Introduction
The early diagnosis of ovarian cancer is complex,
and thus leads to ineffective treatment and the highest mortality
rate of any gynecological malignancy, which poses a serious threat
to the health of females. Detailed investigations of the biological
behavior and mechanisms underlying ovarian cancer have been
performed in order to identify an improved treatment for this
gynecological cancer and recently, the promotion of the cancer
stem-cell theory has provided a novel perspective for determining
the biological behavior of ovarian cancer. Cancer stem cells
possess a self-renewal capacity within the tumor, which enables
unlimited cellular proliferation and differentiation. Therefore,
cancer stem cells are considered to be the source of relapse,
tumorigenesis, tumor invasion, tumor metastasis, cancer drug
resistance and recurrence (1).
Marked invasiveness and metastatic capacity are the predominant
characteristics of cancer stem cells. In addition, the
epithelial-mesenchymal transition (EMT) is important in cancer stem
cell metastasis and recurrence (2).
In the present study, the human epithelial ovarian carcinoma cell
line, HO-8910 was analyzed, which was established from a patient
with poorly differentiated ovarian papillary serous
cystadenocarcinoma. The cells were grown in suspension culture with
paclitaxel-combined serum-free medium (Hangzhou Sijiqing Biology
Engineering Materials Co., Ltd., Hangzhou, China) in order to
successfully screen the ovarian cancer stem cells for the
expression of CD133+ and CD117+ in
vivo and in vitro, prior to further identification of
their specific markers and biological characteristics (3). Our previous study identified that the
WW domain-containing oxidoreductase (WWOX) gene
significantly affects the biological behavior of human ovarian
cancer cells (4). In the current
study, to further investigate the impact of the WWOX gene on
ovarian cancer stem cells, the eukaryotic expression vector,
pcDNA3.1-WWOX, was transfected into ovarian cancer stem cells to
investigate the impact on EMT and its mechanism of action.
Materials and methods
Materials
Human ovarian cancer stem cells were screened and
collected at Central Laboratory of Shandong University School of
Medicine (Jinan, China). The pcDNA3.1-WWOX eukaryotic expression
vector was also prepared and stored in this laboratory. The
LipofectorTM liposomal transfection reagent was provided
by the Beyotime Institute of Biotechnology (Shanghai, China) and
Transwell® chambers were purchased from Chemicon
(Billerica, MA, USA). The E-cadherin, β-catenin, vimentin,
fibronectin, Elf5 and Snail primary antibodies were purchased from
Sigma-Aldrich (St. Louis, MO, USA). This study was approved by the
Ethics Committee of Shandong University (Jinan, China).
Cell culture
The human ovarian cancer stem cells were subcultured
using serum-free medium, and the HO-8910 cell line was incubated
using RPMI-1640 medium (Hyclone, South Logan, UT, USA), in a
thermostat-equipped, humidified incubator containing 5%
CO2 at 37°C.
Western blot analysis to detect the
differential expression of EMT markers in ovarian cancer stem and
HO-8910 cells
In total, two groups of cells were harvested during
the log growth phase and incubated in 200 μl lysis buffer on ice.
Total protein concentrations from cell lysates were determined
using the bicinchoninic acid assay (Hangzhou Sijiqing Biology
Engineering Materials Co., Ltd.). Next, the total protein was
separated using 10% sodium dodecyl sulphate-polyacrylamide gel and
the protein bands were transferred to a nitrocellulose membrane
(Qiagen, Hilden, Germany). The nitrocellulose membranes were
blocked with 5% non-fat milk for 60 min and incubated with the
rabbit anti-human E-cadherin primary monoclonal antibody (1:1,000)
at 4°C overnight. Following three 10 min washes with washing
solution (Hangzhou Sijiqing Biology Engineering Materials Co.,
Ltd., Hangzhou, China), the horseradish peroxidase-conjugated goat
anti-rabbit secondary antibody (1:10,000) was further incubated at
room temperature for 2 h. An enhanced chemiluminescence reagent
(Hangzhou Sijiqing Biology Engineering Materials Co., Ltd.) was
used to visualize the protein blots on highly sensitive X-ray film
(Shanghai Shenggong Biological Engineering Co., Ltd., Shanghai,
China) following exposure and development in the dark. The
detection of β-catenin, N-cadherin, vimentin and fibronectin was
performed according to the aforementioned procedures.
Gene transfection and experimental
groups
The lipofection technique was used to transfect the
eukaryotic expression vector carrying the WWOX gene into the
ovarian cancer stem cells (recombinant plasmid group) according to
the manufacturer’s instructions for the LipofectorTM
liposomal transfection reagent. The stably transfected cells were
selected and further cultured, while the empty plasmid (empty
plasmid group) and non-transfected ovarian cancer stem cells (blank
control group) served as the controls.
Reverse transcription-polymerase chain
reaction (RT-PCR) for detection of WWOX mRNA expression
A total of three groups of cells was harvested
during the log growth phase and total RNA was extracted using
TRIzol reagent (Chemicon, Temecula, CA, USA). The standard
conditions for RT-PCR were followed according to the manufacturer’s
instructions for the reverse-transcription reagent (Promega
Corporation, Madison, WI, USA). The primer sequences used were as
follows: Forward, 5′-CACGCATTTTAGAAGAATGG-3′ and reverse,
5′-GACAGCAGCACAGTACACG-3′ (amplified fragment size of 598 bp) for
WWOX; and forward, 5′-CGGGAAGCTTGTGATCAATGG-3′ and reverse,
5′-GGCAGTGATGGCATGGACTG-3′ (amplified fragment size of 357 bp) for
the housekeeping gene, glyceraldehyde-3-phosphate dehydrogenase
(GAPDH). The RT-PCR reaction conditions used were as
follows: 30 Cycles of 94°C for 45 sec, 55°C for 60 sec; 72°C for 60
sec; and a 72°C primer extension for 10 min. The PCR-amplified cDNA
fragments were detected using 2% agarose gel electrophoresis and
observed under ultraviolet illumination using an image capture
system (4100, Olympus, Tokyo, Japan). The cells expressing the
WWOX gene exhibited an amplified band of 598 bp, whereas
cells without the WWOX gene did not exhibit a specific
amplified band. Finally, the automatic analyzer ChemiImager 5500
imaging software (Alpha Innotech Corp., San Diego, CA, USA) was
used to quantify the amplified bands using the
WWOX/GAPDH content ratio to measure the relative
expression levels of WWOX mRNA.
Methyl thiazolyl tetrazolium (MTT) assay
for detection of ovarian cancer stem cell proliferation
The cells were grouped as aforementioned and the
three groups of cells were seeded in 96-well plates (Hangzhou
Sijiqing Biology Engineering Materials Co., Ltd.) at a density of
1.5×104 cells/well and incubated for various time
periods (one, two, three, four, five or six days). A total of 20 μl
MTT working solution was added at the end of each time point and
incubated in a CO2 incubator at 37°C for an additional 4
h; dimethyl sulfoxide was added to terminate the reaction. The
reaction product was measured in each well at an absorbance (A)
value of 490 nm using an ELISA plate reader (Elx910, Qiagen) and
the corresponding cellular growth curves were plotted.
Analysis of cancer invasion by ovarian
cancer stem cells in vitro using the Transwell® cell
migration/invasion Matrigel assay
A precoated Matrigel insert was placed between the
upper and lower invasion chambers and 200 μl of a HO-8910
single-cell suspension (containing ~1×105 cells) was
plated in the invasion insert and incubated in a CO2
incubator at 37°C for 12 h. The non-invasive cells and Matrigel
medium were subsequently removed from the insert. Next, the insert
was fixed and stained with hematoxylin and eosin to visualize the
invasive cells. The number of invasive cells was measured under a
light microscope (CKX41, Olympus) and each group of cells was
assessed in the three individual inserts, and in triplicate.
Western blot analysis to detect the
expression of EMT markers and regulatory factors, Elf5 and Snail,
in ovarian cancer stem cells
The experiment was divided into three groups: the
recombinant plasmid group, empty plasmid group and the blank
control group. Western blot analysis was performed using the same
method as described previously for the detection of the
differential expression of EMT markers in ovarian cancer stem and
HO-8910 cells
Results
Expression of EMT markers in ovarian
cancer stem and HO-8910 cells
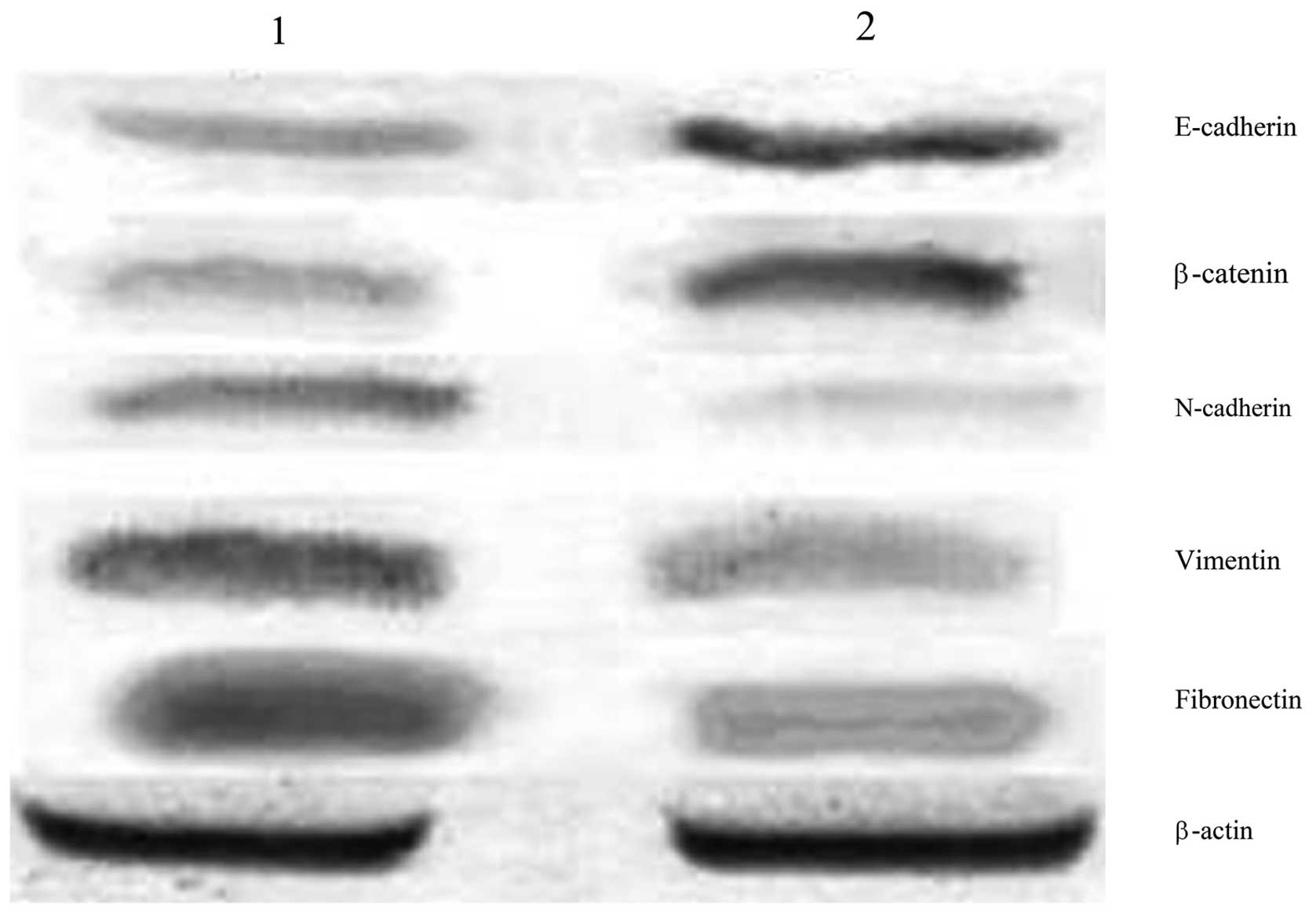
The protein expression of E-cadherin and β-catenin
in ovarian cancer stem cells, as detected by western blot analysis,
was 0.294±0.023 and 0.313±0.017, respectively; significantly lower
than that in the HO-8910 cells (0.771±0.031 for E-cadherin and
0.752±0.011 for β-catenin; P<0.05). Conversely, the expression
of N-cadherin, vimentin and fibronectin in ovarian cancer stem
cells was 0.698±0.012, 0.839±0.021 and 0.847±0.022, respectively;
significantly higher than that in HO-8910 cells (0.228±0.022 for
N-cadherin, 0.353±0.027 for vimentin and 0.322±0.019 for
fibronectin; P<0.05; Fig.
1).
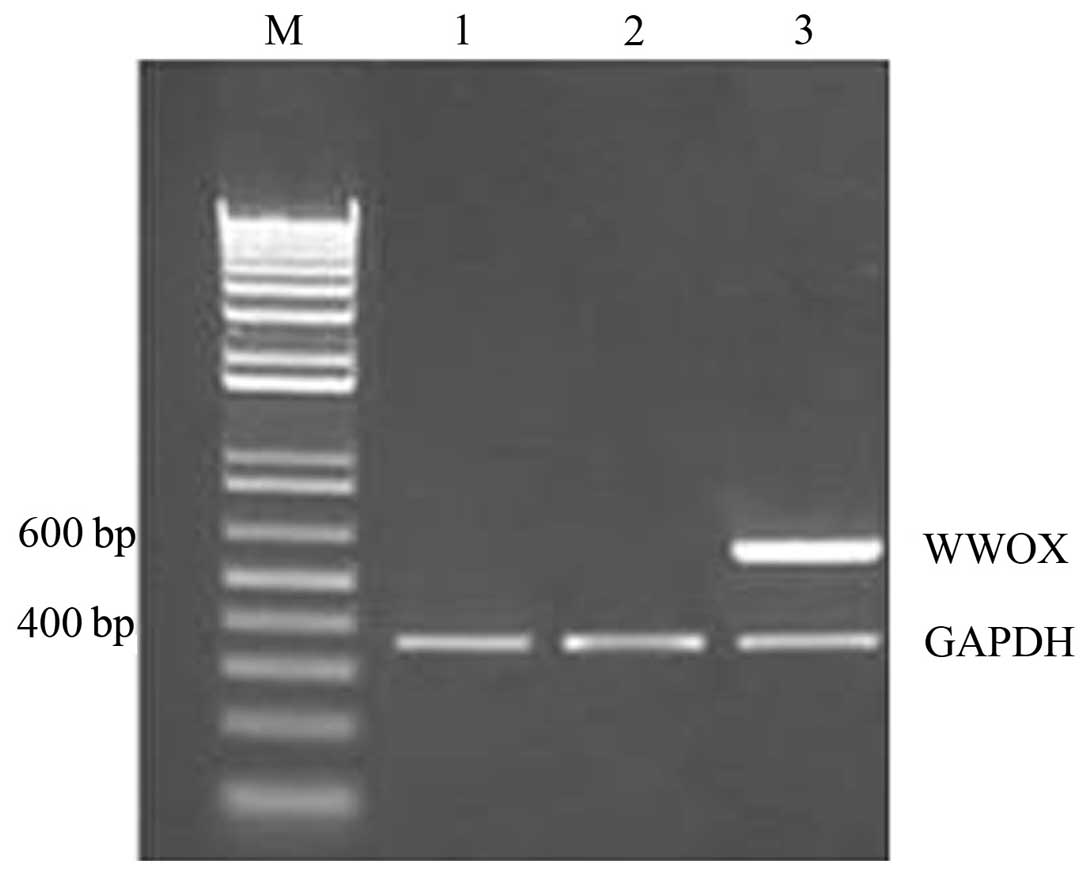
Differences in WWOX mRNA expression
following the transfection of ovarian cancer stem cells with the
WWOX gene
The results of the RT-PCR analysis demonstrated that
WWOX mRNA expression in the recombinant plasmid group was
high, however, WWOX mRNA was not detected in the empty
plasmid or blank control groups (Fig.
2).
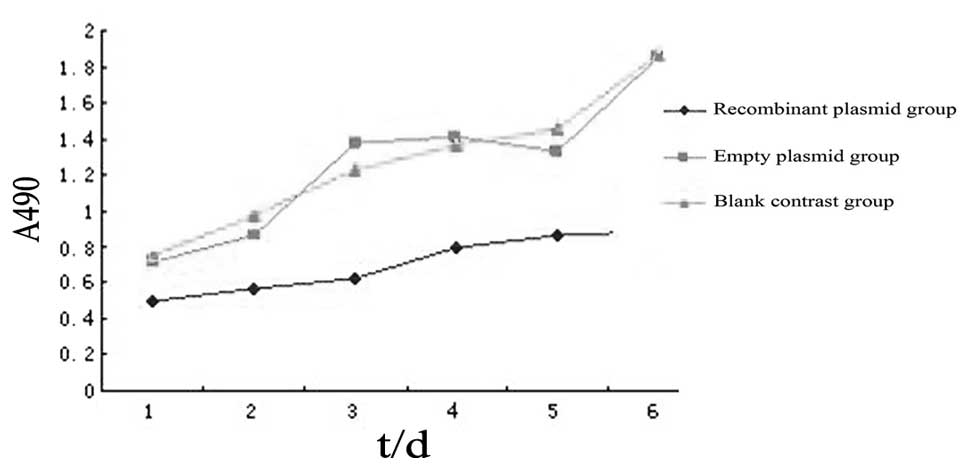
Changes in cell proliferation following
the transfection of ovarian cancer stem cells with the WWOX
gene
The MTT assay revealed that the A values of the
recombinant plasmid group following one, two, three, four, five and
six days of incubation were 0.502±0.004, 0.567±0.011, 0.622±0.016,
0.798±0.002, 0.861±0.022 and 0.892±0.013, respectively;
significantly lower than that of the blank control or empty plasmid
groups at the corresponding time points (P<0.05). No
statistically significant differences were identified between the
empty plasmid and blank control groups (P>0.05; Fig. 3).
Changes in invasive capacity following
the transfection of ovarian cancer stem cells with the WWOX
gene
In vitro invasion assays using the
Transwell® chamber detected the following number of
invasive cells in the recombinant plasmid, empty plasmid and blank
control groups: 105.5±3.1, 199.7±3.4 and 191.4±4.1, respectively.
Statistically significant differences were identified between the
recombinant plasmid and control groups (P<0.05), however, no
statistically significant differences were identified between the
empty plasmid and blank control groups (P>0.05).
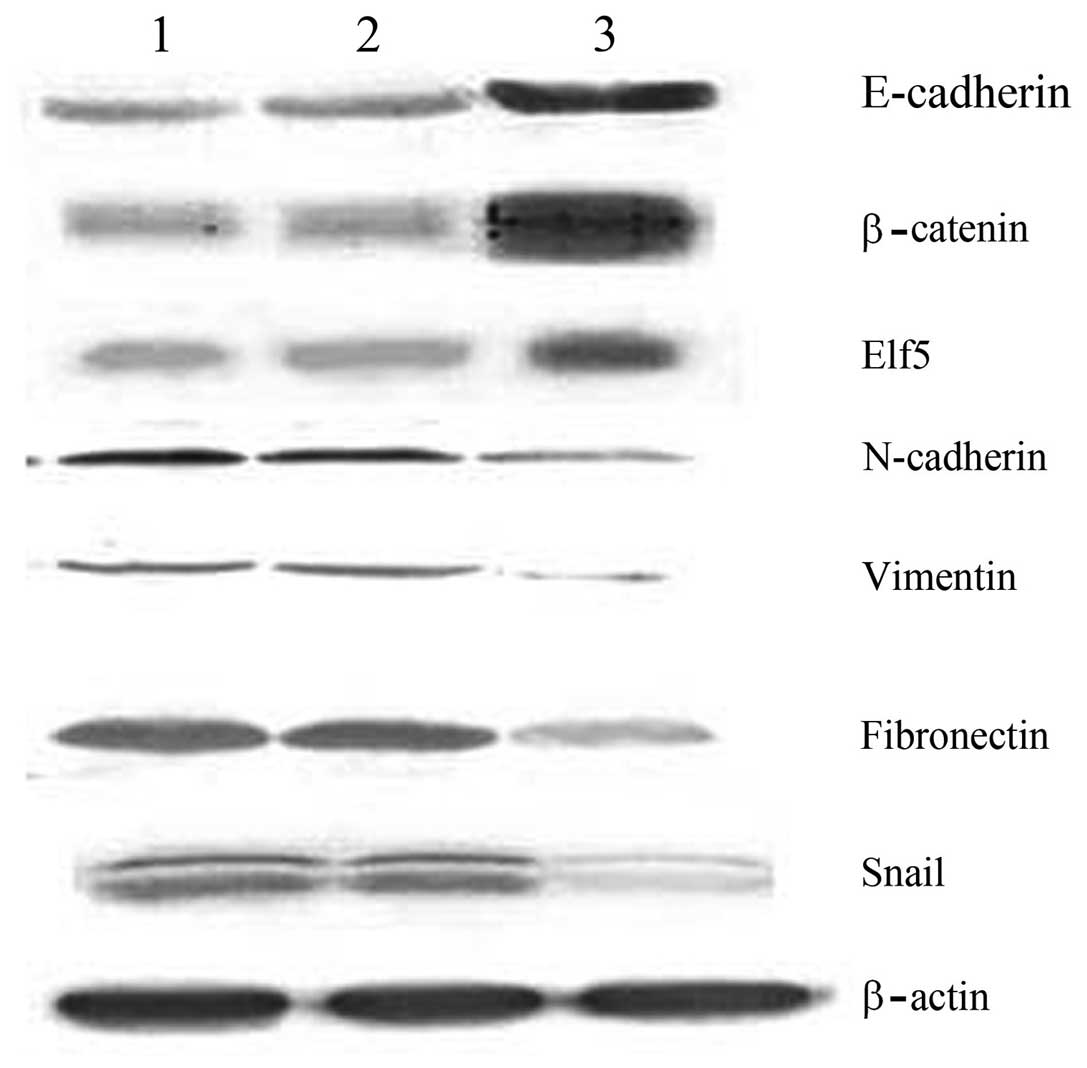
Changes in EMT markers and regulatory
factors following the transfection of ovarian cancer stem cells
with the WWOX gene
The results of western blot analysis revealed that
the expression levels of E-cadherin, β-catenin and Elf5 in the
recombinant plasmid group were 0.762±0.007, 0.911±0.016 and
0.841±0.021, respectively; significantly higher than those of the
control groups (P<0.05). In addition, the expression levels of
N-cadherin, vimentin, fibronectin and Snail in the recombinant
plasmid group were 0.212±0.008, 0.136±0.017, 0.311±0.015 and
0.339±0.027, respectively; significantly lower than those of the
control groups (P<0.05; Fig.
4).
Discussion
The EMT occurs in epithelial cells under specific
physiological or pathological conditions. EMT is a process in which
unique characteristics of certain mesenchymal cells are acquired,
including epithelial cell polarity, intracellular adhesion and loss
of specific cell surface markers. Cytoskeletal remodeling occurs in
these cells and the cells subsequently obtain a mesenchymal-like
phenotype. These changes increase the invasive capacity of tumor
cells and enhance their degree of malignancy (5). The major molecular characteristics of
EMT are the downregulation of the epithelial cell markers,
E-cadherin and β-catenin, and upregulation of the markers of
mesenchymal phenotype, vimentin, fibronectin and N-cadherin. The
downregulation of E-cadherin indicates EMT and is the prerequisite
for epithelial tumor cell invasion (6,7).
E-cadherin, as the most significant EMT marker, is
predominantly affected by the regulation of the transcription
factor, Snail. Snail appears to be a basic helix-loop-helix
transcription factor in Drosophila, rodents and humans,
which belongs to the family of zinc-finger proteins. Snail binds to
the E-cadherin promoter to inhibit the transcription of E-cadherin
and subsequently causes the marked upregulation of N-cadherin,
vimentin and fibronectin, which is a hallmark of EMT (8). Snail, as a transcription factor at the
center of the signaling cascade, is regulated by the upstream
transcription factor, Elf5 (9).
Elf5 is a transcription factor present in mammals that
significantly inhibits breast cancer and certain hormone-related
tumors. Elf5 may directly inhibit the expression of the Snail
transcription factor to further suppress the EMT and thereby reduce
the invasiveness of breast cancer cells (10).
In 2000, the WWOX gene was isolated and
identified as a tumor suppressor gene by Bednarek et al
(11) using shotgun sequencing technology. Furthermore,
the WWOX gene is mapped to the human chromosome
16q23.3–24.1, which covers the entire chromosomal fragile site,
FRA16D. The WWOX peptide contains 414 amino acids with two WW
domains at the N-terminal. WW functional domains are associated
with protein-protein interactions, which are necessary for tumor
inhibition by tumor suppressor genes through various
signal-transduction pathways. Our previous studies confirmed that
the WWOX gene is regulated by genetic and epigenetic
mechanisms (12–17). In order to further investigate the
impact of WWOX genes on ovarian cancer stem cells, the
current study selected human ovarian cancer stem cells and the
human epithelial ovarian carcinoma cell line, HO-8910 as
experimental models. Western blot analyses were used to detect the
differences in EMT markers, including the expression of E-cadherin,
β-catenin, N-cadherin, vimentin and fibronectin, in the two groups.
The results revealed that the expression of E-cadherin and
β-catenin in ovarian cancer stem cells was significantly lower than
that in the HO-8910 cancer cell line, whereas the expression of
N-cadherin, vimentin and fibronectin in ovarian cancer stem cells
was significantly higher than that in the HO-8910 cells. This
indicated that the EMT phenomenon occurs in ovarian cancer stem
cells. The ovarian cancer stem cells were transfected with the
pcDNA3.1-WWOX and pcDNA3.1 eukaryotic expression vectors and the
cells were partitioned into recombinant plasmid, empty plasmid and
blank control groups, according to the plasmid characteristics. The
three cell groups were subsequently tested by MTT assay to measure
the cell proliferation rates, a Transwell® invasion
assay to determine the invasive capacities and western blot
analysis to detect the changes in EMT protein marker expression
levels, as well as expression of EMT regulatory factors, Elf5 and
Snail. The results established that the WWOX gene inhibits
ovarian stem cell proliferation and reduces its invasive capacity
following transfection. In addition, WWOX was found to
significantly upregulate E-cadherin, β-catenin and Elf5, whilst
significantly downregulating N-cadherin, vimentin, fibronectin and
Snail. In conclusion, these results indicated that the WWOX
gene reverses the EMT phenomenon in ovarian cancer stem cells by
regulating the expression of various transcription factors and
reduces tumor invasion, providing a potential novel therapeutic
target for ovarian cancer.
References
|
1
|
Ishii H, Iwatsuki M, Ieta K, et al: Cancer
stem cells and chemoradiation resistance. Cancer Sci. 99:1871–1877.
2008.
|
|
2
|
Cioce M and Ciliberto G: On the
connections between cancer stem cells and EMT. Cell Cycle.
11:4301–4302. 2012.
|
|
3
|
Yan HC, Yu N and Tong JY: Isolation of
cancer stem cells from ovarian cancer cell line HO9810 and
identification of their biological characteristics. Jiang Su Yi
Yao. 38:431–435. 2012.(In Chinese).
|
|
4
|
Yan HC, Xue JQ, Lu XY, et al: Effects of
WWOX gene transfection on cell growth of epithelial ovarian cancer.
Zhonghua Fu Chan Ke Za Zhi. 5:361–365. 2008.(In Chinese).
|
|
5
|
Jordan NV, Johnson GL and Abell AN:
Tracking the intermediate stages of epithelial-mesenchymal
transition in epithelial stem cells and cancer. Cell Cycle.
10:2865–2873. 2011.
|
|
6
|
Jing Y, Han Z, Zhang S, et al:
Epithelial-mesenchymal transition in tumor microenvironment. Cell
Biosci. 1:292011.
|
|
7
|
Jiang J, Tang YL and Liang XH: EMT: a new
vision of hypoxia promoting cancer progression. Cancer Biol Ther.
11:714–723. 2011.
|
|
8
|
Scheel C and Weinberg RA: Cancer stem
cells and epithelial-mesenchymal transition: concepts and molecular
links. Semin Cancer Biol. 22:396–403. 2012.
|
|
9
|
Lee HJ and Ormandy CJ: Elf5, hormones and
cell fate. Trends Endocrinol Metab. 23:292–298. 2012.
|
|
10
|
Chakrabarti R, Hwang J, Andres Blanco M,
et al: Elf5 inhibits the epithelial-mesenchymal transition in
mammary gland development and breast cancer metastasis by
transcriptionally repressing Snail2. Nat Cell Biol. 14:1212–1222.
2012.
|
|
11
|
Bednarek AK, Laflin KJ, Daniel RL, et al:
WWOX, a novel WW domain-containing protein mapping to human
chromosome 16q23.3–24.1, a region frequently affected in breast
cancer. Cancer Res. 60:2140–2145. 2000.
|
|
12
|
Yan HC, Lu XY and Han QY: WWOX mRNA
expression in epithelial ovarian cancer and its clinical
significance. Acta Academiae Medicinae Xuzhou. 2:126–128. 2007.(In
Chinese).
|
|
13
|
Zuo HC, Lu XZ, Han QY and Jin XS:
Construction and Identification of WWOX gene eukaryotic expression
vector. Jiang Su Yi Yao. 3:287–288. 2008.(In Chinese).
|
|
14
|
Zuo HC, Zhang ZZ, Wu XY, et al: Effects of
wwox gene transfection on control mechanism of cell cycle of
epithelial ovarian cancer. Zhonghua Fu Chan Ke Za Zhi. 5:383–385.
2010.
|
|
15
|
Yan H, Yu N and Tong J: Effects of
5-Aza-2′ deoxycytidine on the methylation state and function of the
WWOX gene in the HO-8910 ovarian cancer cell line. Oncol Lett.
6:845–849. 2013.
|
|
16
|
Yan HC and Zhang J: Effects of sodium
valproate on the growth of human ovarian cancer cell line HO8910.
Asian Pac J Cancer Prev. 13:6429–6433. 2012.
|
|
17
|
Yan HC and Sun J: Methylation status of
WWOX gene promoter CpG islands in epithelial ovarian cancer and its
clinical significance. Biomedical Reports. 1:375–378. 2013.
|