Introduction
A novel approach to therapeutic strategy is
emerging, which based on the peculiar metabolism of cancer cells.
Cancer cells are characterized by a high rate of glycolysis, which
is their primary energy source, exceeding the capacity of
mitochondrial oxidative energy metabolism (1). Fructose-bisphosphate aldolase (EC
4.1.2.13) is involved in glycolysis by converting fructose
1,6-diphosphate into dihydroxyacetone phosphate and
glyceraldehyde-3-phosphate (2). The
three aldolase isozymes (ALDOA, ALDOB and ALDOC) have a tetramer
structure with identical molecular weights of ~160 kDa. It is well
known that cancer cells with a high glycolytic rate often exhibit
an aberrant expression of all glycolytic enzymes (2). It has been found that the control of
glycolysis in rapidly growing tumor cells occurs at least partly at
the level of the consuming block (from aldolase to lactate
dehydrogenase) (3). Accumulation of
fructose-1,6-bisphosphate resulting from inhibition of
aldolase-catalyzed cleavage should stop glycolysis and, therefore,
cancer development and progression (2). A previous study has suggested that
aldolase is involved in melanoma cell survival (1).
Angiopoietin-like 4 (ANGPTL4), a secreted protein of
the angiopoietin-like family, is involved in regulating glucose
homeostasis, insulin sensitivity and lipid metabolism through its
capacity to inhibit lipoprotein lipase (4–6). A
previous study has shown that ANGPTL4 expression is regulated by
hypoxia in tumor cells (7).
Moreover, ANGPTL4 mRNA is expressed in the perinecrotic areas of
various human tumors and is highly upregulated in epithelial tumor
cells from clear-cell renal carcinoma (8). A recent study has shown that ANGPTL4
is highly expressed in melanoma brain metastasis and
micrometastasis cells, suggesting that ANGPTL4 is involved in
melanoma metastasis (9).
To the best of our knowledge, the present study is
the first to investigate the relationship between ANGPTL4 and ALDOA
in human melanoma cell invasion and survival.
Materials and methods
Cell lines, plasmids and reagents
WM-115 and WM-266-4 human melanoma cell lines were
purchased from the American Type Culture Collection (Manassas, VA,
USA). Human full-length ANGPTL4 and ALDOA cDNAs (Origene,
Beijing, China) were subcloned into pcDNA 3.1 expression vectors
(Invitrogen Life Technologies, Carlsbad, CA, USA), respectively
(9,10). Human ALDOA
promoter-luciferase reporter (HPRM14783-PG02) and Secrete-Pair
Gaussia Luciferase Assay kit (SPGA-G010) were purchased from
GeneCopoeia (Rockville, MD, USA). Human ANGPTL4 (sc-44664-V) and
human ALDOA (sc-29664-V) shRNA lentiviral particles; control
shRNA lentiviral particles-A (sc-108080); and anti-ANGPTL4 (N-15)
(sc-34113), -ALDOA (N-15) (sc-12059) and -matrix
metalloproteinase-2 (MMP-2) antibodies (sc-53630) were purchased
from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA). DeadEnd™
Fluorometric TUNEL system was purchased from Promega (Madison, WI,
USA). Superfect™ transfection reagent was purchased from Qiagen
(Valencia, CA, USA). Selective protein kinase C (PKC) inhibitor
Go6983 and agonist phorbol 12-myristate 13-acetate (PMA), as well
as puromycin, G418 and cisplatin were purchased from Sigma-Aldrich
(St. Louis, MO, USA).
Transfection and lentiviral
transduction
The ANGPTL4 and ALDOA expression constructs were
transfected into cells using Superfect transfection reagent
(Qiagen) according to the manufacturer’s instructions. Pools of
stable transductants were generated via selection with G418 (800
μg/ml) according to the manufacturer’s protocol. Lentiviral
transduction was performed and pools of stable transductants were
generated via selection with puromycin (5 μg/ml).
Western blot analysis
Immunoblotting was performed with respective
antibodies. Briefly, cells were dissolved in 250 μl of 2× SDS
loading buffer (62.5 mM Tris-HCl, pH 6.8; 2% SDS; 25% glycerol;
0.01% bromphenol blue and 5% 2-mercaptoethanol; Invitrogen Life
Technologies), and incubated at 95°C for 10 min. Equal amount of
proteins for each sample were separated by 10% SDS-polyacrylamide
gel (Invitrogen Life Technologies) electrophoresis and blotted onto
a polyvinylidene difluoride microporous membrane (Millipore,
Billerica, MA, USA). Membranes were incubated for 1 h with a 1/1000
dilution of anti-ANGPTL4 goat polyclonal (N-15; sc-34113),
anti-ALDOA goat polyclonal (N-15; sc-12059) and anti-MMP-2 mouse
monoclonal antibodies (sc-53630) (all Santa Cruz Biotechnology,
Inc.) and then washed and revealed using mouse anti-goat IgG-B
(sc-53799) or donkey anti-mouse IgG-B (sc-2098) secondary
antibodies (Santa Cruz Biotechnology, Inc.) with horseradish
peroxidase conjugate (1/5000, 1 h). Peroxidase was revealed with an
ECL detection plus kit (GE Healthcare, Little Chalfont, UK).
Quantitative polymerase chain reaction
(qPCR)
RNA was prepared from cells using TRIzol reagent
(Invitrogen Life Technologies) followed by purification with Turbo
DNA-free kit (Ambion, Austin, TX, USA). The cDNAs were synthesized
using SuperScript II reverse transcriptase (Invitrogen Life
Technologies). Real-time qPCR was performed using an Abi-Prism 7700
sequence detection system (Applied Biosystems, Foster City, CA,
USA), using the fluorescent dye SYBR Green Master Mix (PE
Biosystems, Framingham, MA, USA) as described by the manufacturer.
The results were normalized against that of the housekeeping gene
glyceraldehyde-3-phosphate dehydrogenase (GAPDH) in the same
sample. The primers used are as follows: Forward,
5′-TCATCCTCTTCCATGAGACACTCT-3′ and reverse,
5′-ATTCTGCTGGCAGATACTGGCATAA-3′ for human ALDOA; forward,
5′-GACTCATGACCACAGTCCATGC-3′ and reverse,
5′-AGAGGCAGGGATGATGTTCTG-3′ for human GAPDH. Each experiment was
repeated twice and performed in triplicate.
Luciferase Assay
WM-115 and WM-266-4 cells were transfected with
human ALDOA promoter-luciferase reporter constructs using
Superfect transfection reagent (Qiagen). Plasmid PRL-CMV encoding
Renilla reniformis luciferase (at one-fifth molar ratio to
test plasmids) was co-transfected with test plasmids in each
transfection as an internal control for data normalization.
Luciferase assays were performed with a Secrete-Pair Gaussia
Luciferase Assay kit (GeneCopoeia) according to the manufacturer’s
instructions. Each experiment was repeated three times and
performed in triplicate.
In vitro cell invasion assay
Transwell® cell-culture chambers with
8-μm pore size (BD Biosciences, Bedford, MA, USA) for 24-well
plates were coated with 50 μl Matrigel (10 mg/ml; BD Biosciences;
diluted 1:3 in RPMI-1640; Life Technologies, Grand Island, NY,
USA). WM-115 and WM-266-4 cells were seeded in the upper chamber at
a density of 5×105 cells per well in RPMI-1640
serum-free medium. Complete medium (600 μl; RPMI-1640 with 5% fetal
bovine serum) was added to the lower chamber. Cells were allowed to
migrate for 24 h followed by fixation and staining with 1% crystal
violet (Sigma-Aldrich). Invaded cells were counted in 10 random
fields per chamber under a microscope (BX51-P; Olympus, Guangzhou,
China). Each experiment was repeated three times and performed in
triplicate.
Measurement of apoptosis by TUNEL
assay
The TUNEL assay was performed using the DeadEnd
Fluorometric TUNEL system according to the manufacturer’s
instructions (Promega). Cells were treated with cisplatin (10 nM)
for 8 h. Apoptotic cells exhibit a strong nuclear green
fluorescence that could be detected using a standard fluorescein
filter. All cells stained with DAPI exhibit a strong blue nuclear
fluorescence. The slides were observed under fluorescence
microscopy (AF6000; Leica Microsystems, Beijing, China) with
relative apoptotic cells determined by counting TUNEL-positive
cells in five random fields (magnification, ×100) per sample.
Statistical analysis
Statistical analyses were performed with SPSS 10.0
for Windows (SPSS, Inc., Chicago, IL, USA). Data values are
expressed as the mean ± standard deviation. Comparisons of means
among multiple groups were performed with one-way analysis of
variance followed by post hoc pairwise comparisons using Tukey’s
tests. A two-tailed P<0.05 was considered to indicate a
statistically significant difference.
Results
Effect of overexpression and knockdown of
ANGPTL4 on ALDOA expression in human melanoma cells
We employed WM-115 and WM-266-4 human melanoma cells
as cellular models in this study. WM-115 was established from a
primary melanoma, and WM266-4 was derived from a skin metastatic
site of the same tumor from which WM-115 was derived. Western blot
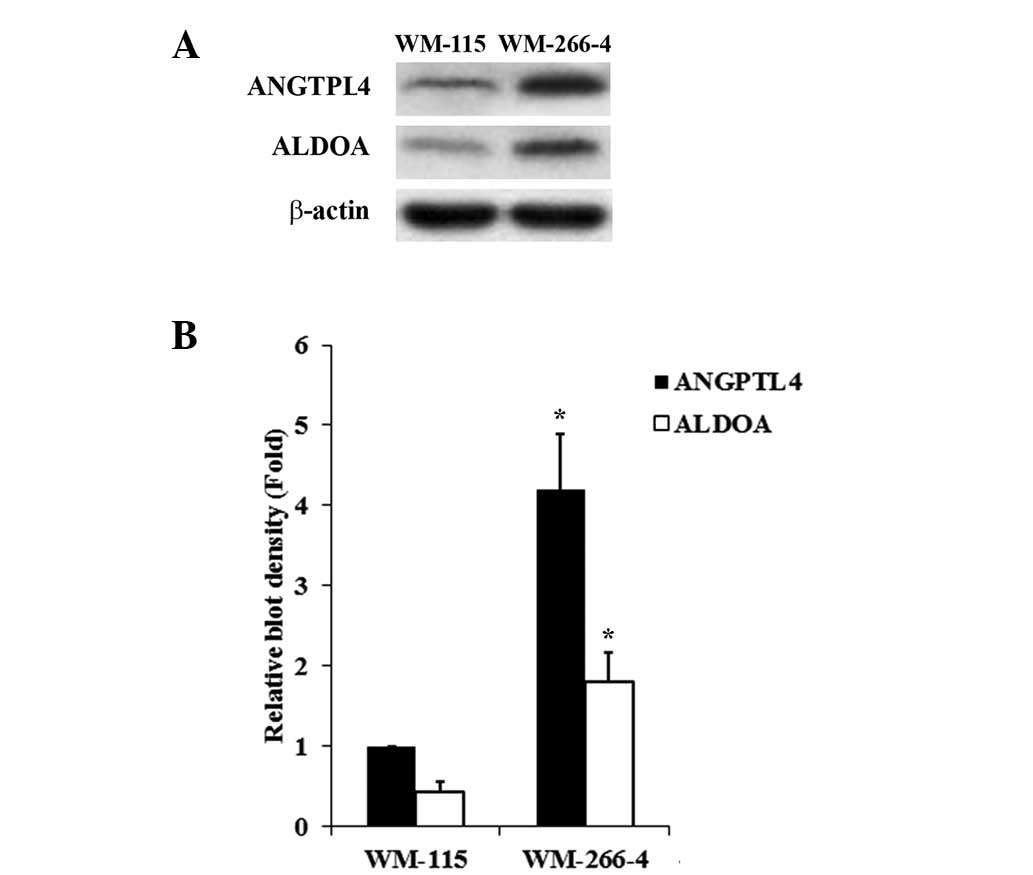
analyses showed that WM-115 cells had lower constitutive expression
of ANGPTL4 and ALDOA than WM-266-4 cells (Fig. 1). The two cell lines would allow
specific ANGPTL4 knockdown or overexpression studies to be
performed in the context of the study goals. Thus, we stably
transfected WM-115 cells with an ANGPTL4 expression vector to
overexpress ANGPTL4, and stably transduced WM-266-4 cells with
ANGPTL4-shRNA to knock down ANGPTL4. Western blot analysis showed
that stable transfection of ANGPTL4 led to an over two-fold
increase of ANGPTL4 expression in WM-115 cells, which was not
affected by selective PKC inhibitor Go6983 (500 nM). On the other
hand, knockdown of ANGPTL4 by shRNA resulted in a >80% decrease
of endogenous ANGPTL4 in WM-266-4 cells, which was not affected by
selective PKC agonist PMA (500 nM) (Fig. 2). The ALDOA expression in WM-115
cells increased in parallel with ANGPTL4 overexpression, which was
inhibited by Go6983. In WM-266-4 cells, the ALDOA expression
decreased in parallel with ANGPTL4 knockdown, which was rescued by
PMA (Fig. 2). A similar data trend
was observed with ALDOA mRNA levels in the cells (Fig. 3).
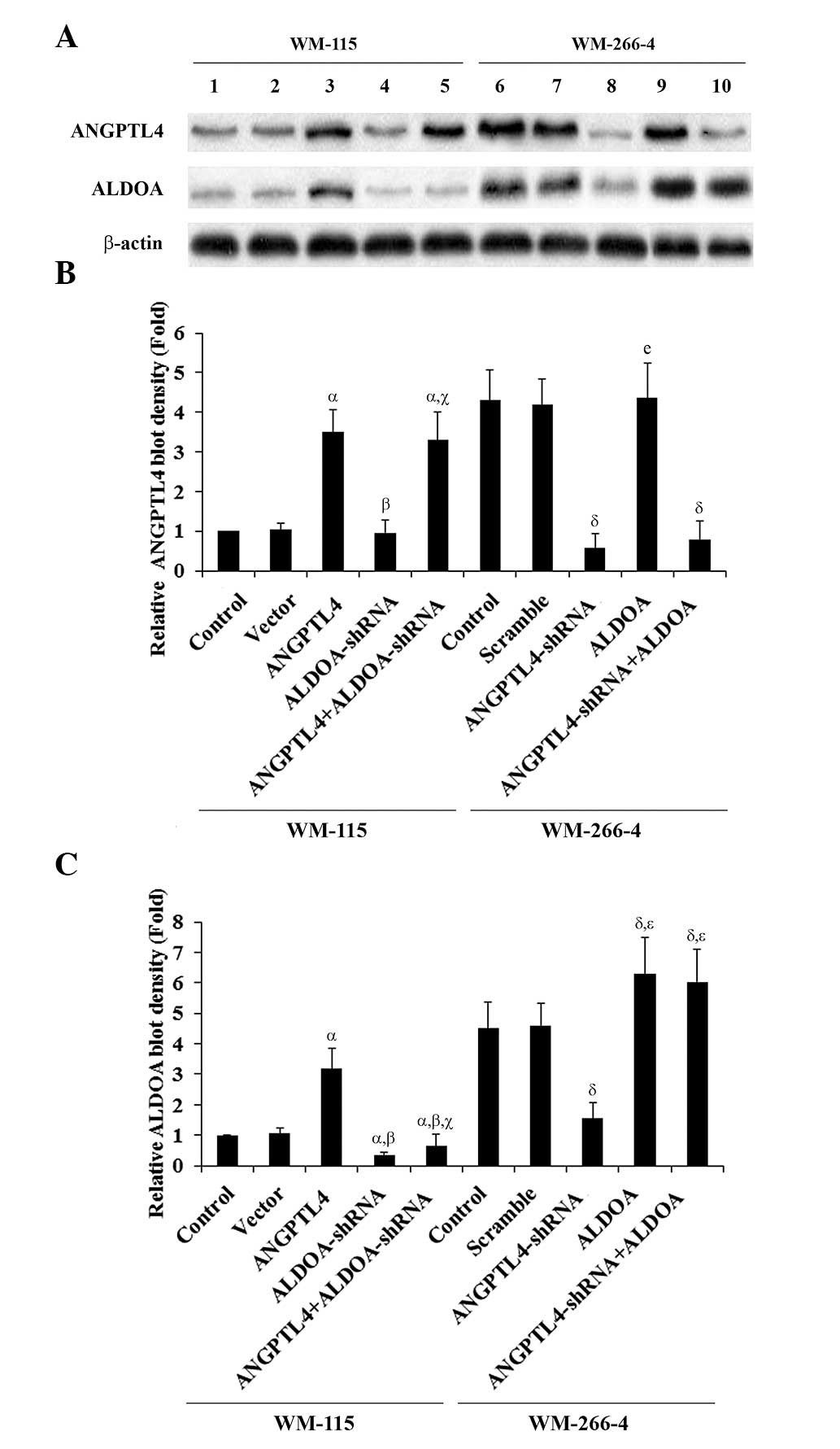 | Figure 2Angiopoietin-like 4 (ANGPTL4) and
aldolase A (ALDOA) expression in melanoma cells with overexpression
and knockdown of ANGPTL4. (A) WM-115 cells: Expression of ANGPTL4
and ALDOA in control cells (lane 1), cells stably transfected with
empty pcDNA3 vector (lane 2), cells overexpressing ANGPTL4 (lane
3), cells treated with selective protein kinase C (PKC) inhibitor
Go6983 (500 nM, 24 h; lane 4), and cells overexpressing ANGPTL4 and
simultaneously treated with Go6983 (500 nM, 24 h; lane 5), was
analyzed by western blotting. WM-266-4 cells: Expression of ANGPTL4
and ALDOA in control cells (lane 6), cells stably transduced with
scramble control shRNA (lane 7), cells stably expressing
ANGPTL4-shRNA (lane 8), cells treated with selective PKC agonist
phorbol 12-myristate 13-acetate (PMA; 500 nM, 24 h; lane 9), and
cells stably expressing ANGPTL4-shRNA and simultaneously treated
with PMA (500 nM, 24 h; lane 10), was analyzed by western blotting.
β-actin blotting was used as a loading control. (B and C) Density
of the ANGPTL4 (B) and ALDOA (C) blots was normalized against that
of β-actin to obtain a relative blot density, respectively, which
was expressed as the fold-change to the relative ANGPTL4 (B) or
ALDOA (C) blot density of WM-115 control cells (designated as 1).
WM-115 cells: αP<0.05, compared with Control and
Vector; βP<0.05, compared with ANGPTL4;
γP<0.05, compared with PKC inhibitor. WM-266-4 cells:
δP<0.05, compared with Control and Scramble;
χP<0.05, compared with ANGPTL4-shRNA. |
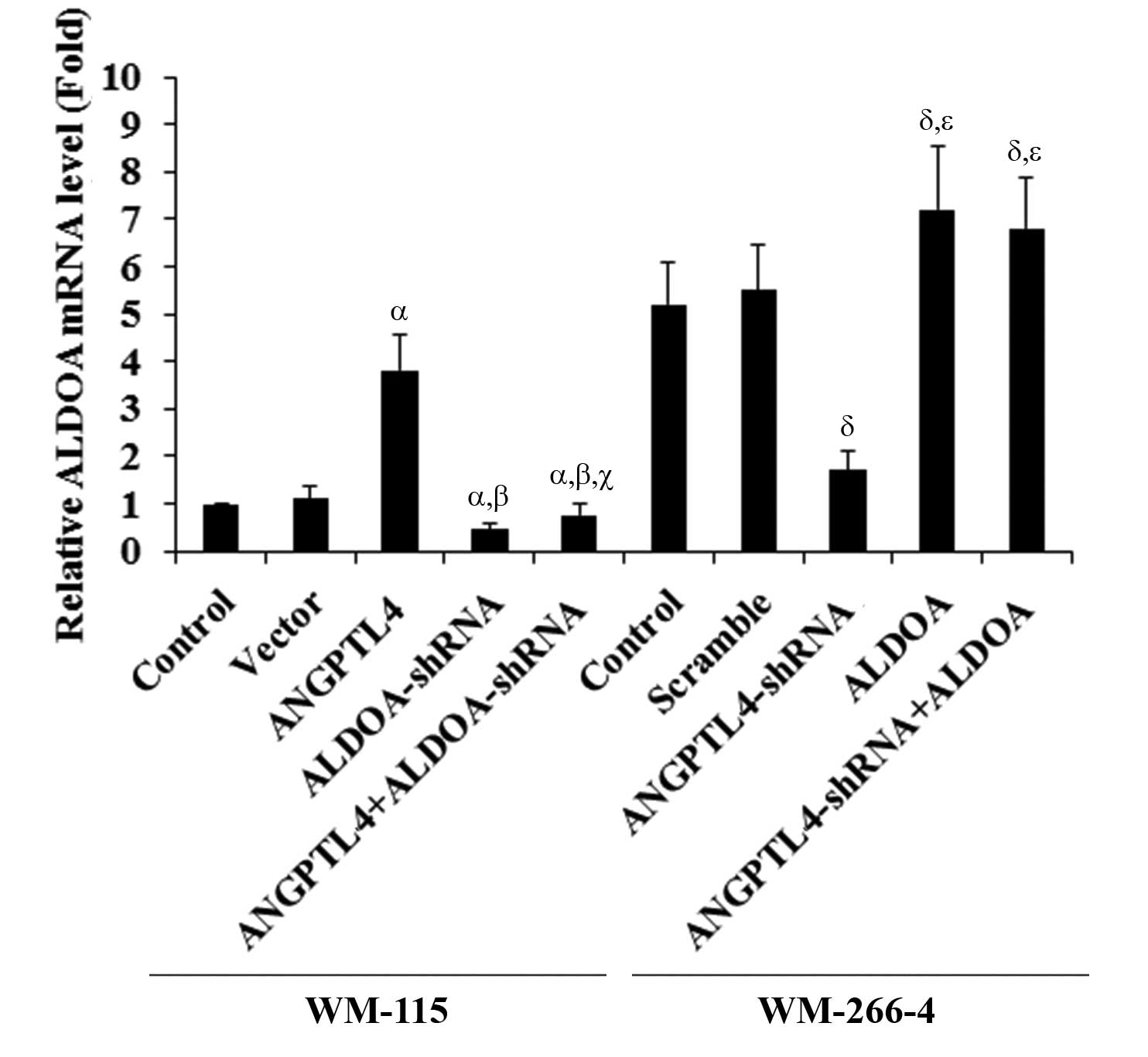 | Figure 3Aldolase A (ALDOA) mRNA expression in
melanoma cells with overexpression and knockdown of
angiopoietin-like 4 (ANGPTL4). WM-115 cells: The ALDOA mRNA
level in control cells, cells stably transfected with empty pcDNA3
vector, cells overexpressing ANGPTL4, cells treated with selective
protein kinase C (PKC) inhibitor Go6983 (500 nM, 24 h), and cells
overexpressing ANGPTL4 and simultaneously treated with Go6983 (500
nM, 24 h), was analyzed by quantitative polymerase chain reaction
(qPCR). WM-266-4 cells: The ALDOA mRNA level in control
cells, cells stably transduced with scramble control shRNA, cells
stably expressing ANGPTL4-shRNA, cells treated with selective PKC
agonist phorbol 12-myristate 13-acetate (PMA; 500 nM, 24 h, and
cells stably expressing ANGPTL4-shRNA and simultaneously treated
with PMA (500 nM, 24 h), was analyzed by qPCR. The ALDOA
mRNA level is shown as the fold-change to that of WM-115 control
cells (designated as 1). WM-115 cells: αP<0.05,
compared with Control and Vector; βP<0.05, compared
with ANGPTL4; γP<0.05, compared with PKC inhibitor.
WM-266-4 cells: δP<0.05, compared with Control and
Scramble; χP<0.05, compared with ANGPTL4-shRNA. |
Effect of overexpression and knockdown of
ANGPTL4 on ALDOA gene promoter activities in human melanoma
cells
To determine whether ANGPTL4 regulates ALDOA
expression in human melanoma cells by altering the ALDOA
gene promoter activity, we transfected WM-115 and WM-266-4 cells
with human ALDOA promoter-luciferase reporter plasmids.
Luciferase assays showed that the ALDOA gene promoter activity in
WM-115 cells was increased by ANGPTL4 overexpression, which was
inhibited by Go6983 (500 nM). In WM-266-4 cells, the ALDOA
gene promoter activity was decreased by ANGPTL4 knockdown, which
was completely restored by PMA (500 nM) (Fig. 4).
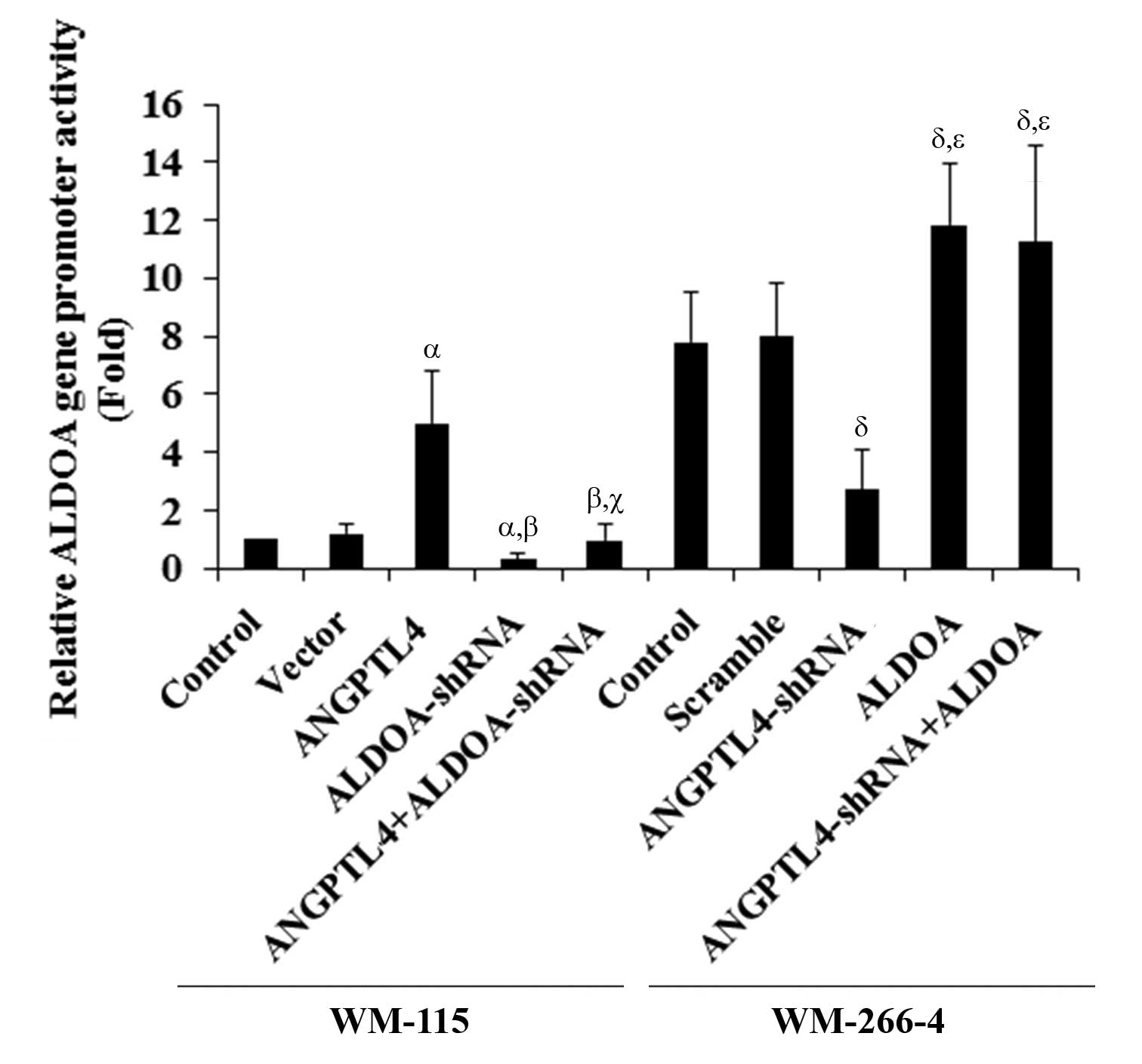 | Figure 4Effect of angiopoietin-like 4
(ANGPTL4) on human aldolase A (ALDOA) promoter activities. WM-115
and WM-266-4 cells were transfected with human ALDOA
promoter-luciferase reporter plasmids. After 24 h, luciferase
assays were performed. WM-115 cells: Luciferase activity in control
cells, cells stably transfected with empty pcDNA3 vector, cells
overexpressing ANGPTL4, cells treated with selective protein kinase
C (PKC) inhibitor Go6983 (500 nM, 24 h), and cells overexpressing
ANGPTL4 and simultaneously treated with Go6983 (500 nM, 24 h), was
analyzed. WM-266-4 cells: Luciferase activity in control cells,
cells stably transduced with scramble control shRNA, cells stably
expressing ANGPTL4-shRNA, cells treated with selective PKC agonist
phorbol 12-myristate 13-acetate (PMA; 500 nM, 24 h), and cells
stably expressing ANGPTL4-shRNA and simultaneously treated with PMA
(500 nM, 24 h), was analyzed. The luciferase activity was expressed
as the fold-change to that of WM-115 control cells (designated as
1). WM-115 cells: αP<0.05, compared with Control and
Vector; βP<0.05, compared with ANGPTL4;
γP<0.05, compared with PKC inhibitor. WM-266-4 cells:
δP<0.05, compared with Control and Scramble;
χP<0.05, compared with ANGPTL4-shRNA. |
Functional role of ALDOA in
ANGPTL4-enhanced cell invasion and MMP-2 expression in human
melanoma cells
To examine the functional roles of ANGPTL4 and ALDOA
in melanoma cell invasion, we performed in vitro cell
invasion assays, which showed that ANGPTL4 overexpression increased
cell invasion in WM-115 cells by over two-fold, which was reversed
by knocking down ALDOA (Fig. 5). In
WM-266-4 cells, knockdown of ANGPTL4 decreased cell invasion by
over 65%, which was completely restored by overexpression of ALDOA
(Fig. 5). A similar data trend was
observed with MMP-2 expression in the cells (Fig. 6).
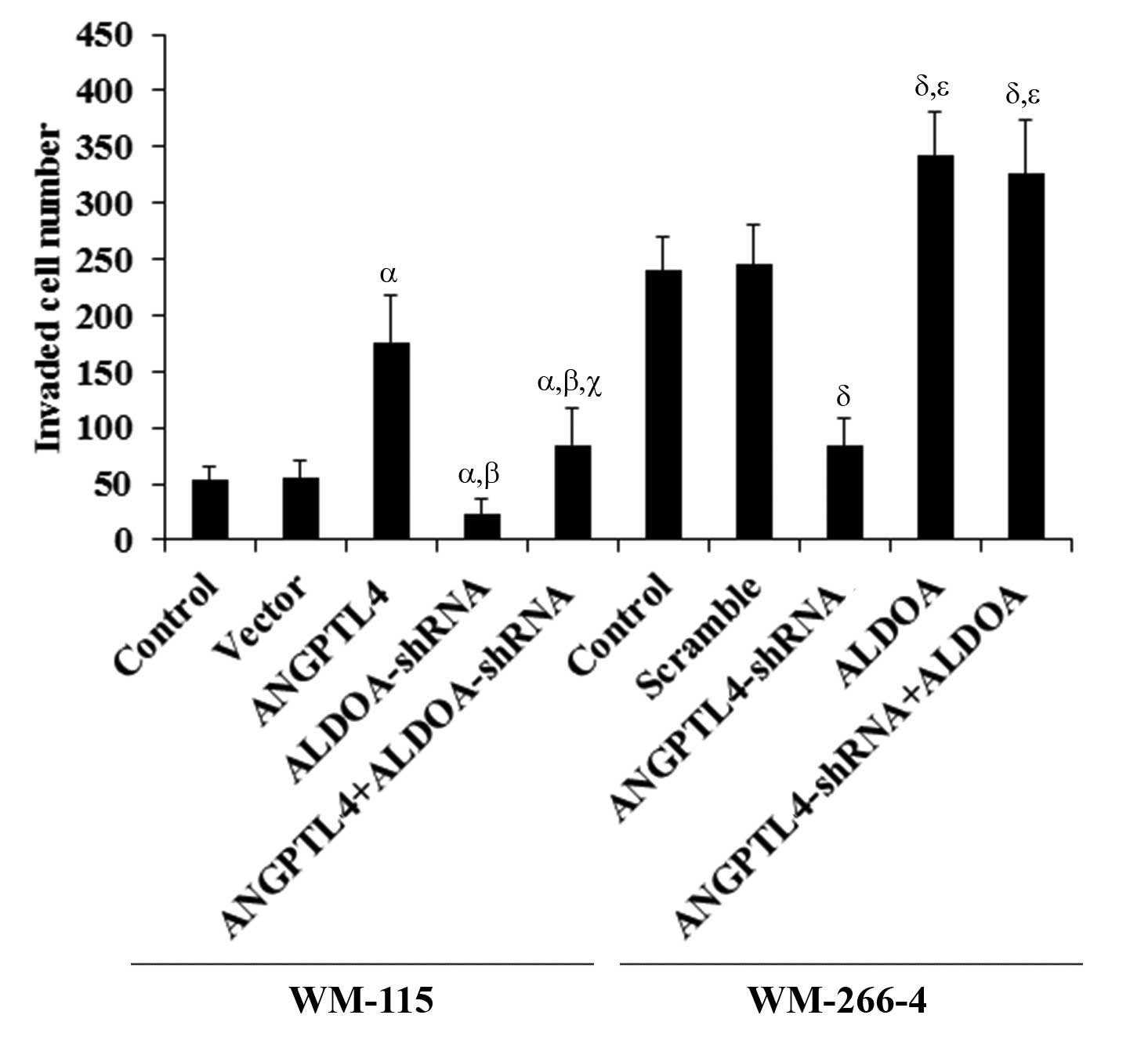 | Figure 5In vitro cell invasion in
melanoma cells with overexpression and knockdown of
angiopoietin-like 4 (ANGPTL4) and/or aldolase A (ALDOA) WM-115
cells: In vitro cell invasion assays were performed in
control cells, cells stably transfected with empty pcDNA3 vector,
cells overexpressing ANGPTL4, cells stably expressing ALDOA-shRNA,
and cells overexpressing ANGPTL4 plus stably expressing
ALDOA-shRNA. WM-266-4 cells: In vitro cell invasion assays
were performed in control cells, cells stably transduced with
scramble control shRNA, cells stably expressing ANGPTL4-shRNA,
cells overexpressing ALDOA, and cells stably expressing
ANGPTL4-shRNA plus overexpressing ALDOA. Invaded cell numbers were
counted. WM-115 cells: αP<0.05, compared with Control
and Vector; βP<0.05, compared with ANGPTL4;
γP<0.05, compared with ALDOA-shRNA. WM-266-4 cells:
δP<0.05, compared with Control and Scramble;
χP<0.05, compared with ANGPTL4-shRNA. |
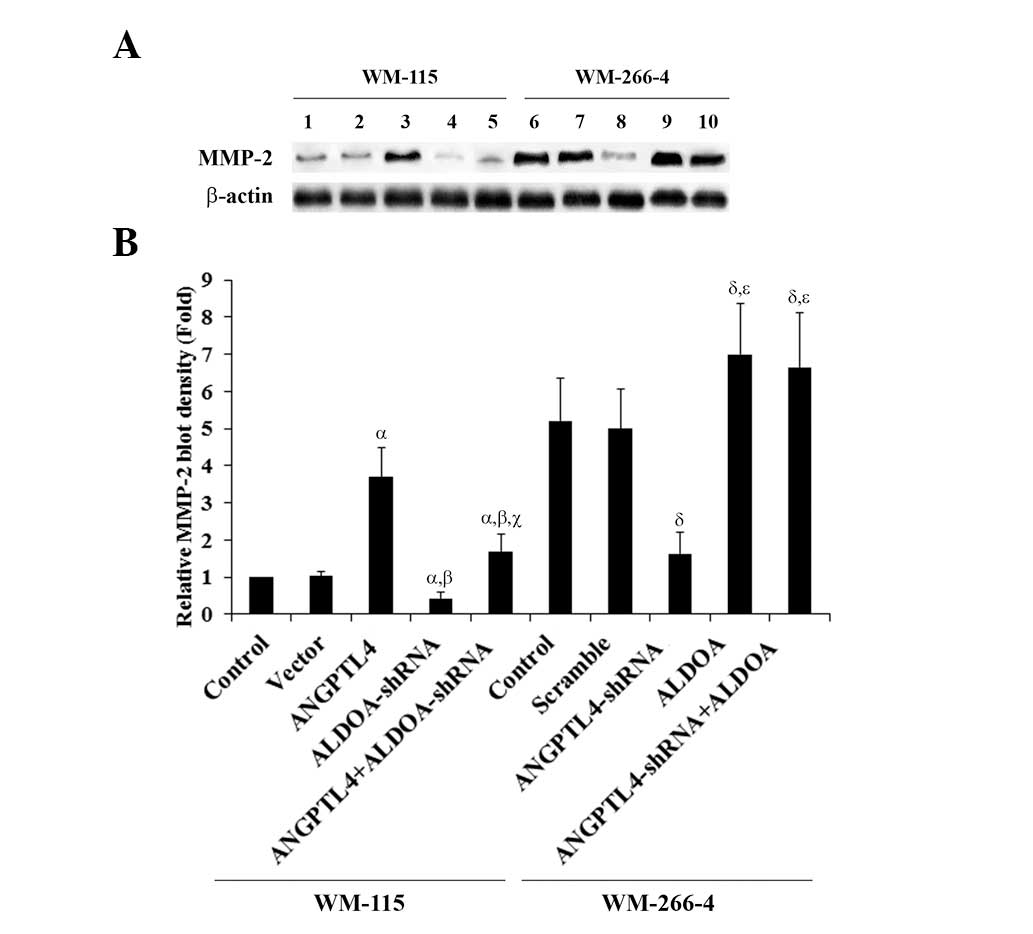 | Figure 6Matrix metalloproteinase-2 (MMP-2)
expression in melanoma cells with overexpression and knockdown of
angiopoietin-like 4 (ANGPTL4) and/or aldolase A (ALDOA). (A) WM-115
cells: MMP-2 expression was determined in control cells (lane 1),
cells stably transfected with empty pcDNA3 vector (lane 2), cells
overexpressing ANGPTL4 (lane 3), cells stably expressing
ALDOA-shRNA (lane 4), and cells overexpressing ANGPTL4 plus stably
expressing ALDOA-shRNA (lane 5), by western blot analysis. WM-266-4
cells: MMP-2 expression was determined in control cells, cells
stably transduced with scramble control shRNA (lane 7), cells
stably expressing ANGPTL4-shRNA (lane 8), cells overexpressing
ALDOA (lane 9), and cells stably expressing ANGPTL4-shRNA plus
overexpressing ALDOA (lane 10), by western blot analysis. β-actin
blotting was used as a loading control. (B) The density of the
MMP-2 blot was normalized against that of β-actin to obtain a
relative blot density, which was expressed as the fold-change to
the relative MMP-2 blot density of WM-115 control cells (designated
as 1). WM-115 cells: αP<0.05, compared with Control
and Vector; βP<0.05, compared with ANGPTL4;
γP<0.05, compared with ALDOA-shRNA. WM-266-4 cells:
δP<0.05, compared with Control and Scramble;
χP<0.05, compared with ANGPTL4-shRNA. |
Functional role of ALDOA in
ANGPTL4-enhanced cell survival against cisplatin in human melanoma
cells
To investigate the functional roles of ANGPTL4 and
ALDOA in melanoma cell survival against apoptotic stress, we
examined cell apoptosis in melanoma cells treated with 10 nM of
cisplatin, an apoptosis-inducing chemotherapeutic agent commonly
used to treat melanoma. Overexpression or knockdown of ANGPTL4
and/or ALDOA did not significantly alter cell apoptosis in both
WM-115 and WM-266-4 cells under normal culture conditions (Fig. 7). However, in WM-115 cells treated
with cisplatin, overexpression of ANGPTL4 significantly decreased
cell apoptosis compared with the controls, which was reversed by
knocking down ALDOA (Fig. 8A). In
WM-266-4 cells, knockdown of ANGPTL4 significantly increased
cisplatin-induced cell apoptosis, which was reversed by
overexpression of ALDOA (Fig.
8B).
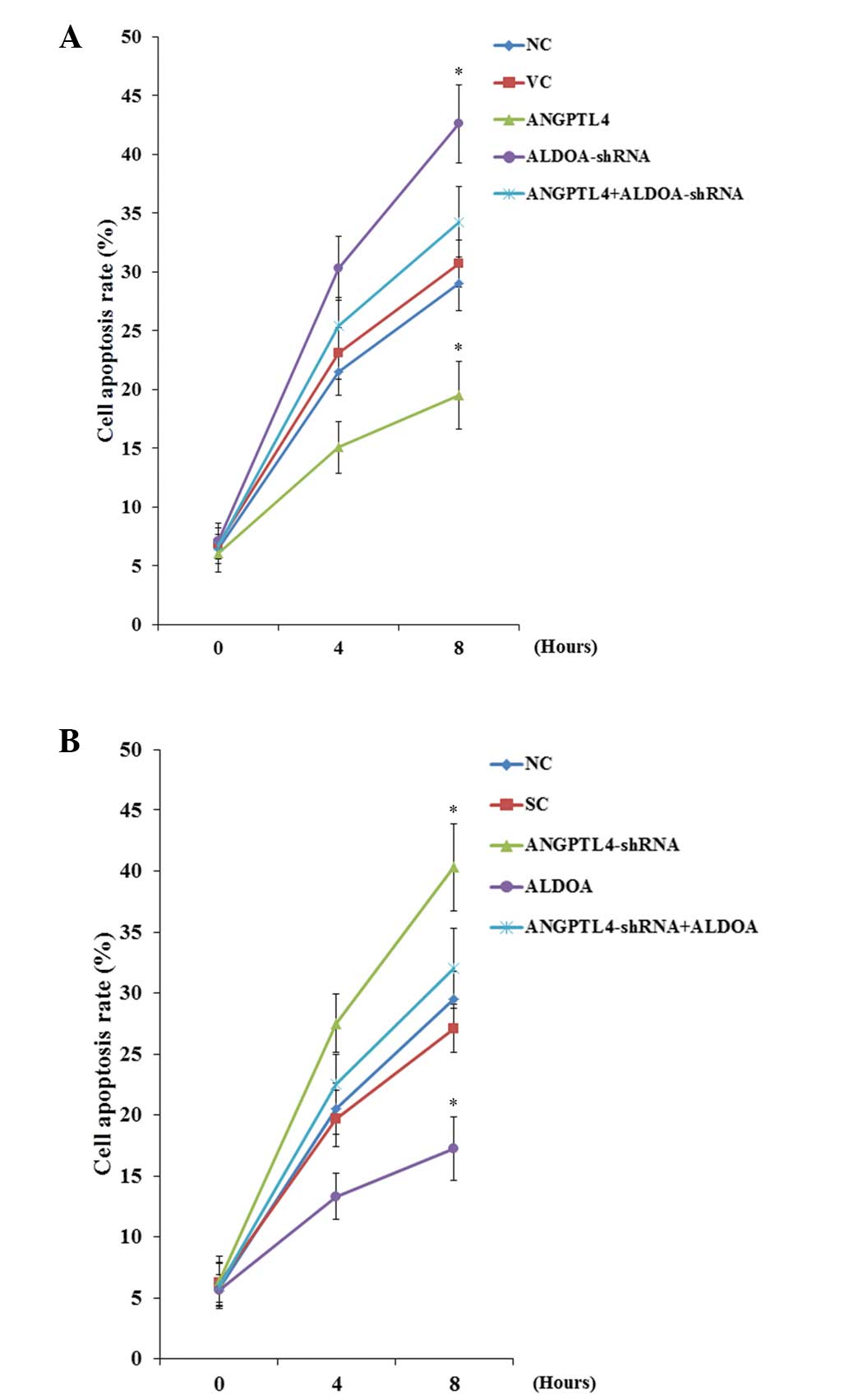 | Figure 8Cisplatin-induced apoptosis in
melanoma cells with overexpression or knockdown of
angiopoietin-like 4 (ANGPTL4) and/or aldolase A (ALDOA). (A) WM-115
cells: TUNEL assays were performed in control cells, cells stably
transfected with empty pcDNA3 vector, cells overexpressing ANGPTL4,
cells stably expressing ALDOA-shRNA, and cells overexpressing
ANGPTL4 plus stably expressing ALDOA-shRNA. (B) WM-266-4 cells:
TUNEL assays were performed in control cells, cells stably
transduced with scramble control shRNA, cells stably expressing
ANGPTL4-shRNA, cells overexpressing ALDOA, and cells stably
expressing ANGPTL4-shRNA plus overexpressing ALDOA. The cells were
treated with 10 nM of cisplatin for 8 h. Cell apoptosis rates at 4
and 8 h were shown as the percentage of TUNEL positive cells in
total cells. WM-115 cells: *P<0.05, compared with
Control and Vector. WM-266-4 cells: *P<0.05, compared
with Control and Scramble. |
Discussion
Inhibiting cancer cell glycolysis is an emerging
therapeutic strategy for cancer (2). A previous study suggested that
aldolase is involved in melanoma cell survival (1). ANGPTL4 reportedly is involved in
melanoma metastasis (9). To the
best of our knowledge, the present study provides the first
evidence that ANGPTL4 upregulates ALDOA expression in human
melanoma cells, and that a major part of the promoting effect of
ANGPTL4 on melanoma cell invasion and survival is mediated by
ALDOA.
WM-115 and WM-266-4 cells were utilized as melanoma
cell models in this study. The two cell lines were respectively
established from a primary melanoma and a skin metastatic site of
the same tumor in the same patient, which gives them a more
comparable genetic background. In addition, WM-115 cells express a
relatively low level of ANGPTL4 compared with WM-266-4 cells. Thus,
overexpression and knockdown of ANPTL-4 were respectively performed
in the two cell lines to approach the study objectives from
different angles.
ANGPTL4 reportedly modulates epidermal
differentiation through stimulating the expression of PKC (11), and stimulation of PKC has been shown
to promote ALDOA gene transcription (12). In the present study, ALDOA
expression at both the mRNA and the protein levels was
significantly increased and decreased in parallel with
overexpression and knockdown of ANGPTL4 in melanoma cells, which
was blocked by selective PKC inhibitor and restored by PKC agonist,
respectively. The results suggest that ANGPTL4 expression may
affect ALDOA expression in human melanoma cells at the gene
transcription level through a PKC-dependent mechanism. Luciferase
assays confirmed that ANGPTL4 could enhance ALDOA gene
promoter/transcriptional activities in melanoma cells through a
PKC-dependent mechanism. However, the mechanism by which ANGPTL4
modulates the ALDOA promoter activities remains unclear and
will be further investigated in our future studies. In addition,
although it has been reported that activation of PKC can induce
ANGPTL4 expression in human airway smooth muscle cells (13), our data indicate that PKC does not
modulate ANTPTL-4 expression in melanoma cells.
A previous study suggested that ANGPTL4 may promote
melanoma metastasis (9). Since our
findings had suggested that ALDOA was a downstream effector of
ANGPTL4/PKC signaling, we investigated the functional roles of
ANGPTL4 and ALDOA in melanoma cell invasion. ALDOA knockdown almost
canceled the effects of increased cell invasion and MMP-2
expression caused by ANGPTL4 overexpression in WM-115 cells, while
ALDOA overexpression restored the decreased cell invasion and MMP-2
expression caused by ANGPTL4 knockdown in WM-266-4 cells. The
results suggest that ALDOA is a critical mediator of the promoting
effect of ANGPTL4 on melanoma cell invasion, likely through
upregulating the MMP-2 expression.
Cell survival against apoptotic stress is critical
for cancer progression and metastasis (14). In the current study, a relatively
small concentration of cisplatin (10 nM) was used to induce
apoptotic stress without killing the majority of the cells. In the
presence of cisplatin, ALDOA knockdown almost canceled the effects
of increased cell survival caused by ANGPTL4 overexpression in
WM-115 cells, while ALDOA overexpression restored the decreased
cell survival caused by ANGPTL4 knockdown in WM-266-4 cells. The
results not only suggest an important functional role of ALDOA in
ANGPTL4-enhanced melanoma cell survival, but also implicate ANGPTL4
and ALDOA in the development of melanoma chemoresistance. Cisplatin
elicits DNA repair mechanisms by crosslinking DNA, which in turn
activates apoptosis when repair proves impossible (14). It remains unclear whether ANGPTL4
and ALDOA may impact melanoma cell survival against other types of
chemotherapy agents. Further studies with additional types of
chemotherapy agents and melanoma cell lines would elaborate this
issue.
The aldolase isozymes (ALDOA, ALDOB and ALDOC) are
encoded by three different genes, differentially expressed during
development. ALDOA is mainly produced by the developing embryo and
in adult muscle; ALDOB is produced by the liver, kidney and
intestine; and ALDOC is mainly produced by the brain and other
nervous tissue. ALDOA and ALDOB have been associated with poor
prognosis of osteosarcoma and hepatocarcinoma, respectively
(15,16). It would be interesting to explore in
future studies whether and how ALDOB and ALDOC are involved in
melanoma cell invasion and survival.
In conclusion, the present study demonstrates that
ANGPTL4 upregulates ALDOA expression in human melanoma cells at the
ALDOA gene promoter/transcriptional level through a PKC-dependent
mechanism, and that ALDOA is a critical mediator of the promoting
effect of ANGPTL4 on melanoma cell invasion, likely through
upregulating the MMP-2 expression. Additionally, our results also
suggest that ALDOA plays an important role in ANGPTL4-enhanced
melanoma cell survival against cisplatin-induced apoptotic stress,
which implicates ANGPTL4 and ALDOA in the development of melanoma
chemoresistance.
Acknowledgements
This study was supported by the Fundamental Research
Funds for the Central Universities of Central South University
(grant no. 201322ts084).
References
|
1
|
Schwartz D and Beitner R: Detachment of
the glycolytic enzymes, phosphofructokinase and aldolase, from
cytoskeleton of melanoma cells, induced by local anesthetics. Mol
Genet Metab. 69:159–164. 2000.
|
|
2
|
Scatena R, Bottoni P, Pontoglio A,
Mastrototaro L and Giardina B: Glycolytic enzyme inhibitors in
cancer treatment. Expert Opin Investig Drugs. 17:1533–1545.
2008.
|
|
3
|
Marin-Hernández A, Rodríguez-Enríquez S,
Vital-González PA, Flores-Rodríguez FL, Macías-Silva M,
Sosa-Garrocho M and Moreno-Sánchez R: Determining and understanding
the control of glycolysis in fast-growth tumor cells. Flux control
by an over-expressed but strongly product-inhibited hexokinase.
FEBS J. 273:1975–1988. 2006.
|
|
4
|
Mandard S, Zandbergen F, van Straten E,
Wahli W, Kuipers F, Muller M and Kersten S: The fasting-induced
adipose factor/angiopoietin-like protein 4 is physically associated
with lipoproteins and governs plasma lipid levels and adiposity. J
Biol Chem. 281:934–944. 2006.
|
|
5
|
Yoshida K, Shimizugawa T, Ono M and
Furukawa H: Angiopoietin-like protein 4 is a potent
hyperlipidemia-inducing factor in mice and inhibitor of lipoprotein
lipase. J Lipid Res. 43:1770–1772. 2002.
|
|
6
|
Xu A, Lam MC, Chan KW, et al:
Angiopoietin-like protein 4 decreases blood glucose and improves
glucose tolerance but induces hyperlipidemia and hepatic steatosis
in mice. Proc Natl Acad Sci USA. 102:6086–6091. 2005.
|
|
7
|
Lal A, Peters H, St Croix B, et al:
Transcriptional response to hypoxia in human tumors. J Natl Cancer
Inst. 93:1337–1343. 2001.
|
|
8
|
Le Jan S, Amy C, Cazes A, et al:
Angiopoietin-like 4 is a proangiogenic factor produced during
ischemia and in conventional renal cell carcinoma. Am J Pathol.
162:1521–1528. 2003.
|
|
9
|
Izraely S, Sagi-Assif O, Klein A, et al:
The metastatic microenvironment: brain-residing melanoma metastasis
and dormant micrometastasis. Int J Cancer. 131:1071–1082. 2012.
|
|
10
|
Sakakibara M, Takahashi I, Takasaki Y,
Mukai T and Hori K: Construction and expression of human aldolase A
and B expression plasmids in Escherichia coli host. Biochim
Biophys Acta. 1007:334–342. 1989.
|
|
11
|
Pal M, Tan MJ, Huang RL, Goh YY, Wang XL,
Tang MB and Tan NS: Angiopoietin-like 4 regulates epidermal
differentiation. PLoS One. 6:e253772011.
|
|
12
|
Costanzo P, Lupo A, Medugno L, D’Agostino
P, Zevino C and Izzo P: PKC-dependent phosphorylation of the p97
repressor regulates the transcription of aldolase A L-type
promoter. FEBS Lett. 454:61–66. 1999.
|
|
13
|
Stapleton CM, Joo JH, Kim YS, Liao G,
Panettieri RA Jr and Jetten AM: Induction of ANGPTL4 expression in
human airway smooth muscle cells by PMA through activation of PKC
and MAPK pathways. Exp Cell Res. 316:507–516. 2010.
|
|
14
|
Wu H, Yang L, Liao D, Chen Y, Wang W and
Fang J: Podocalyxin regulates astrocytoma cell invasion and
survival against temozolomide. Exp Ther Med. 5:1025–1029. 2013.
|
|
15
|
Chen X, Yang TT, Zhou Y, et al: Proteomic
profiling of osteosarcoma cells identifies ALDOA and SULT1A3 as
negative survival markers of human osteosarcoma. Mol Carcinog.
53:138–144. 2014.
|
|
16
|
Peng SY, Lai PL, Pan HW, Hsiao LP and Hsu
HC: Aberrant expression of the glycolytic enzymes aldolase B and
type II hexokinase in hepatocellular carcinoma are predictive
markers for advanced stage, early recurrence and poor prognosis.
Oncol Rep. 19:1045–1053. 2008.
|