Introduction
It had been indicated that antitumor agents do not
have a beneficial effect on patient survival in cases of kidney
cancer. Only cytokine therapies, including interferon-α and
interleukin-2, have been used for patients with metastatic (or
surgically unresectable) kidney cancer (MKC). However, these agents
demonstrate insufficient efficacy (1–3).
Subsequent to the phase III trial investigating the effects of
sorafenib on MKC, the vascular endothelial growth factor (VEGF)
signaling pathway inhibitors have emerged as leading treatments for
MKC (4–8). Three VEGF receptor-tyrosine kinase
inhibitors (VEGFR-TKI), sorafenib, sunitinib and axitinib, are
available for patients with MKC in Japan, as of 2012. The affinity
and selectivity of VEGFR-TKI for VEGFR are varied, and accordingly
the incidence and severity of adverse events (AE) also differ
(5–7). Hypertension (HT) is the most common AE
associated with VEGFR-TKI therapy and it occasionally becomes a
critical factor for the discontinuation of the treatment (5–8). By
contrast, the onset of HT following the initiation of VEGFR-TKI
treatment has been reported as a possible biomarker of a good
response to VEGFR-TKI (9).
Therefore, the control of HT is extremely significant for the
continued use of VEGFR-TKI and to achieve the optimal outcome in
MKC treatment. The present study was performed to identify the
predictive factors of VEGFR-TKI-induced HT, and to determine the
classes of antihypertensive agents (AHTAs) that demonstrate the
optimum efficacy against secondary HT.
Patients and methods
Study population
All studies were performed retrospectively in
Kanazawa University (Kanazawa, Japan) using the charts of patients
who were hospitalized at the Department of Urology. Patients with
MKC who underwent VEGFR-TKI (sorafenib, sunitinib and axitinib)
therapy were analyzed. The AHTAs that were administered were
categorized according to their mechanisms of action. The study was
in accordance with the Declaration of Helsinki Guidelines.
Definition of HT
HT was defined as systolic blood pressure (BP) of
>140 mmHg, corresponding to Grade 2 of the National Cancer
Institute-Common Terminology Criteria for Adverse Events version
4.0 (10). The policy of the
Department of Integrative Cancer Therapy and Urology, Kanazawa
University Graduate School of Medical Science (Kanazawa, Japan) for
commencing AHTA administration was also the same as the definition
of HT. The BP of all cases was reviewed prior to VEGFR-TKI
administration (baseline), between the onset of de novo HT
and commencing AHTA administration, and on HT improvement following
AHTA administration. The average BP levels at identical times on
three consecutive days were calculated and used for analyses;
however, single BP measurements were also used if the patient was
discharged and became an outpatient.
Statistical analysis
Statistical analyses were performed using
commercially available software (Prism; GraphPad Software, Inc.,
San Diego, CA, USA). Comparisons between two groups were performed
by unpaired two-sided t-test, Fisher’s exact test and χ2
test to identify trends. The probability of administrating AHTA was
estimated using the Kaplan-Meier method. In all analyses, P<0.05
was considered to indicate a statistically significant
difference.
Results
Patient characteristics
In total, 50 VEGFR-TKI administration events from 41
patients were analyzed and the patient demographic data are shown
in Table I. The number of cases of
sorafenib, sunitinib and axitinib administration were 18, 27 and 5,
respectively. A total of 22 patients had pre-existing HT, and one
or two AHTA had previously been prescribed. The probability of AHTA
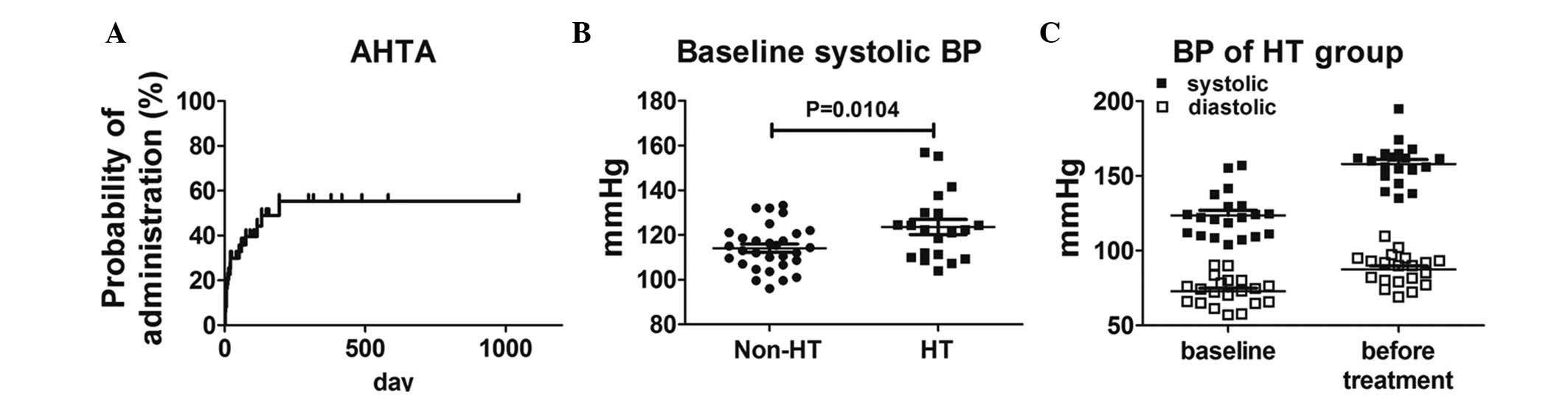
administration is shown in Fig.
1A.
 | Table IPatient demographics. |
Table I
Patient demographics.
| Demographic | Value |
|---|
| Number | 50 |
| Median age, year | 65 (26–85) |
| Gender |
| Male | 43 |
| Female | 7 |
| Prior
nephrectomy |
| Yes | 30 |
| No | 20 |
| TKI |
| Sorafenib | 18 |
| Sunitinib | 27 |
| Axitinib | 5 |
| Median TKI
administration days | 102 (7–1117) |
| Median initial
BP |
| Systolic | 116 (96–157) |
| Diastolic | 72 (57–90) |
| Number of prior
AHTA |
| 0 | 28 |
| 1 | 13 |
| 2 | 9 |
| Prior AHTA |
| CCB | 17 |
| ARB | 9a |
| ACEI | 1 |
| Others | 4 |
| TKI-induced HT |
| Yes | 20 |
| No | 30 |
Predictive factor of VEGFR-TKI-induced
HT
Of the 50 cases, 20 had HT subsequent to VEGFR-TKI
administration, and their backgrounds were compared with the 30
non-HT cases (Table II). The
median systolic BP at baseline was significantly higher in 20 HT
cases (P=0.0104), and the distributions of systolic BP in the
non-HT and HT groups are shown in Fig.
1B. In total, 2 cases exhibited Grade 2 HT at baseline and
commenced AHTA following the deterioration of HT to Grade 3
(systolic BP, >160 mmHg). The distributions of BP at baseline
and prior to AHTA administration in the HT group are also shown in
Fig. 1C.
 | Table IIComparison of the backgrounds between
HT and non-HT patients. |
Table II
Comparison of the backgrounds between
HT and non-HT patients.
| Background | Non-HT | HT | P-value |
|---|
| Number | 30 | 20 | |
| Median age, year | 65 (26–80) | 66 (47–85) | 0.5992 |
| Gender |
| Male | 26 | 17 | 1.0000 |
| Female | 4 | 3 | |
| Prior
nephrectomy |
| Yes | 17 | 13 | 0.7688 |
| No | 13 | 7 | |
| TKI |
| Sorafenib | 11 | 7 | 0.9923 |
| Sunitinib | 16 | 11 | |
| Axitinib | 3 | 2 | |
| Median TKI
administration days | 69 (5–1047) | 188 (21–1117) | 0.1895 |
| Median initial
BP |
| Systolic | 114 (96–133) | 122 (104–157) | 0.0104 |
| Diastolic | 70 (58–83) | 74 (57–90) | 0.2555 |
| Number of prior
AHTA |
| 0 | 15 | 13 | 0.3486 |
| 1 | 10 | 3 | |
| 2 | 5 | 4 | |
| Administered
AHTA |
| CCB | 13 | 4 | 0.3127 |
| ARB | 5a | 4 | |
| ACEI | 0 | 1 | |
| Others | 2 | 2 | |
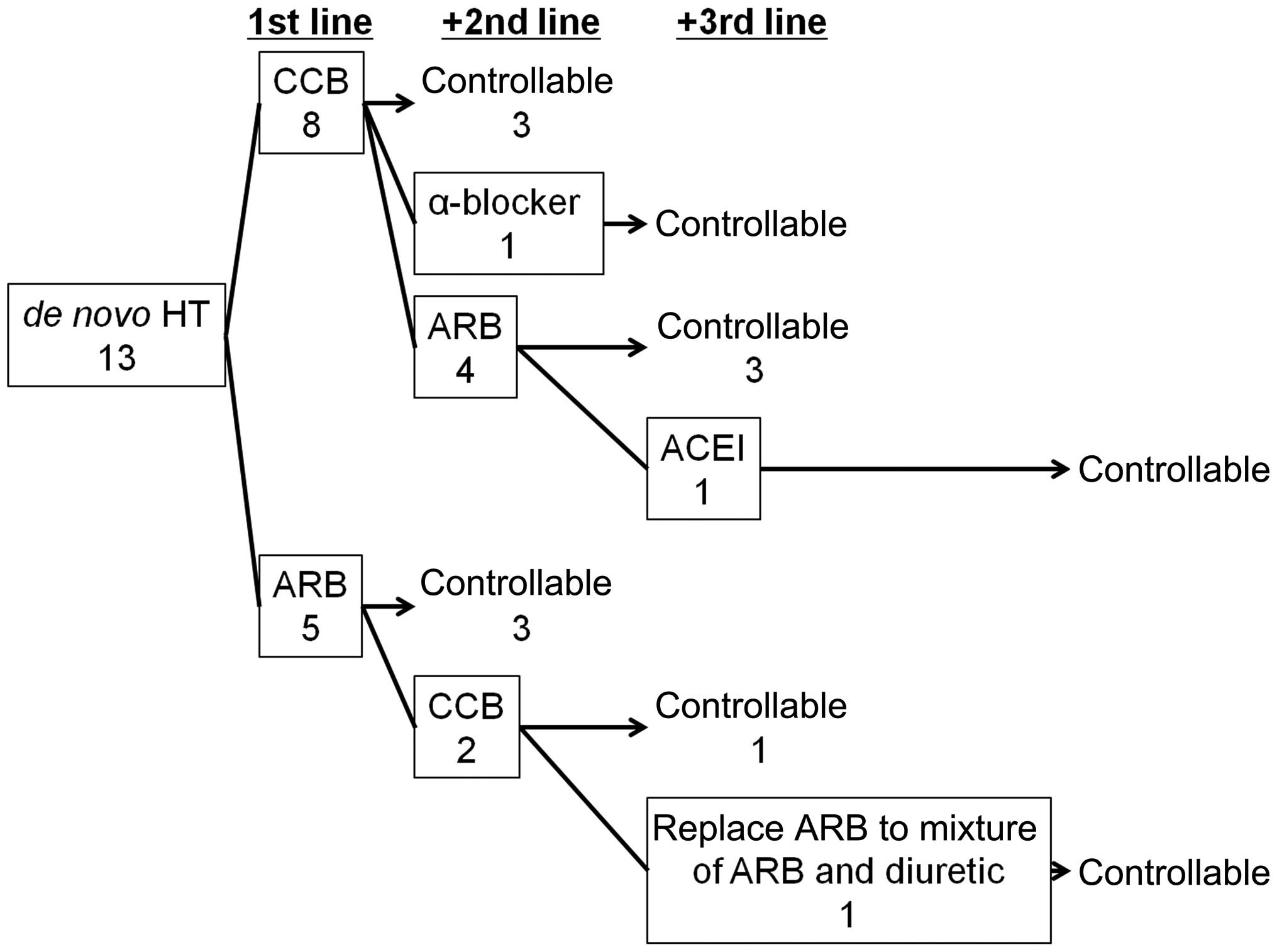
Administration of AHTA
The variations in efficacy, between AHTA
administration in 13 cases of de novo HT and no AHTA
administration prior to initiation of VEGFR-TKI therapy, were
analyzed. The first-line AHTA treatment was either calcium channel
blockers (CCBs) or angiotensin receptor II blockers (ARBs). There
was no significant difference identified between the control rate
of CCB and ARB as first-line treatments (3/8 for CCB treatment and
3/5 for ARB, P=0.5921; Fig. 2).
Discussion
It is important to identify the predictive factors
for key AE that are associated with VEGF-TKI to prevent treatment
discontinuation, as well as to predict the population that may show
a good response to these agents. Furthermore, it may contribute to
an improved outcome. In a study using axitinib treatment for
Japanese patients with MKC, Tomita et al (11) indicated that baseline proteinuria
and soluble VEGFR-2 levels may be predictive factors of
axitinib-induced proteinuria, which may also be a predictive factor
of a good response to axitinib. With regard to HT, a study of the
VEGFR-TKI, cediranib, for non-small cell lung cancer indicated that
predictors of VEGFR-TKI-induced HT were as follows: Eastern
Cooperative Oncology Group performance status 0; female; normal
lactate dehydrogenase levels; and no prior peripheral vascular
disease (12). A meta-analysis of
sunitinib indicated a significantly higher incidence of
sunitinib-induced HT in MKC compared with gastrointestinal stromal
tumors (13). These studies
indicated that predictors of VEGFR-TKI-induced HT in patients with
MKC should exist, and should be identified for extended VEGFR-TKI
use during MKC treatment. In the present study, a high baseline
systolic BP was the only predictive factor of VEGFR-TKI-induced HT.
This result is reasonable and indicated that the evaluation of BP
at baseline is significant for managing VEGFR-TKI administration.
As controversy remains regarding the optimal treatment for
VEGFR-TKI-induced HT, the category of AHTA that is preferable for
treatment of secondary HT, based on the charts of 13 de novo
HT cases, was investigated. As expected, two major categories of
AHTA were used as first-line treatments for VEGFR-TKI-induced HT,
CCB and ARB, and there was no difference in efficacy identified
between these two AHTA categories. Although certain review studies
proposed the use of AHTA for VEGFR-TKI-induced HT, there is no
evidence that the specific usage of AHTA is a requirement for
VEGFR-TKI-induced HT (14–16). However, the unique situation of
VEGFR-TKI-induced HT should be considered. It has been reported
that ARB may have antitumor effects due to the inhibition of
angiotensin II signaling (17). A
systematic review indicated that ARB improved progression-free
survival in patients with MKC, and that ARB administration was
protective against prostate-specific antigen failure in patients
with prostate cancer (18). In
addition, ARB decreased pressure in the glomerulus, and reduced
proteinuria, which consequently inhibited the deterioration of
renal function (19,20). As proteinuria is a critical AE of
VEGFR-TKI and HT (7,11), ARB may be preferable for patients
that are treated with VEGFR-TKI. By contrast, ARB cannot be used
for patients with bilateral renal artery stenosis or solitary
kidney associated with renal artery stenosis, or for patients with
an elevated creatinine level (>2.0 mg/dl), therefore, CCB may be
appropriate in such cases. Although this was a retrospective study
with a small sample size, it was shown that baseline BP may predict
VEGFR-TKI-induced HT. Furthermore, no difference in efficacy was
identified between CCB and ARB for VEGFR-TKI-induced HT. These
findings may aid clinicians with predicting the onset of
VEGFR-TKI-induced HT and for its management via the primary use of
CCB or ARB.
References
|
1
|
No authors listed. Interferon-alpha and
survival in metastatic renal carcinoma: early results of a
randomised controlled trial. Medical Research Council Renal Cancer
Collaborators. Lancet. 353:14–17. 1999.
|
|
2
|
Yang JC, Sherry RM, Steinberg SM, Topalian
SL, Schwartzentruber DJ, Hwu P, et al: Randomized study of
high-dose and low-dose interleukin-2 in patients with metastatic
renal cancer. J Clin Oncol. 21:3127–3132. 2003.
|
|
3
|
McDermott DF, Regan MM, Clark JI, Flaherty
LE, Weiss GR, Logan TF, et al: Randomized phase III trial of
high-dose interleukin-2 versus subcutaneous interleukin-2 and
interferon in patients with metastatic renal cell carcinoma. J Clin
Oncol. 23:133–141. 2005.
|
|
4
|
Rini BI, Halabi S, Rosenberg JE, Stadler
WM, Vaena DA, Archer L, et al: Phase III trial of bevacizumab plus
interferon alfa versus interferon alfa monotherapy in patients with
metastatic renal cell carcinoma: final results of CALGB 90206. J
Clin Oncol. 28:2137–2143. 2010.
|
|
5
|
Escudier B, Eisen T, Stadler WM, Szczylik
C, Oudard S, Staehler M, et al: Sorafenib for treatment of renal
cell carcinoma: Final efficacy and safety results of the phase III
treatment approaches in renal cancer global evaluation trial. J
Clin Oncol. 27:3312–3318. 2009.
|
|
6
|
Motzer RJ, Hutson TE, Tomczak P,
Michaelson MD, Bukowski RM, Oudard S, et al: Overall survival and
updated results for sunitinib compared with interferon alfa in
patients with metastatic renal cell carcinoma. J Clin Oncol.
27:3584–3590. 2009.
|
|
7
|
Rini BI, Escudier B, Tomczak P, Kaprin A,
Szczylik C, Hutson TE, et al: Comparative effectiveness of axitinib
versus sorafenib in advanced renal cell carcinoma (AXIS): a
randomised phase 3 trial. Lancet. 378:1931–1939. 2011.
|
|
8
|
Sternberg CN, Davis ID, Mardiak J,
Szczylik C, Lee E, Wagstaff J, et al: Pazopanib in locally advanced
or metastatic renal cell carcinoma: results of a randomized phase
III trial. J Clin Oncol. 28:1061–1068. 2010.
|
|
9
|
Horsley L, Marti K and Jayson GC: Is the
toxicity of anti-angiogenic drugs predictive of outcome? A review
of hypertension and proteinuria as biomarkers of response to
anti-angiogenic therapy. Expert Opin Drug Metab Toxicol. 8:283–293.
2012.
|
|
10
|
National Cancer Institute. Common
Terminology Criteria for Adverse Events v.3.0 and v.4.0 (CTCAE).
http://ctep.cancer.gov/protocolDevelopment/adverse_effects.htm.
Accessed March 14, 2014
|
|
11
|
Tomita Y, Uemura H, Fujimoto H, Kanayama
HO, Shinohara N, Nakazawa H, et al; Japan Axitinib Phase II Study
Group. Key predictive factors of axitinib (AG-013736)-induced
proteinuria and efficacy: a phase II study in Japanese patients
with cytokine-refractory metastatic renal cell Carcinoma. Eur J
Cancer. 47:2592–2602. 2011.
|
|
12
|
Goodwin R, Ding K, Seymour L, LeMaître A,
Arnold A, Shepherd FA, et al; NCIC Clinical Trials Group.
Treatment-emergent hypertension and outcomes in patients with
advanced non-small-cell lung cancer receiving chemotherapy with or
without the vascular endothelial growth factor receptor inhibitor
cediranib: NCIC Clinical Trials Group Study BR24. Ann Oncol.
21:2220–2226. 2010.
|
|
13
|
Zhu X, Stergiopoulos K and Wu S: Risk of
hypertension and renal dysfunction with an angiogenesis inhibitor
sunitinib: systematic review and meta-analysis. Acta Oncol.
48:9–17. 2009.
|
|
14
|
Larochelle P, Kollmannsberger C, Feldman
RD, Schiffrin EL, Poirier L, Patenaude F, et al: Hypertension
management in patients with renal cell cancer treated with
anti-angiogenic agents. Curr Oncol. 19:202–208. 2012.
|
|
15
|
Maitland ML, Bakris GL, Black HR, Chen HX,
Durand JB, Elliott WJ, et al; Cardiovascular Toxicities Panel,
Convened by the Angiogenesis Task Force of the National Cancer
Institute Investigational Drug Steering Committee. Initial
assessment, surveillance, and management of blood pressure in
patients receiving vascular endothelial growth factor signaling
pathway inhibitors. J Natl Cancer Inst. 102:596–604. 2010.
|
|
16
|
Izzedine H, Ederhy S, Goldwasser F, Soria
JC, Milano G, Cohen A, et al: Management of hypertension in
angiogenesis inhibitor-treated patients. Ann Oncol. 20:807–815.
2009.
|
|
17
|
Miyajima A, Kikuchi E, Kosaka T and Oya M:
Angiotensin II type 1 receptor antagonist as an angiogenic
inhibitor in urogenital cancer. Rev Recent Clin Trials. 4:75–78.
2009.
|
|
18
|
Mc Menamin ÚC, Murray LJ, Cantwell MM and
Hughes CM: Angiotensin-converting enzyme inhibitors and angiotensin
receptor blockers in cancer progression and survival: a systematic
review. Cancer Causes Control. 23:221–230. 2012.
|
|
19
|
Julius S, Kjeldsen SE, Weber M, Brunner
HR, Ekman S, Hansson L, et al; VALUE trial group. Outcomes in
hypertensive patients at high cardiovascular risk treated with
regimens based on valsartan or amlodipine: the VALUE randomised
trial. Lancet. 363:2022–2031. 2004.
|
|
20
|
Lindholm LH, Ibsen H, Dahlöf B, Devereux
RB, Beevers G, de Faire U, et al; LIFE Study Group. Cardiovascular
morbidity and mortality in patients with diabetes in the Losartan
Intervention For Endpoint reduction in hypertension study (LIFE): a
randomised trial against atenolol. Lancet. 359:1004–1010. 2002.
|