germany) with the following
conditions: 40 cycles of 95°C for 30 sec, 55°C for 30 sec and 72°C
for 30 sec. Each sample was determined in triplicate. Target mRNA
expression was calculated as target gene copies/GAPDH copies. The
relative standard curve method was used to determine the relative
mRNA expression of CXCR4 and Foxp3 genes.
Cell proliferation assays
The LAN-5 or SK-N-SH cells in RPMI-1640 medium
supplemented with 10% FCS were plated at 1×104
cells/well in a 96-well flat-bottom tissue culture plate and
incubated for 24 h at 37°C. CTX or THP was added to the culture
medium and the cells were incubated for 72 h at 37°C. To each well,
10 μl of 5 mg/ml MTT (Sigma-Aldrich, St. Louis, MO, USA) was added
and, after 6 h of incubation, 100 μl of dimethylsulfoxide
(Sigma-Aldrich) was added to each well and absorbance was measured
at 570 nm on a microplate reader (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA).
Flow cytometry
A total of 1×106 LAN-5 or SK-N-SH cells
were collected and 1 μl of mouse phycoerythrin (PE) labeled
monoclonal anti-human CXCR4 antibody (BioLegend) was added to each
sample and incubated at 4°C for 30 min. The cells were washed three
times and analyzed on a Becton-Dickinson FACSCalibur flow cytometer
(BD Biosciences, Franklin Lakes, NJ, USA).
To detect the intracellular Foxp3 expression, the
cells were first permeablized with the permeabilization buffer
according to the manufacturer’s instructions (eBioscience, San
Diego, CA, USA). Mouse PE labeled monoclonal anti-human Foxp3
antibody (1 μl; BioLegend) was added to the cells and incubated at
4°C for 30 min. After three washes, the cells were analyzed on the
FACSCalibur flow cytometer.
Statistical analysis
Differences between cases and controls regarding the
means and proportions were compared using Student’s t-test and the
χ2 test. SPSS software, version 17.0 (SPSS, Inc.,
Chicago, IL, USA) was used for statistical analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of CXCR4 and Foxp3 in LAN-5
and SK-N-SH cells
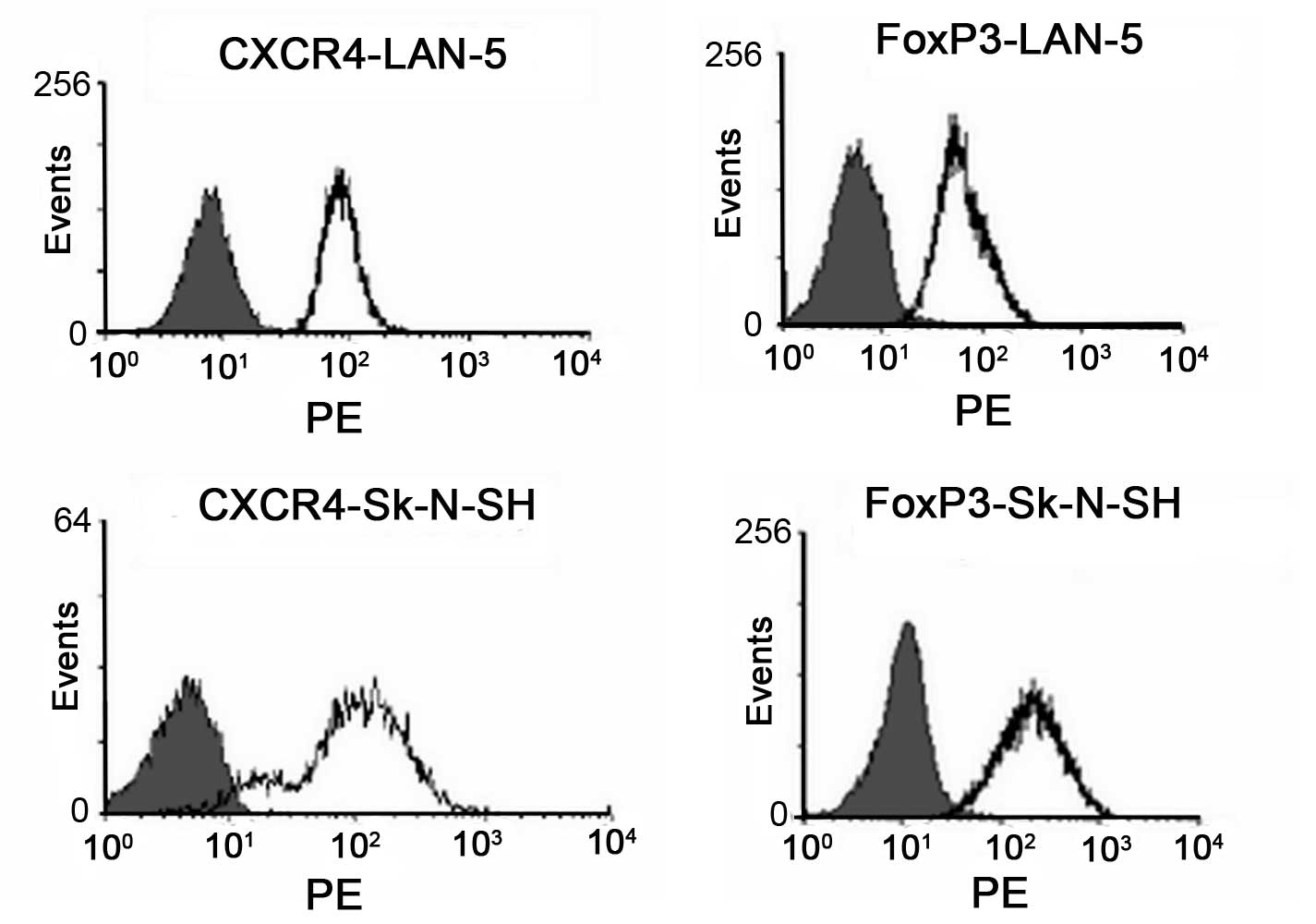
To investigate the role of CXCR4 in the progression
and metastasis of neuroblastoma, the expression of CXCR4 in LAN-5
and SK-N-SH cells was analyzed by FACS. As shown in Fig. 1, CXCR4 was highly expressed in the
two cell lines. Foxp3 was also highly expressed in the LAN-5 and
SK-N-SH cell lines (Fig. 1).
Chemotherapeutic drugs inhibit the
proliferation of neuroblastoma cells
The inhibition effects of CTX and THP on the
proliferation of LAN-5 and SK-N-SH cells were analyzed by the MTT
assay. CTX inhibited the proliferation of LAN-5 and SK-N-SH cells
at the half maximal inhibitory (IC50) value of 6.7 and
3.8 μmol/l, respectively. THP inhibited the proliferation of LAN-5
and SK-N-SH cells at the IC50 value of 0.067 μg/ml.
Chemotherapy treatment downregulates the
expression of CXCR4 and Foxp3
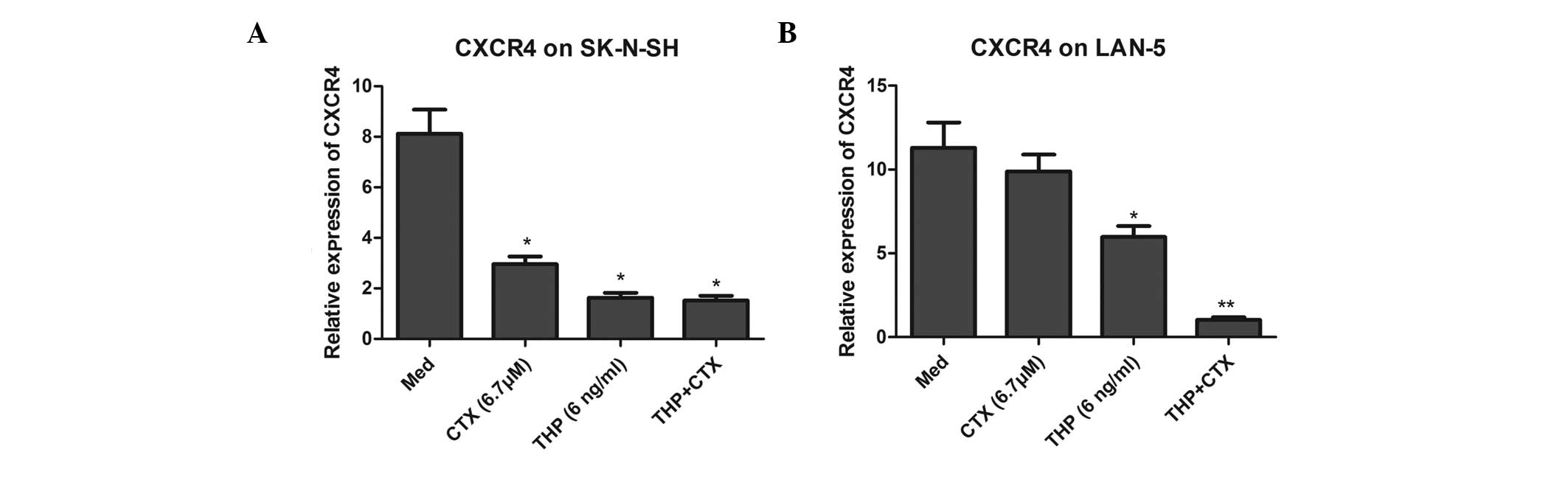
As shown in Fig. 2,
at the protein level, CTX and THP decreased the expression of CXCR4
on the surface of LAN-5 cells (P<0.05). However, CTX and THP did
not affect the protein level of CXCR4 in the SK-N-SH cells. THP,
but not CTX, significantly reduced the mRNA level of CXCR4 in LAN-5
cells. However, THP and CTX significantly downregulated the mRNA
expression of CXCR4 in the SK-N-SH cells (Fig. 3).
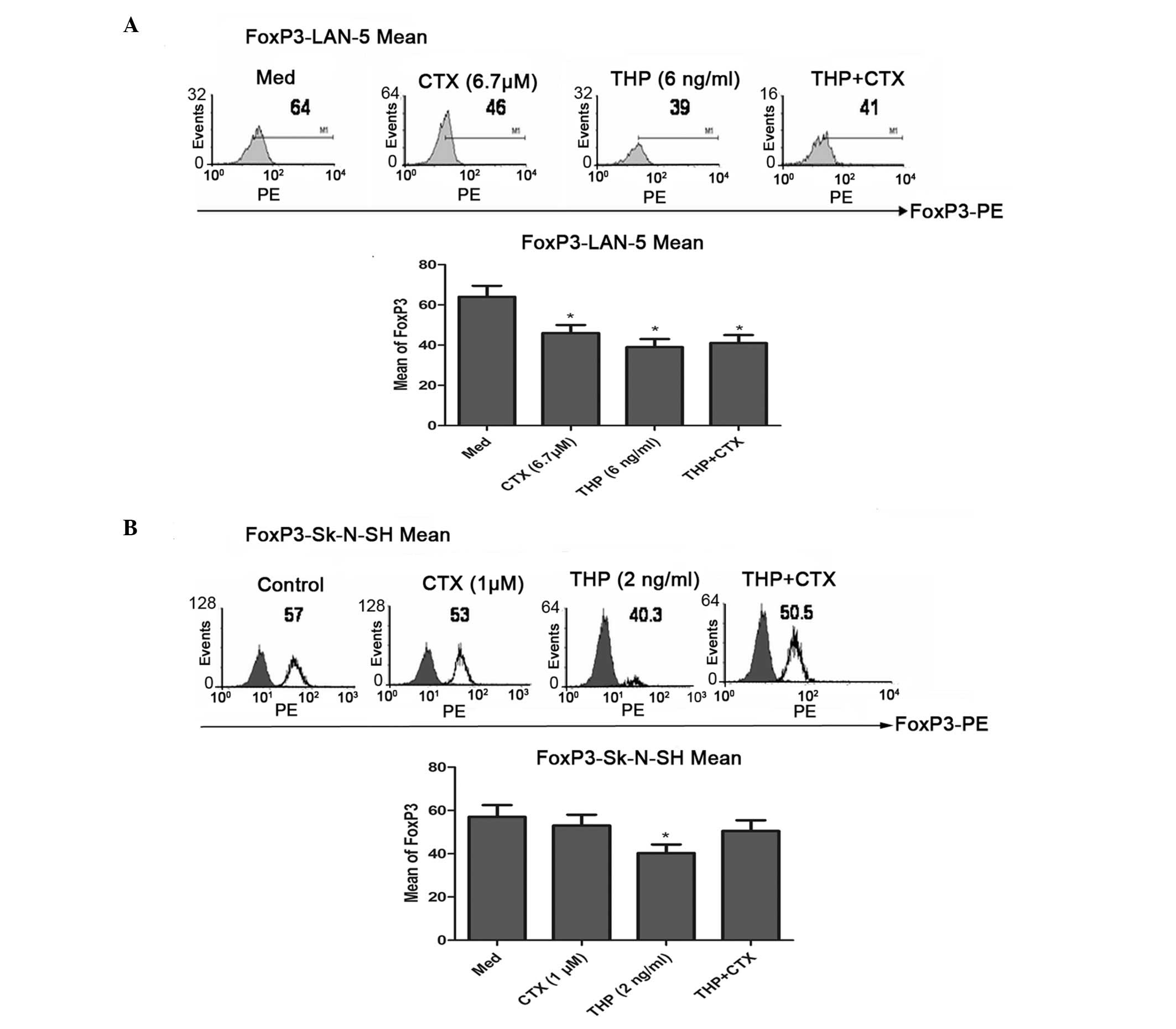
FACS data showed that CTX and THP significantly
downregulated the expression of Foxp3 in the LAN-5 cells
(P<0.05) (Fig. 4), while CTX and
THP did not affect the expression of Foxp3 in the SK-N-SH cells.
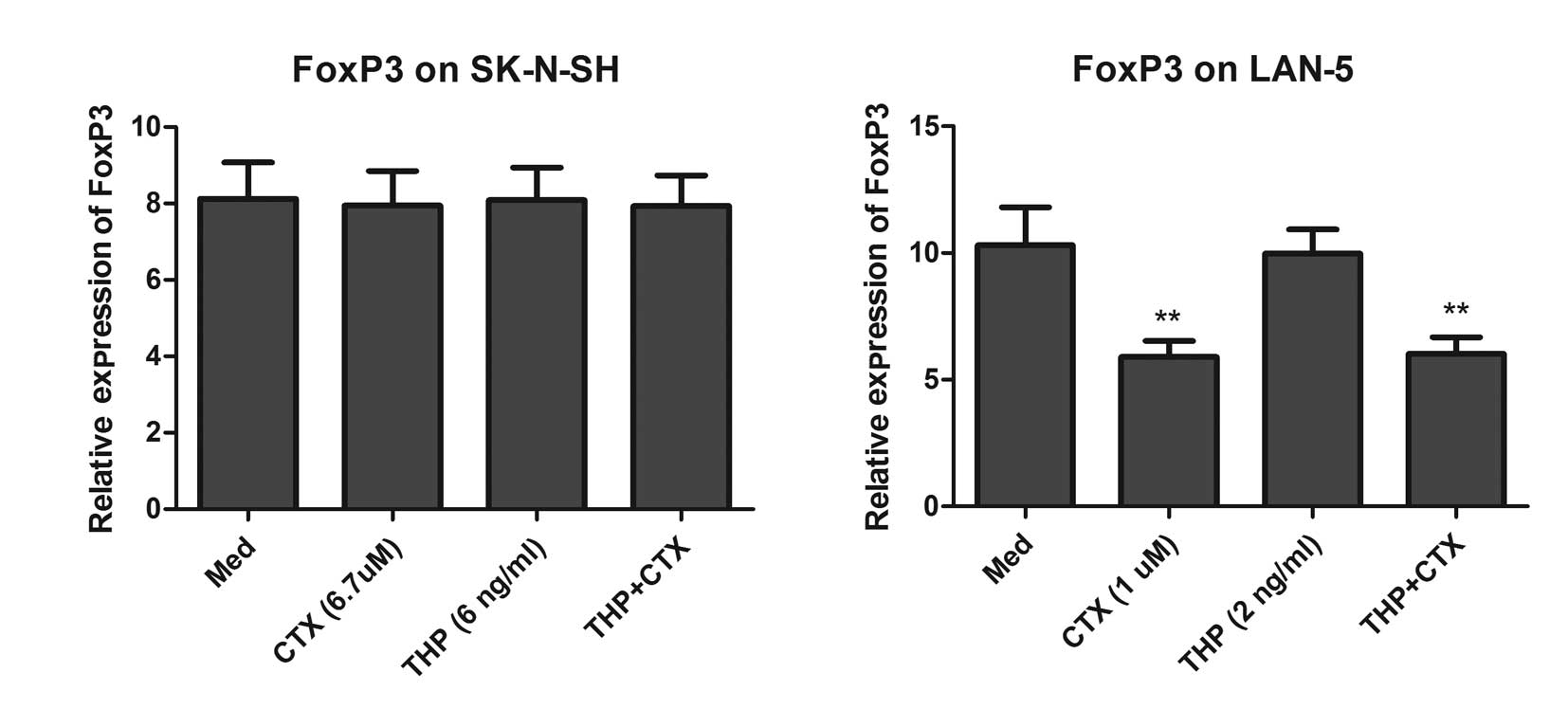
qPCR analysis showed that only CTX significantly downregulated the
expression of Foxp3 in the LAN-5 cells (Fig. 5).
Discussion
Neuroblastoma is a type of cancer that originates in
certain primitive nerve cells found in an embryo or fetus. This
type of cancer occurs in infants and young children. It is rarely
found in children >10 years old (19,20).
Although more than one half of children have localized tumors with
a good prognosis, the remaining patients have metastatic disease
with a poor long-term survival rate of ~30% worldwide (21,22).
Therefore the presence of metastatic diseases in children with
neuroblastoma is associated with a poor prognosis. The most
frequent distant metastatic sites are bone marrow and cortical bone
(23). However, the mechanisms
underlying the high frequency of metastasis in patients with
neuroblastoma remain unclear. Understanding the mechanisms of bone
and bone marrow metastasis of neuroblastoma may affect current and
future therapies of this disease.
CXCR4, a receptor for a chemokine CXCL12, is
important for the migration of hematopoietic cells to bone marrow
and neuronal migration (24,25).
Emerging evidence suggests that CXCR4 may also play an important
role in the metastasis of a variety of tumors. CXCR4 expression has
been found to be associated with bone marrow metastases of breast
(26) and prostate (27) cancer, and rhabdomyosarcoma (28). Thus, studying the role of CXCR4 in
the metastasis of neuroblastoma is of great importance. In the
present study, CXCR4 was found to be highly expressed in the LAN-5
and SK-N-SH neuroblastoma cell lines. Notably, the expression of
CXCR4 was decreased after the cells were treated with chemotherapy
drugs, CTX and THP, in association with the inhibition of cell
proliferation; therefore, the expression of CXCR4 may be involved
in the metastasis of neuroblastoma.
Immune evasion of tumors contributes to the survival
of cancer cells. Tregs plays an important role in the suppression
of immune responses and are also involved in immune evasion in
patients with cancer. The increased prevalence of Tregs may be
induced in patients with tumors (29). The transcription factor, Foxp3, is a
key factor for the differentiation of Tregs and is expressed in T
cells only. However, previous studies have demonstrated that Foxp3
was also expressed in tumor cells, such as pancreatic cancer
(30), melanoma (31) and other tumor cell lines (32,33).
The Foxp3-expressing cancer cells inhibited the proliferation of
CD4+CD25− T cells, potentially contributing
to immune evasion of the tumor cells. However, the role of Foxp3 in
neuroblastoma is largely unknown. In this study, the expression of
Foxp3 in neuroblastoma cells was investigated and our findings
indicated that Foxp3 was expressed in the LAN-5 and SK-N-SH
neuroblastoma cell lines. When the proliferation of tumor cells was
inhibited by CTX and THP, the expression of Foxp3 also
significantly decreased. Merlo et al reported that Foxp3
expression may be associated with the metastatic potential of the
tumor rather than suppression of a specific immune response
(34). The authors proposed that
Foxp3 expressed in cancer cells may modulate expression of
chemokine receptors and other genes, and thus influence invasion
and metastasis of tumor cells. In our study, Foxp3 and CXCR4 were
expressed in neuroblastoma cells; therefore, it may be possible
that Foxp3 expressed in these cells upregulated CXCR4 and
contributed to the higher frequency of neuroblastoma.
In conclusion, the present study demonstrates that
CXCR4 and Foxp3 exhibit higher expression in neuroblastoma cells,
and therefore, exposure to chemotherapy agents may reduce their
expression. In addition, the results suggest that CXCR4 and Foxp3
may present as potential targets for neuroblastoma
chemotherapy.
References
|
1
|
Guerreiro R, Santos-Costa Q and
Azevedo-Pereira JM: The chemokines and their receptors:
characteristics and physiological functions. Acta Med Port.
24(Suppl 4): 967–976. 2011.(In Portugese).
|
|
2
|
Ito H: Chemokines in mesenchymal stem cell
therapy for bone repair: a novel concept of recruiting mesenchymal
stem cells and the possible cell sources. Mod Rheumatol.
21:113–121. 2011.PubMed/NCBI
|
|
3
|
Salazar N, Castellan M, Shirodkar SS and
Lokeshwar BL: Chemokines and chemokine receptors as promoters of
prostate cancer growth and progression. Crit Rev Eukaryot Gene
Expr. 23:77–91. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Singh R, Lillard JW Jr and Singh S:
Chemokines: key players in cancer progression and metastasis. Front
Biosci (Schol Ed). 3:1569–1582. 2011. View
Article : Google Scholar : PubMed/NCBI
|
|
5
|
Felix AS, Edwards R, Bowser R and Linkov
F: Chemokines and cancer progression: a qualitative review on the
role of stromal cell-derived factor 1-alpha and CXCR4 in
endometrial cancer. Cancer Microenviron. 3:49–56. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wald O, Shapira OM and Izhar U:
CXCR4/CXCL12 axis in non small cell lung cancer (NSCLC) pathologic
roles and therapeutic potential. Theranostics. 3:26–33. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mo W, Chen J, Patel A, et al: CXCR4/CXCL12
mediate autocrine cell-cycle progression in NF1-associated
malignant peripheral nerve sheath tumors. Cell. 152:1077–1090.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Li B, Saouaf SJ, Samanta A, Shen Y,
Hancock WW and Greene MI: Biochemistry and therapeutic implications
of mechanisms involved in FOXP3 activity in immune suppression.
Curr Opin Immunol. 19:583–588. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Zeestraten EC, Van Hoesel AQ, Speetjens
FM, et al: FoxP3- and CD8-positive infiltrating immune cells
together determine clinical outcome in colorectal cancer. Cancer
Microenviron. 6:31–39. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Milne K, Köbel M, Kalloger SE, et al:
Systematic analysis of immune infiltrates in high-grade serous
ovarian cancer reveals CD20, FoxP3 and TIA-1 as positive prognostic
factors. PLoS One. 4:e64122009. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mercer F and Unutmaz D: The biology of
FoxP3: a key player in immune suppression during infections,
autoimmune diseases and cancer. Adv Exp Med Biol. 665:47–59. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Garaventa A, Parodi S, De Bernardi B, et
al: Outcome of children with neuroblastoma after progression or
relapse. A retrospective study of the Italian neuroblastoma
registry. Eur J Cancer. 45:2835–2842. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Cabioglu N, Sahin A, Doucet M, et al:
Chemokine receptor CXCR4 expression in breast cancer as a potential
predictive marker of isolated tumor cells in bone marrow. Clin Exp
Metastasis. 22:39–46. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Porvasnik S, Sakamoto N, Kusmartsev S, et
al: Effects of CXCR4 antagonist CTCE-9908 on prostate tumor growth.
Prostate. 69:1460–1469. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tao H, Mimura Y, Aoe K, et al: Prognostic
potential of FOXP3 expression in non-small cell lung cancer cells
combined with tumor-infiltrating regulatory T cells. Lung Cancer.
75:95–101. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Droeser R, Zlobec I, Kilic E, et al:
Differential pattern and prognostic significance of
CD4+, FOXP3+ and IL-17+ tumor
infiltrating lymphocytes in ductal and lobular breast cancers. BMC
Cancer. 12:1342012.PubMed/NCBI
|
|
17
|
Li YG, He JH, Yu L, et al: microRNA-202
suppresses MYCN expression under the control of E2F1 in the
neuroblastoma cell line LAN-5. Mol Med Rep. 9:541–546.
2014.PubMed/NCBI
|
|
18
|
Chen YX, Chen XW, Li CG, Yue LJ, Mai HR
and Wen FQ: Effect of tumor gangliosides on tyrosine
phosphorylation of p125FAK in platelet adhesion to collagen. Oncol
Rep. 29:343–348. 2013.PubMed/NCBI
|
|
19
|
Li K, Dong K, Gao J, Yao W, Xiao X and
Zheng S: Neuroblastoma management in Chinese children. J Invest
Surg. 25:86–92. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Navalkele P, O’Dorisio MS, O’Dorisio TM,
Zamba GK and Lynch CF: Incidence, survival, and prevalence of
neuroendocrine tumors versus neuroblastoma in children and young
adults: nine standard SEER registries, 1975–2006. Pediatr Blood
Cancer. 56:50–57. 2011.PubMed/NCBI
|
|
21
|
Gains J, Mandeville H, Cork N, Brock P and
Gaze M: Ten challenges in the management of neuroblastoma. Future
Oncol. 8:839–858. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Øra I and Eggert A: Progress in treatment
and risk stratification of neuroblastoma: impact on future clinical
and basic research. Semin Cancer Biol. 21:217–228. 2011.PubMed/NCBI
|
|
23
|
Sharp SE, Gelfand MJ and Shulkin BL:
Pediatrics: diagnosis of neuroblastoma. Semin Nucl Med. 41:345–353.
2011. View Article : Google Scholar
|
|
24
|
Hesselgesser J, Liang M, Hoxie J, et al:
Identification and characterization of the CXCR4 chemokine receptor
in human T cell lines: ligand binding, biological activity, and
HIV-1 infectivity. J Immunol. 160:877–883. 1998.PubMed/NCBI
|
|
25
|
Zeelenberg IS, Ruuls-Van Stalle L and Roos
E: The chemokine receptor CXCR4 is required for outgrowth of colon
carcinoma micrometastases. Cancer Res. 63:3833–3839.
2003.PubMed/NCBI
|
|
26
|
Hiller DJ, Meschonat C, Kim R, Li BD and
Chu QD: Chemokine receptor CXCR4 level in primary tumors
independently predicts outcome for patients with locally advanced
breast cancer. Surgery. 150:459–465. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jung SJ, Kim CI, Park CH, et al:
Correlation between chemokine receptor CXCR4 expression and
prognostic factors in patients with prostate cancer. Korean J Urol.
52:607–611. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tarnowski M, Grymula K, Reca R, et al:
Regulation of expression of stromal-derived factor-1 receptors:
CXCR4 and CXCR7 in human rhabdomyosarcomas. Mol Cancer Res. 8:1–14.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li H, Zhao H, Yu J, et al: Increased
prevalence of regulatory T cells in the lung cancer
microenvironment: a role of thymic stromal lymphopoietin. Cancer
Immunol Immunother. 60:1587–1596. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Ikemoto T, Yamaguchi T, Morine Y, et al:
Clinical roles of increased populations of
Foxp3+CD4+ T cells in peripheral blood from
advanced pancreatic cancer patients. Pancreas. 33:386–390.
2006.PubMed/NCBI
|
|
31
|
Lucas S, van Baren N, de Smet C and Coulie
PG: Demethylation of the FOXP3 gene in human melanoma cells
precludes the use of this epigenetic mark for quantification of
Tregs in unseparated melanoma samples. Int J Cancer. 130:1960–1966.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zuo T, Liu R, Zhang H, et al: FOXP3 is a
novel transcriptional repressor for the breast cancer oncogene
SKP2. J Clin Invest. 117:3765–3773. 2007.PubMed/NCBI
|
|
33
|
Fu HY, Li C, Yang W, et al: FOXP3 and TLR4
protein expression are correlated in non-small cell lung cancer:
implications for tumor progression and escape. Acta Histochem.
115:151–157. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Merlo A, Tagliabue E, Mènard S and Balsari
A: Matured human monocyte-derived dendritic cells (MoDCs) induce
expansion of CD4+CD25+FOXP3+ T
cells lacking regulatory properties. Immunol Lett. 117:106–113.
2008. View Article : Google Scholar : PubMed/NCBI
|