Introduction
Techniques for percutaneous ethanol injection
therapy (PEIT) have been widely established to treat hepatocellular
carcinoma (HCC) and have once been applied in larger size cancer
nodules using a multiple-insertion technique (1). With the development of the
radiofrequency ablation (RFA) technique, the adoption of PEIT
became limited to small-sized HCC nodules or those locating in the
vascular-rich portions of the liver, including the hepatic hilus
(2). Small early-stage HCC have
been detected frequently by periodic surveillance of cirrhotic
patients (3). Among these cases,
there were certain cases where the PEIT needle unavoidably
penetrated the large vessels between the cancer nodules during
PEIT. For the treatment of these special cases, a curved PEIT
needle was created to avoid the unnecessary adverse effects that
are associated with this treatment modality.
Patients and methods
Patient 1
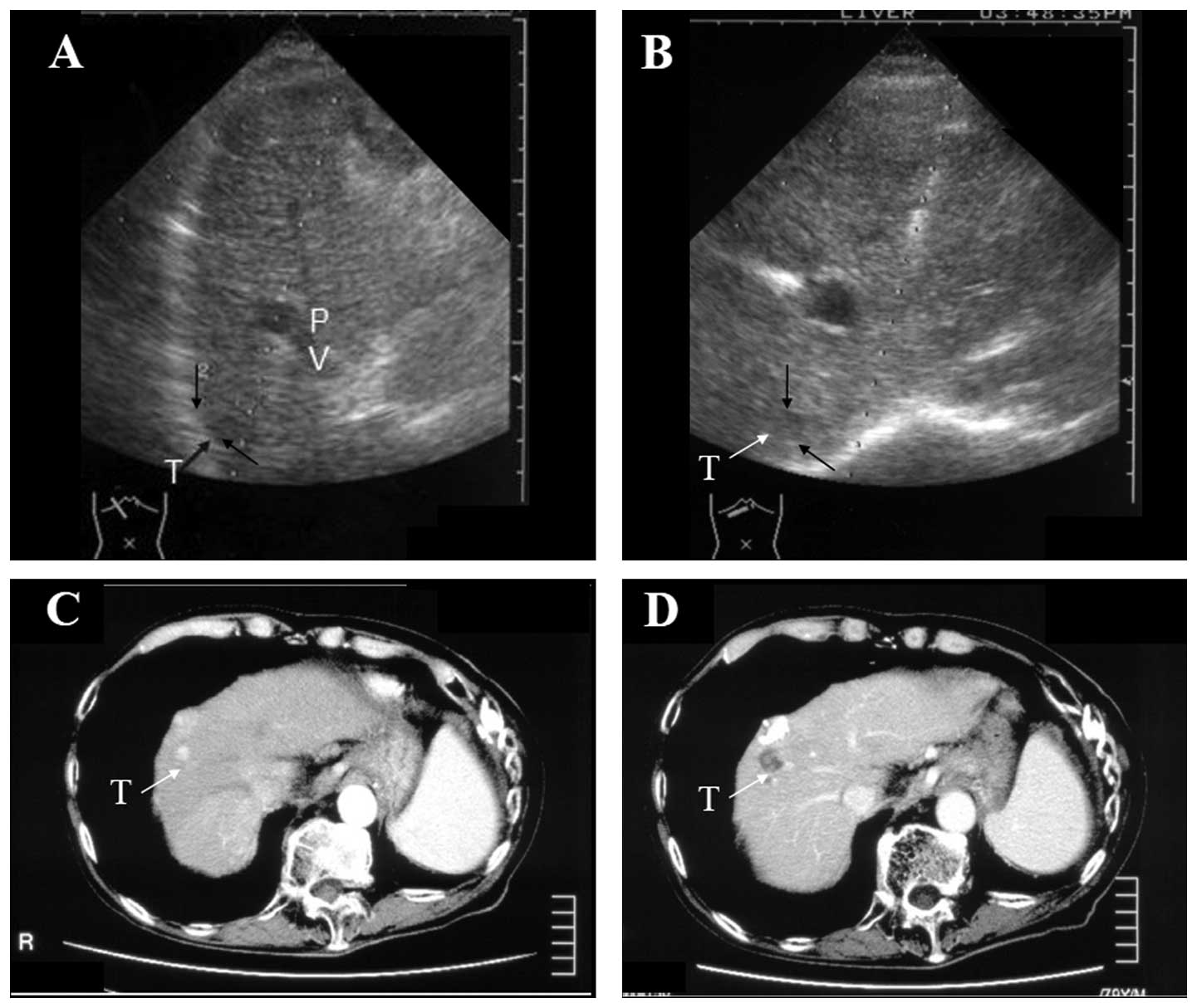
A 79-year-old male, exhibiting a HCC that was 2.6 cm
in diameter and located in Couinaud’s S4 subsegment (S4), underwent
conventional PEIT with a mixture of 5 ml of 99.5% ethanol and 0.6
ml of iodized oil (Lipiodol) in January 2005 (4). The Lipiodol mixture method was adopted
to improve the visibility of the HCC nodule during computed
tomography (CT) scanning (5). The
patient was treated twice in 2001 with RFA for HCCs that were 1.5
and 2.5 cm in diameter, and located in S2 and S8, respectively. The
histology of a biopsy specimen obtained at that time showed a
well-differentiated HCC that was positive for the hepatitis C virus
and exhibited liver cirrhosis.
In January 2005, an additional HCC (size, 1.6×1.6
cm) was identified behind the treated HCC, which was located just
beneath a branch of the large left portal vein on the approaching
line of the ultrasound image (Fig.
1A). A curved PEIT needle was used for the treatment of this
HCC.
Patient 2
A 56-year-old male who was positive for the
hepatitis C virus and exhibited liver cirrhosis was referred to the
Kagawa University Hospital (Miki-cho, Japan) for the treatment of
recurrent HCC. In 2000, the patient was treated with RFA twice for
HCCs that were 2.0 and 1.6 cm in diameter, and located in S8 and
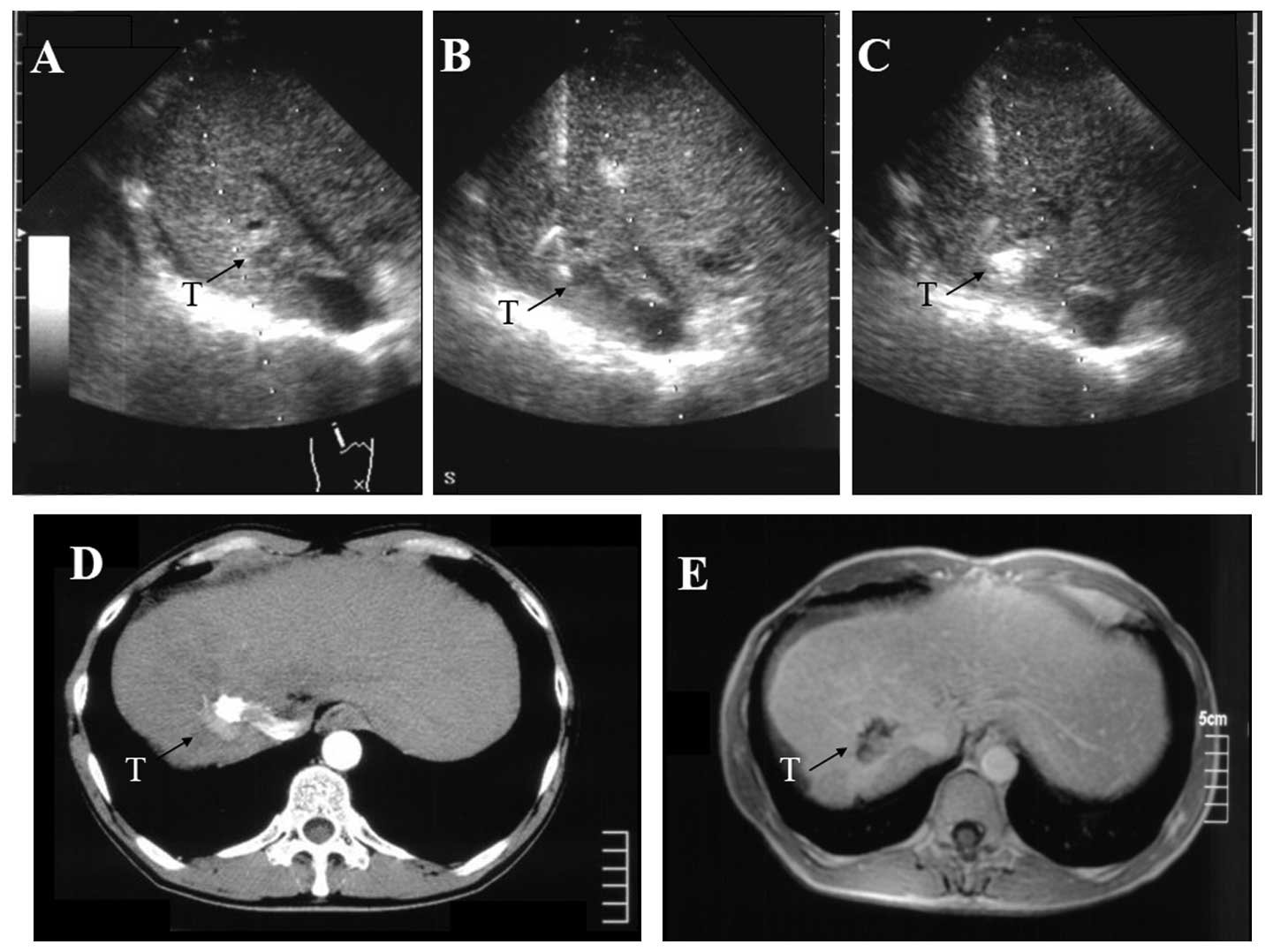
S7, respectively. An additional HCC, 1.9 cm in diameter, was
identified in S7 and treated angiographically using a mixture of
Lipiodol and a lipophilic anticancer agent, styrene maleic acid
neocarzinostatin (Lipiodol; Yamanouchi Pharmaceutical Co., Ltd.,
Tokyo, Japan), which was followed by a transcatheter embolization
of the tumor (6) in February 2005.
Three months later, another tumor (diameter, 1.3 cm) was observed
in S7 on the posterior side of the branch of the right portal vein
(Fig. 2A). This portal vein was
located just above the tumor, and a conventional straight PEIT
needle would intersect the puncture point of the tumor on the
ultrasound image. This HCC was treated using a curved PEIT needle.
As the patient’s renal function was low, dynamic magnetic resonance
imaging (MRI) was employed to evaluate the therapeutic effects.
Procedure
Prior to performing the procedure, written informed
consent was obtained from each patient’s family and the study was
approved by the clinal ethics committee of Kagawa University
Hospital (Kagawa, Japan). The patients were treated with the same
method as follows.
An 18-gauge PEIT needle (20-cm long; Hakko Co.,
Ltd., Chikuma, Japan), which was manually curved into a fishhook
shape, was prepared (Fig. 3). A
curved portion of the 18-gauge needle was pushed inside the
straight 16-gauge overcoat needle (shortened to a 12-cm length;
Bard Biopty-Cut Needle, Discovery Bay, CA, USA). Prior to
commencing the hand-assisted maneuver, the curved PEIT needle was
drawn back into the overcoat needle. The procedure was guided via
the ultrasound monitor to assess whether the curved PEIT needle,
which extended from the overcoat needle, approached the cancer
nodule.
Subsequent to receiving local anesthetic, the
coaxially prepared needles were held by the overcoat needle and
inserted with ultrasonic guidance into the lateral side of the
interposing vessel edge, above the cancer nodule. The curved PEIT
needle was extended slowly from the overcoat needle towards the
HCC. The quantity of ethanol that was injected by a single shot was
0.5–1.0 ml. A total of 5 ml of 99.5% ethanol was injected in small
doses through the curved PEIT needle.
Results
A large portal vein intersected the dermal insertion
point of the PEIT needle and the cancer nodule in the two patients.
To reinforce the straight structure of the outer needle following
insertion of the inner curved PEIT needle, a 16-gauge outer needle
was required. As the curved PEIT needle was extended from the
overcoat needle into the parenchyma of the liver at the lateral
side of a large vessel neighboring the HCC, the needle did not
maintain the original curve that was previously visualized. The
curved PEIT needle gradually lost its shape and weakened in the
liver parenchyma, resulting in the angle of the anticipating
approach curve becoming a weak curved line in the fibrotic liver.
Therefore, it was required that the inner PEIT needle was fixed as
a stronger curve in advance, or that an overcoat needle was
inserted nearer to the target than the simulation line. As a result
of the curve in the needle, it detoured the large vessel adjacent
to the HCC (Figs. 1B and 2B). Immediately following the ethanol
injection, the tumor area became increasingly hyperechoic (Fig. 2C). The arterial phase of an
abdominal dynamic CT image in patient 1 prior to and following the
therapy is shown in Fig. 1C and D.
The treated HCC evolved into a low-density area without contrast
enhancement. The arterial phase of an abdominal dynamic CT image in
patient 2 prior to therapy is shown in Fig. 2D. Compared with the HCC of the prior
treatment, the tumor evolved into a low-intensity area without
enhancement as shown in the abdominal dynamic MRI image following
therapy (Fig. 2E). The two patients
were successfully treated with this method without any specific
training required for performing the procedure.
Discussion
Ultrasound-guided percutaneous ablation therapy has
developed during the past two decades (7). Prior to the introduction of RFA
therapy, PEIT was the most effective method for the initial
treatment of patients with well-differentiated HCC (tumor size,
<15 mm in greatest dimension) (8). Duplex color Doppler ultrasound is
effective for identifying tortuous vessels that supply HCCs in the
cirrhotic liver. Previously, PEIT was performed for palliative
ablation of tumors supplying vessels in the HCC nodule and to
minimize the tumor vascularization using duplex color Doppler
ultrasound (9).
In multivariate analysis, the significant prognostic
factors have been determined as local recurrence, and tumor size
and number. This indicates that successfully attaining a complete
treatment for HCC during the first treatment is significant for
improving the prognosis of patients with HCC (10). RFA exhibits superior therapeutic
results compared with PEIT. The overall survival rate was higher in
patients treated with RFA compared with those that were treated
with PEIT. Furthermore, the local recurrence rate is higher in
patients treated with PEIT compared with those treated with RFA
(11). RFA has been widely
investigated as an alternative to PEIT, however, due to its mild
invasiveness to the patient, PEIT retained its validity for the
treatment of small HCC. PEIT is widely used for encapsulated small
tumors in livers (12,13). PEIT can also be considered as a
treatment of choice for small HCCs, particularly in patients with
poor liver reserve or comorbidity that make them potentially poor
surgical candidates (14,15). In addition, PEIT is a useful
alternative where RFA is unavailable (16). Repeated PEIT is permitted in
patients with an adequate liver function. In addition, during
follow-up the intrahepatic recurrence of HCC is the predominant
factor that affects survival rate (17). The prognosis of patients with HCC
who undergo incurative therapy is extremely poor.
Furthermore, for patients with small HCC, PEIT may
produce a survival rate comparable to surgical resection (18,19).
By contrast, a previous study on hepatic resection showed higher
survival rates compared with non-surgical therapies in small HCC.
In clinical stage I cases with a solitary tumor <2 cm in
diameter, in all clinical stages with a solitary tumor >2 cm and
in the clinical stage II cases with two tumors >2 cm, the
hepatic resection showed higher survival rates compared with the
non-surgical groups (20).
PEIT-associated adverse effects are not as serious
compared with those of RFA therapies, however, studies regarding
vascular or bile duct damage, such as hepatic infarction (21) or portal branch venous thrombosis
(22), have been reported. Since
complications include acute and delayed vascular injury following
ethanol injection, patients require a long period of follow-up
subsequent to treatment (23). To
avoid adverse effects, PEIT may be useful in those patients with
small HCC where taking a straight approach line, guided by
ultrasound imaging, is difficult due to intervening vessels between
the puncture point and the HCC. A study by Zuo et al
(24) also reported that CT-guided
PEIT with a curved needle is effective for the treatment of
malignant liver neoplasms, which strongly supports the use of a
novel ultrasound-guided PEIT with a curved needle in the present
study. Although the procedure is not simple, it is feasible for
physicians proficient in performing the regular PEIT technique.
In conclusion, the present study reports two cases
of HCC treated with a curved PEIT needle. For the purpose of
achieving PEIT safely, a novel PEIT needle was created with an
overcoat needle and a coaxial curved PEIT needle for the treatment
of small HCC adjacent to an intrahepatic large vessel. In cases of
patients with small HCC, which is difficult to approach with a
conventional strait PEIT needle, the curved PEIT needle presented
in the current study may be effective in avoiding unnecessary
adverse effects
References
|
1
|
Shiina S, Hata Y, Niwa Y, et al:
Multiple-needle insertion method in percutaneous ethanol injection
therapy for liver neoplasms. Gastroenterol Jpn. 26:47–50.
1991.PubMed/NCBI
|
|
2
|
Tateishi R, Shiina S, Teratani T, et al:
Percutaneous radiofrequency ablation for hepatocellular carcinoma.
An analysis of 1000 cases. Cancer. 103:1201–1209. 2005.PubMed/NCBI
|
|
3
|
Kuo YH, Lu SN, Chen CL, et al:
Hepatocellular carcinoma surveillance and appropriate treatment
options improve survival for patients with liver cirrhosis. Eur J
Cancer. 46:744–751. 2010. View Article : Google Scholar
|
|
4
|
Couinaud C: Liver lobes and segments:
notes on the anatomical architecture and surgery of the liver.
Presse Med. 62:709–712. 1954.(In French).
|
|
5
|
Kurokohchi K, Masaki T, Miyauchi Y, et al:
Percutaneous ethanol and lipiodol injection therapy for
hepatocellular carcinoma. Int J Oncol. 24:381–387. 2004.PubMed/NCBI
|
|
6
|
Jinno K, Moriwaki S, Tanada M, et al:
Clinicopathological study on combination therapy consisting of
arterial infusion of lipiodol-dissolved SMANCS and transcatheter
arterial embolization for hepatocellular carcinoma. Cancer
Chemother Pharmacol. 31(Suppl): S7–S12. 1992. View Article : Google Scholar
|
|
7
|
Massarweh NN, Park JO, Farjah F, et al:
Trends in the utilization and impact of radiofrequency ablation for
hepatocellular carcinoma. J Am Coll Surg. 210:441–448. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Hasegawa S, Yamasaki N, Hiwaki T, et al:
Factors that predict intrahepatic recurrence of hepatocellular
carcinoma in 81 patients initially treated by percutaneous ethanol
injection. Cancer. 86:1682–1690. 1999. View Article : Google Scholar
|
|
9
|
Rustemović N, Vucelić B, Opacić M, et al:
Palliative treatment of hepatocellular carcinoma with percutaneous
ethanol injection using tumor’s feeding artery occlusion under the
ultrasonic color Doppler guidance. Coll Antropol. 28:781–791.
2004.
|
|
10
|
Arimura E, Kotoh K, Nakamuta M, et al:
Local recurrence is an important prognostic factor of
hepatocellular carcinoma. World J Gastroenterol. 11:5601–5606.
2005.PubMed/NCBI
|
|
11
|
Orlando A, Leandro G, Olivo M, Andriulli A
and Cottone M: Radiofrequency thermal ablation vs. percutaneous
ethanol injection for small hepatocellular carcinoma in cirrhosis:
meta-analysis of randomized controlled trials. Am J Gastroenterol.
104:514–524. 2009. View Article : Google Scholar
|
|
12
|
Ebara M, Ohto M, Sugiura N, et al:
Percutaneous ethanol injection for the treatment of small
hepatocellular carcinoma. Study of 95 patients. J Gastroenterol
Hepatol. 5:616–626. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Khan KN, Yatsuhashi H, Yamasaki K, et al:
Prospective analysis of risk factors for early intrahepatic
recurrence of hepatocellular carcinoma following ethanol injection.
J Hepatol. 32:269–278. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Huang GT, Lee PH, Tsang YM, et al:
Percutaneous ethanol injection versus surgical resection for the
treatment of small hepatocellular carcinoma. Ann Surg. 242:36–42.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livraghi T: Percutaneous ethanol injection
in the treatment of hepatocellular carcinoma in cirrhosis.
Hepatogastroenterology. 48:20–24. 2001.PubMed/NCBI
|
|
16
|
Lin SM and Lin DY: Percutaneous local
ablation therapy in small hepatocellular carcinoma. Chang Gung Med
J. 26:308–314. 2003.PubMed/NCBI
|
|
17
|
Pompili M, Rapaccini GL, Covino M, et al:
Prognostic factors for survival in patients with compensated
cirrhosis and small hepatocellular carcinoma after percutaneous
ethanol injection therapy. Cancer. 92:126–135. 2001. View Article : Google Scholar
|
|
18
|
Livraghi T, Bolondi L, Lazzaroni S, et al:
Percutaneous ethanol injection in the treatment of hepatocellular
carcinoma in cirrhosis. A study on 207 patients. Cancer.
69:925–929. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shiina S, Teratani T, Obi S, et al:
Percutaneous ethanol injection therapy for liver tumors. Eur J
Ultrasound. 13:95–106. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Arii S, Yamaoka Y, Futagawa S, et al:
Results of surgical and nonsurgical treatment for small-sized
hepatocellular carcinomas: a retrospective and nationwide survey in
Japan. The Liver Cancer Study Group of Japan. Hepatology.
32:1224–1229. 2000. View Article : Google Scholar
|
|
21
|
Seki T, Wakabayashi M, Nakagawa T, et al:
Hepatic infarction following percutaneous ethanol injection therapy
for hepatocellular carcinoma. Eur J Gastroenterol Hepatol.
10:915–918. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Castroagudin JF, Delgado M, Villanueva A,
et al: Safety of percutaneous ethanol injection as neoadjuvant
therapy for hepatocellular carcinoma in waiting list liver
transplant candidates. Transplant Proc. 37:3871–3873. 2005.
View Article : Google Scholar
|
|
23
|
Koda M, Okamoto K, Miyoshi Y and Kawasaki
H: Hepatic vascular and bile duct injury after ethanol injection
therapy for hepatocellular carcinoma. Gastrointest Radiol.
17:167–169. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zuo CJ, Wang PJ, Shao CW, et al: CT-guided
percutaneous ethanol injection with disposable curved needle for
treatment of malignant liver neoplasms and their metastases in
retroperitoneal lymph nodes. World J Gastroenterol. 10:58–61.
2004.
|