Introduction
Interleukin 21 (IL-21) belongs to the type I
cytokine family and plays critical roles in the human immune
system. IL-21 was originally believed to be produced by cluster of
differentiation 4-positive (CD4+) T cells, among which,
T-follicular helper cells could be a major source. Previous studies
have shown that IL-21 is also produced by Th17 cells and by natural
killer T cells, and that IL-21 mRNA can be detected in stromal
cells in the lymph nodes. All these studies indicate the
significance of IL-21 in innate and adaptive immune responses
(1–3).
The IL-21 receptor (IL-21R) contains six tyrosines
in the human cytoplasmic domains, Y281, Y361, Y369, Y397, Y317 and
Y510 (4,5). Previous studies have reported that
IL-21R and the common γ-chain (γc; CD132) function together as a
heterodimer for IL-21 (6,7). Upon binding to the IL-21R expressed on
cells lacking the γc, IL-21 is unable to transduce any
intracytoplasmic signals, whereas in γc-transfected cells, IL-21
binds to the IL-21R and then activates signals downstream (7). Furthermore, a study on chemical
cross-linking has previously demonstrated the direct binding of
IL-21 to the γc (7). The expression
of the IL-21R complex can be detected in various lymphoid organs,
including the spleen and thymus. IL-21R is also expressed on T
cells, B cells, dendritic cells, natural killer cells,
keratinocytes and macrophages. A further study revealed that the
functional IL-21R is expressed on CD4+ T cells and
CD8+ T cells, and that this expression can be
upregulated by the activation of the T cell receptor (3).
IL-21 plays significant roles in antitumor activity
through the regulation of CD8+ T cells. It has been
shown that IL-21 promotes CD8+ T-cell dependent tumor
responses against solid tumors in a mouse model (8,9). IL-21
therapy in mice increases the number of tumor-infiltrating
CD8+ T cells, expands the number of tumor-specific
CD8+ T cells and protects the IL-21-treated mice against
a recurrence of the same tumor (9).
IL-21 treatment in humans causes the increased expression of
perforin and granzyme B in CD8+ T cells, which induce
apoptosis of B-cell chronic lymphocytic leukemia cells (10,11).
These data indicate that CD8+ T cells may be
incorporated into the IL-21-based therapies of hematological
cancers. In the present study, the serum level of IL-21 and the
expression of IL-21 on IL-21R with the pathogenesis of diffuse
large B-cell lymphoma (DLBCL) was investigated.
Materials and methods
Study subjects
This study included 72 patients with a confirmed
diagnosis of DLBCL and 62 healthy controls. The diagnosis of DLBCL
was confirmed by histopathological examinations and, in the
majority of cases, by supplementary immunohistochemistry analyses.
The patient group was categorized according to the Ann Arbor
staging system. The control group was recruited from healthy
subjects who came to the same hospital for general health exams.
All the control subjects were matched with the patient population
based on age, gender and area of residence. Subjects who were
relatives were excluded. All patients and controls were
consecutively recruited from the East Hospital and Changhai
Hospital (Shanghai, China), and were of Han Chinese ethnicity.
Written informed consent was obtained from each subject. The study
was approved by the Ethics Committee Board of Changhai Hospital
(2005116) and Shanghai East Hospital (0582).
Serum assay
Serum samples were collected from all 72 DLBCL
patients and the 62 healthy controls. All samples were immediately
stored at −80°C. Serum IL-21 was assessed using enzyme-linked
immunosorbent assay (eBioscience, Inc., San Diego, CA, USA), with a
detection threshold of 50 pg/ml.
Flow cytometric analysis
The monoclonal antibodies (MoAbs) used in the
present study included fluorescein isothiocyanate-conjugated
anti-CD3 (BD Pharmingen; BD Biosciences, San Diego, CA, USA),
phycoerythrin-conjugated anti-CD8 [clone RPA-T8, mouse
immunoglobulin G (IgG1); BD Pharmingen], peridinin chlorophyll
protein complex-conjugated goat anti-mouse IgG MoAb (BD Pharmingen)
and anti-human IL-21R MoAb (mouse IgG1; R&D Systems,
Minneapolis, MN, USA). Peripheral blood mononuclear cells were
resuspended in phosphate-buffered saline supplemented with 0.2%
bovine serum albumin and 0.1% NaN3 at a concentration of
1×106 cells/ml. The cells were then stained with optimal
concentrations of fluorochrome-labeled MoAbs and isotype-matched
control MoAb, fixed in 3% paraformaldehyde buffer and determined by
flow cytometry (FACSCalibur) using CellQuest software (BD
Biosciences).
Statistical analysis
The SPSS statistical software package version 13.0
(SPSS, Inc., Chicago, IL, USA) was used for the statistical
analysis. Student’s t-test and the Mann-Whitney non-parametric U
test were used for comparison. Pearson’s correlation analysis was
used to calculate the correlation coefficient. P<0.05 was
considered to indicate a statistically significant difference.
Results
Clinical characteristics of the study
subjects
Selected characteristics of the 72 patients with
DLBCL and 62 controls are presented in Table I. The cases and controls did not
reveal any statistical significance with regard to age (P>0.05)
and gender (P>0.05). Of the 72 patients, eight were in Ann Arbor
stage I, 10 were in stage II, 25 were in stage III and 29 were in
stage IV. A total of 18 cases exhibited bone marrow involvement and
30 patients presented with B symptoms.
 | Table ICharacteristics of the patients (n=72)
and control subjects (n=62). |
Table I
Characteristics of the patients (n=72)
and control subjects (n=62).
| Characteristics | Patients, n (%) | Controls, n (%) | P-value |
|---|
| Age, years | | | |
| ≥60 | 45 (62.5) | 39 (62.9) | >0.05 |
| <60 | 27 (37.5) | 23 (37.1) | |
| Gender | | | |
| Male | 42 (58.3) | 34 (54.8) | >0.05 |
| Female | 30 (41.7) | 28 (45.2) | |
| Ann Arbor stage | | | |
| I | 8 (11.1) | | |
| II | 10 (13.9) | | |
| III | 25 (34.7) | | |
| IV | 29 (40.3) | | |
| Bone marrow
involvement | | | |
| No | 54 (75.0) | | |
| Yes | 18 (25.0) | | |
| B symptoms | | | |
| With | 30 (41.7) | | |
| Without | 42 (58.3) | | |
Serum level of IL-21 and expression of
IL-21R on CD8+ T cells in cases and controls
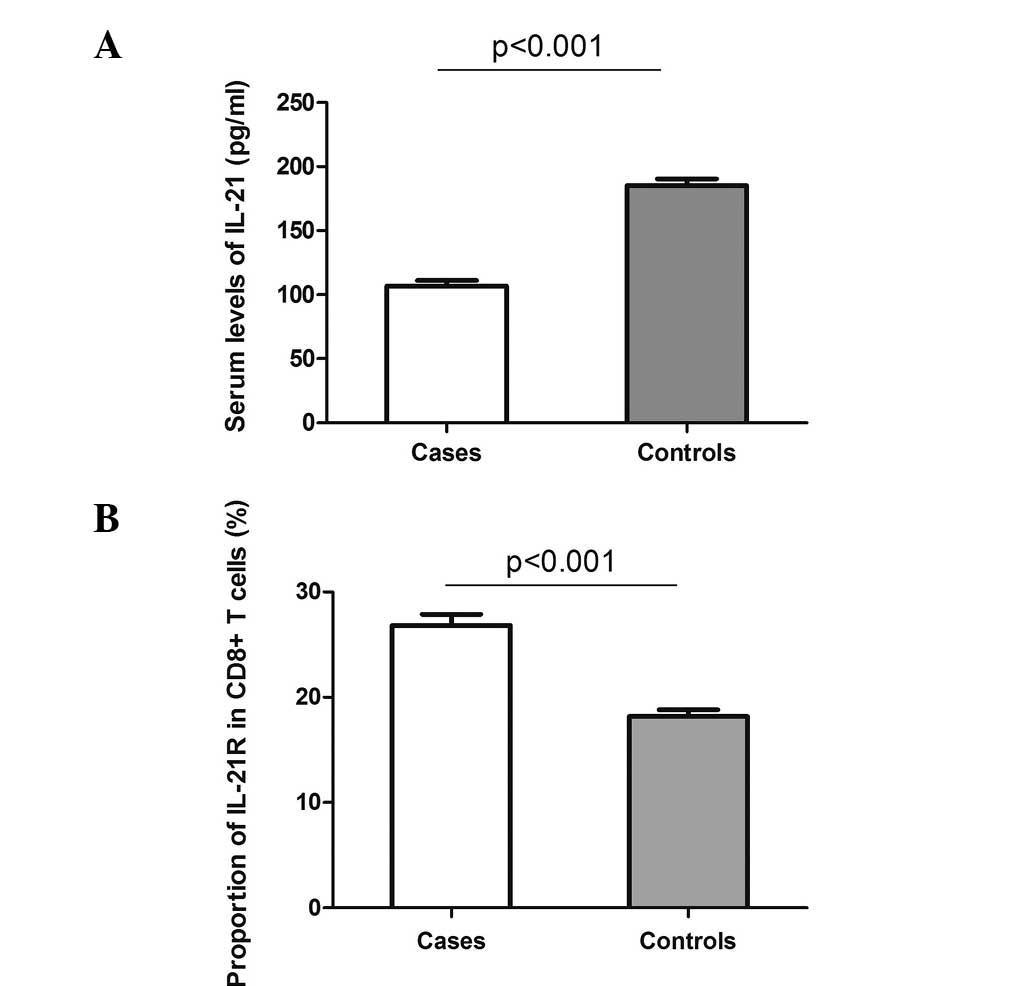
The serum levels of IL-21 were investigated in the
72 cases and 62 controls. As shown in Fig. 1A, a decreased level of IL-21 was
detected in the patients with DLBCL compared with the controls
(mean ± standard error of the mean, 106.8±4.4 vs. 185.3±5.3 pg/ml;
P<0.001). However, when comparing the expression of IL-21R on
the CD8+ T cells between the cases and controls, a
clearly elevated proportion of IL-21R was observed on the
CD8+ T cells in the DLBCL cases (26.8±1.1%) compared
with the controls (18.2±0.6%; Fig.
1B). These data indicate a potential involvement of IL-21 and
IL-21R in the pathogenesis of DLBCL.
Serum level of IL-21 and expression of
IL-21R on CD8+ T cells in patients with various tumor
stages
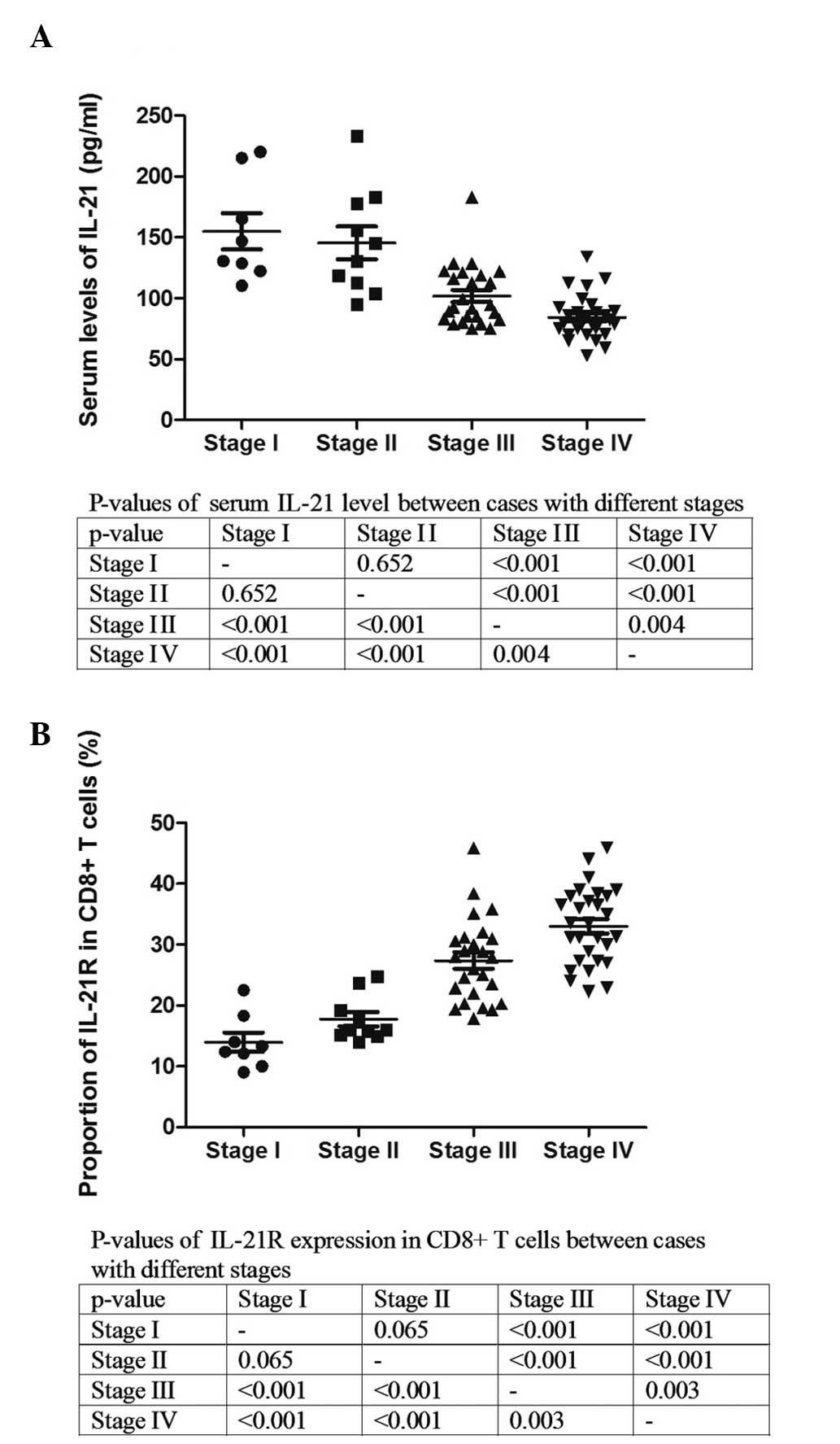
The Ann Arbor staging system is a standardized way
for a cancer care team to summarize information with regard to how
far a cancer has spread (11).
Stage I is indicative of a cancer that is located in a single
region, typically one lymph node and the surrounding area, whereas
stage IV is indicative of the diffuse or disseminated involvement
of one or more extralymphatic organs. The serum level of IL-21 and
the expression of IL-21R on the CD8+ T cells were
analyzed in the patients with various stages (Fig. 2). The serum level of IL-21 was
significantly lower in the patients with stage III and IV compared
with those with stages I and II (Fig.
2A). The analysis of IL-21R demonstrated that the expression of
IL-21R on the CD8+ T cells was clearly elevated with
increasing stage (Fig. 2B). These
results indicate that IL-21 and IL-21R could be associated with the
progression of DLBCL.
Correlation between serum level of IL-21
and expression of IL-21R on CD8+ T cells in DLBCL
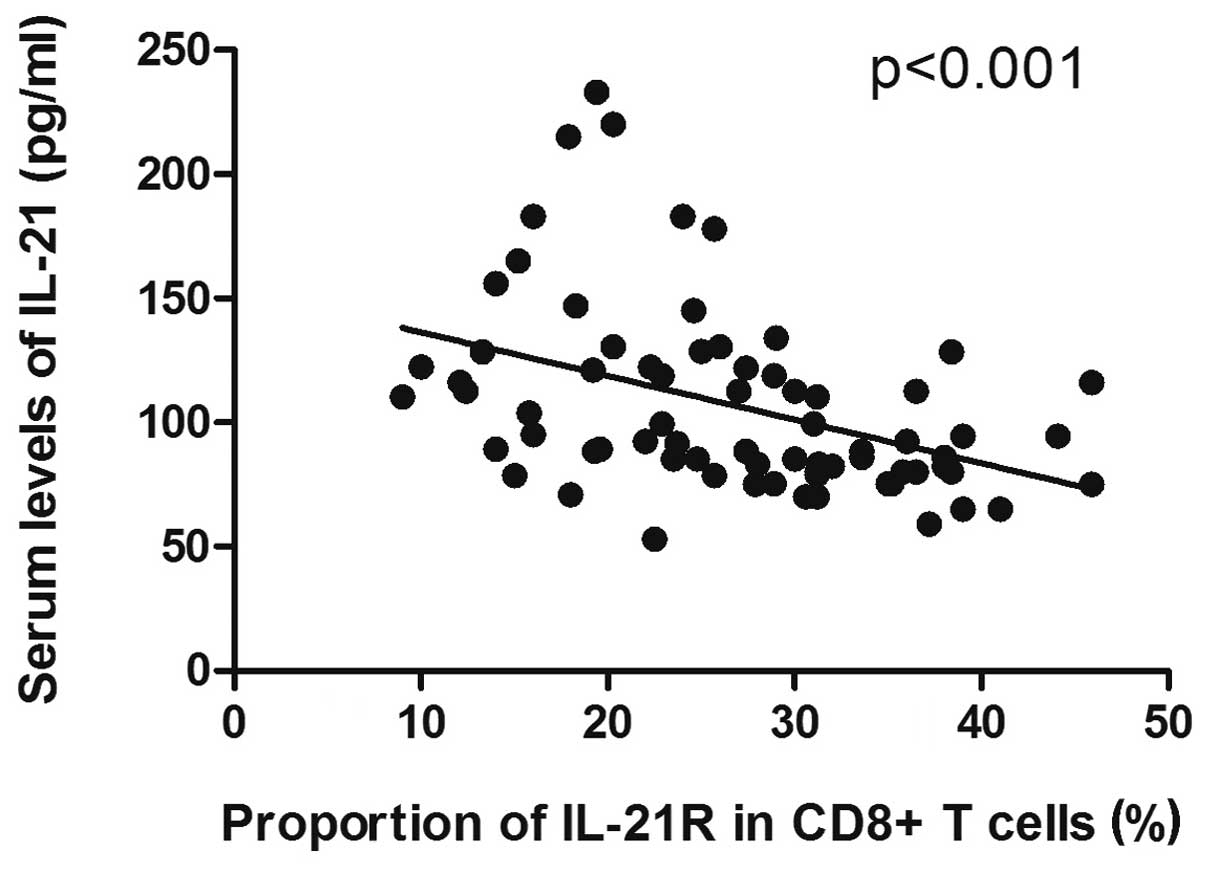
Since a decreased level of serum IL-21, but
increased expression of IL-21R on CD8+ T cells, was
observed in the DLBCL samples, and due to the diverse correlations
with disease progression, we hypothesized that a potential
correlation existed between the serum level of IL-21 and the
expression of IL-21R on the CD8+ T cells in the patients
with DLBCL. The results data showed that the decrease in the serum
level of IL-21 was significantly correlated with the increase in
the expression of IL-21R on the CD8+ T cells in the
patients with DLBCL (P<0.001; Fig.
3).
Serum level of IL-21 and expression of
IL-21R on CD8+ T cells in patients with systemic
symptoms
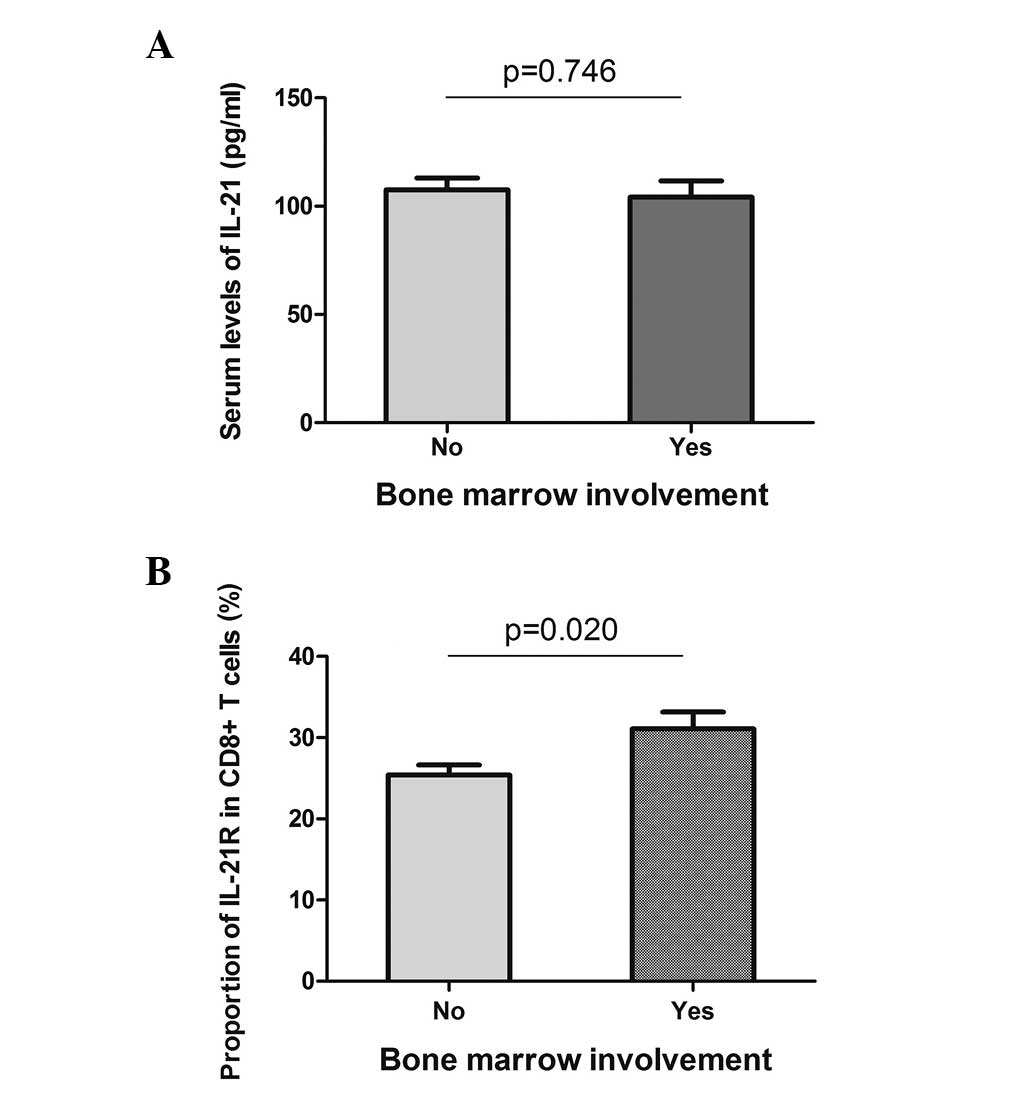
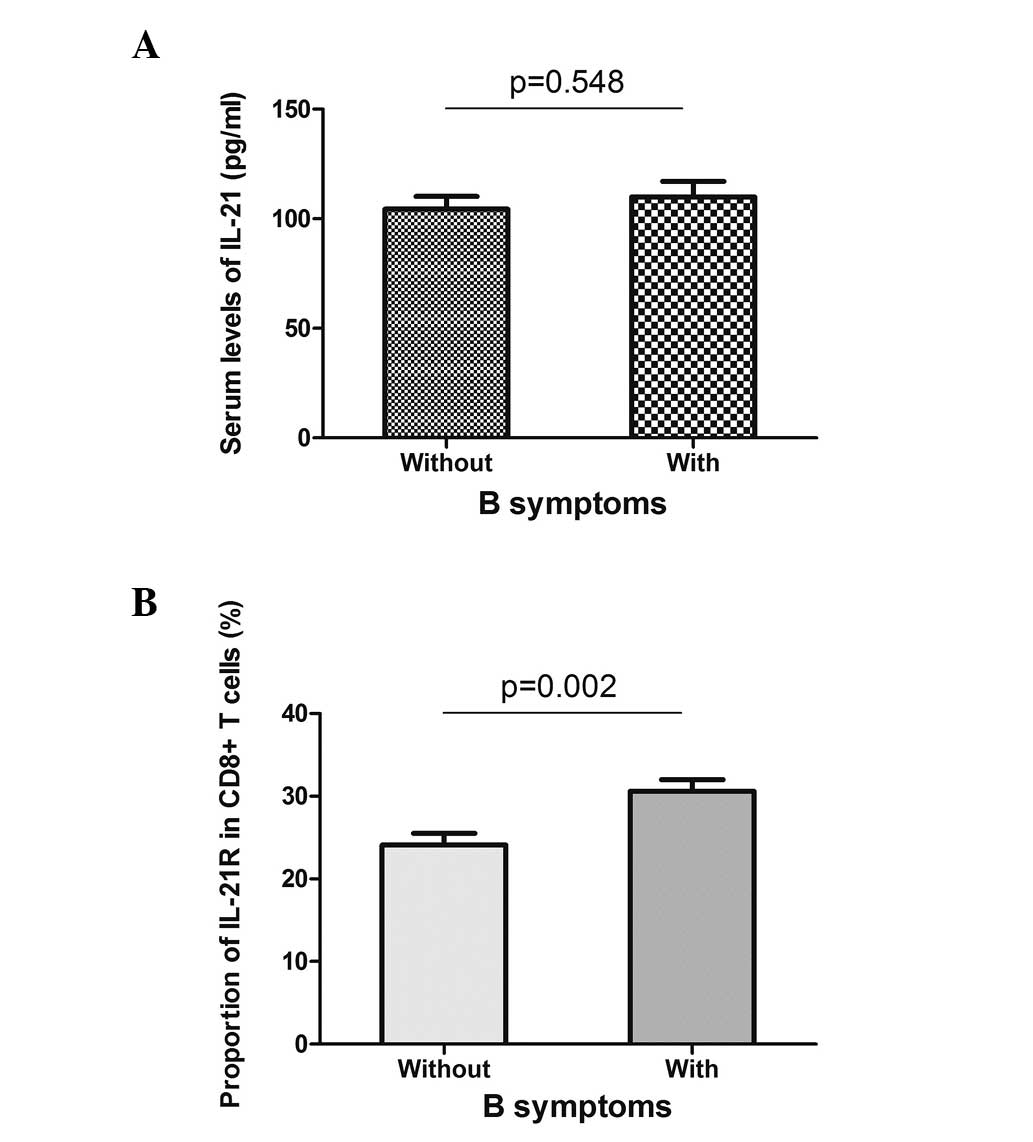
Bone marrow involvement and B symptoms are two
systemic symptoms of DLBCL, and they indicate a poor prognosis of
the disease. The serum level of IL-21 and the expression of IL-21R
on the CD8+ T cells were analyzed in the patients with
or without systemic symptoms. With regard to bone marrow
involvement, the serum level of IL-21 did not reveal a difference
between the two groups (Fig. 4A),
while the expression of IL-21R on the CD8+ T cells was
higher in the positive group (31.1±2.1 vs. 25.4±1.2%, P=0.020;
Fig. 4B). Similarly, the patients
with or without B symptoms did not present different serum levels
of IL-21, but showed a higher expression of IL-21R when B symptoms
were present (Fig. 5). These data
indicated that the expression of IL-21R on CD8+ T cells
may be used as a prognostic marker for DLBCL.
Discussion
In the present study, IL-21 and its receptor were
analyzed in CD8+ T cells, and it was identified that
IL-21/IL-21R are closely involved in the development and
progression of DLBCL.
Several studies conducted on murine models have
confirmed the antitumor role of IL-21 (12–14).
DLBCL is a heterogeneous disease with multiple subtypes and varying
clinical outcomes. It has been reported that IL-21R expression is
positive on primary CD10+ DLBCL cells and DLBCL cell
lines (15–17). IL-21 is known to induce apoptosis in
de novo DLBCL primary tumors, however, it may not effect the
viability of human healthy B cells (15). Additionally, IL-21 is able to induce
tumor regression and increase the survival of mice with xenograft
DLBCL tumors (17). IL-21
stimulates the apoptosis of the DLBCL cell line, CRL-2632, by
activating JAK1, JAK3, STAT1 and STAT3 (15). In addition, it appears that the
anti-lymphoma effects of IL-21 are dependent on a mechanism
involving the IL-21-activated STAT3 upregulation of c-Myc, in which
c-Myc is a highly prognostic marker in DLBCL (17,18).
The present study confirmed the critical role of IL-21 in the
development of DLBCL by identifying a decreased serum level of
IL-21 in the patient group (Fig.
1A). A negative correlation was also found between the serum
level of IL-21 and the tumor stage, indicating the involvement of
this cytokine in the progression of the disease (Fig. 2A).
The effects of IL-21 on CD8+ T cells are
varied. IL-21 increases the proliferation of murine or human mature
T cells stimulated with anti-CD3 or its antigen (19). In conjunction with T-cell receptor
stimulation and other common γc cytokines, including IL-7 and
IL-15, IL-21 is able to augment the proliferation and
differentiation of human and mouse CD8+ T cells into
potent cytolytic effectors (19,20).
In the present study, an elevated level of IL-21R was observed on
the peripheral CD8+ T cells in the DLBCL cases (Fig. 2B), and this positively correlated
with the tumor grade. Notably, the level of IL-21R on the
peripheral CD8+ T cells, but not the serum level of
IL-21, was associated with bone marrow involvement and B symptoms,
indicating that IL-21R on peripheral CD8+ T cells may
play more significant roles in the progression of DLBCL.
In conclusion, the present study identified a
decreased level of serum IL-21, but an increased level of IL-21R
expression on the CD8+ T cells in the patients with
DLBCL. Additionally, the analyses revealed opposite correlations
between IL-21 and IL-21R with disease progression, in which IL-21R
on CD8+ T cells may further reflect the prognosis of the
disease. These data shed light on understanding the pathogenesis of
DLBCL and provide knowledge for the use of IL-21 as a novel
therapy.
References
|
1
|
Parrish-Novak J, Dillon SR, Nelson A, et
al: Interleukin 21 and its receptor are involved in NK cell
expansion and regulation of lymphocyte function. Nature. 408:57–63.
2000.
|
|
2
|
Spolski R and Leonard WJ: Interleukin-21:
basic biology and implications for cancer and autoimmunity. Annu
Rev Immunol. 26:57–79. 2008.
|
|
3
|
Mehta DS, Wurster AL and Grusby MJ:
Biology of IL-21 and the IL-21 receptor. Immunol Rev. 202:84–95.
2004.
|
|
4
|
Ozaki K, Kikly K, Michalovich D, et al:
Cloning of a type I cytokine receptor most related to the IL-2
receptor beta chain. Proc Natl Acad Sci USA. 97:11439–11444.
2000.
|
|
5
|
Parrish-Novak J, Foster DC, Holly RD and
Clegg CH: Interleukin-21 and the IL-21 receptor: novel effectors of
NK and T cell responses. J Leukoc Biol. 72:856–863. 2002.
|
|
6
|
Zeng R, Spolski R, Casas E, et al: The
molecular basis of IL-21-mediated proliferation. Blood.
109:4135–4142. 2007.
|
|
7
|
Asao H, Okuyama C, Kumaki S, et al:
Cutting edge: the common gamma-chain is an indispensable subunit of
the IL-21 receptor complex. J Immunol. 167:1–5. 2001.
|
|
8
|
Noguchi M, Yi H, Rosenblatt HM, et al:
Interleukin-2 receptor gamma chain mutation results in X-linked
severe combined immunodeficiency in humans. Cell. 73:147–157.
1993.
|
|
9
|
Skak K, Kragh M, Hausman D, et al:
Interleukin 21: combination strategies for cancer therapy. Nat Rev
Drug Discov. 7:231–240. 2008.
|
|
10
|
Davis ID, Skrumsager BK, Cebon J, et al:
An open-label, two-arm, phase I trial of recombinant human
interleukin-21 in patients with metastatic melanoma. Clin Cancer
Res. 13:3630–3636. 2007.
|
|
11
|
Jahrsdörfer B, Blackwell SE, Wooldridge
JE, et al: B-chronic lymphocytic leukemia cells and other B cells
can produce granzyme B and gain cytotoxic potential after
interleukin-21-based activation. Blood. 108:2712–2719. 2006.
|
|
12
|
Ugai S, Shimozato O, Kawamura K, et al:
Expression of the interleukin-21 gene in murine colon carcinoma
cells generates systemic immunity in the inoculated hosts. Cancer
Gene Ther. 10:187–192. 2003.
|
|
13
|
Kishida T, Asada H, Itokawa Y, et al:
Interleukin (IL)-21 and IL-15 genetic transfer synergistically
augments therapeutic antitumor immunity and promotes regression of
metastatic lymphoma. Mol Ther. 8:552–558. 2003.
|
|
14
|
He H, Wisner P, Yang G, et al: Combined
IL-21 and low dose IL-2 therapy induces anti-tumor immunity and
long term curative effects in a murine melanoma tumor model. J
Transl Med. 4:242006.
|
|
15
|
Lamprecht B, Kreher S, Anagnostopoulos I,
et al: Aberrant expression of the Th2 cytokine IL-21 in Hodgkin
lymphoma cells regulates STAT3 signaling and attracts Treg cells
via regulation of MIP-3alpha. Blood. 112:3339–3347. 2008.
|
|
16
|
Akamatsu N, Yamada Y, Hasegawa H, et al:
High IL-21 receptor expression and apoptosis induction by IL-21 in
follicular lymphoma. Cancer Lett. 256:196–206. 2007.
|
|
17
|
Sarosiek KA, Malumbres R, Nechushtan H, et
al: Novel IL-21 signaling pathway up-regulates c-Myc and induces
apoptosis of diffuse large B-cell lymphomas. Blood. 115:570–580.
2010.
|
|
18
|
Akasaka T, Akasaka H, Ueda C, et al:
Molecular and clinical features of non-Burkitt’s, diffuse
large-cell lymphoma of B-cell type associated with the
c-MYC/immunoglobulin heavy-chain fusion gene. J Clin Oncol.
18:510–518. 2000.
|
|
19
|
Barker BR, Parvani JG, Meyer D, et al:
IL-21 induces apoptosis of antigen-specific CD8+ T lymphocytes. J
Immunol. 179:3596–3603. 2007.
|
|
20
|
Li Y and Yee C: IL-21 mediated Foxp3
suppression leads to enhanced generation of antigen-specific CD8+
cytotoxic T lymphocytes. Blood. 111:229–235. 2008.
|