Introduction
Renal cell carcinoma (RCC) accounts for 2–3% of all
adult malignant neoplasms. Annually, RCC affects ~150,000
individuals and causes ~78,000 mortalities worldwide (1). In addition, 25–30% of patients present
with metastatic RCC at diagnosis (2). Although treatment with multikinase
inhibitors has been shown to prolong progression-free survival
rates, effective therapy for patients with metastatic
advanced-stage RCC remains limited (3,4). Based
on previous genetic and molecular studies, it has been postulated
that additional tumorigenic events are required for the genesis of
RCC, and investigations into these pathways may lead to the
development of novel agents (5).
The Notch pathway is highly conserved and plays a
crucial role in multiple cellular processes (6). Notch signaling is initiated through
the interactions between the plasma-embedded Notch receptors
(Notch1-4) and cell surface ligands (Jagged1 and Jagged2 and δ-like
1, 2 and 4) present on adjacent cells (6). This results in a conformational change
in Notch to reveal the cleavage site 2 for metalloproteases (ADAM10
and ADAM17), which leaves a 12 amino acid stub of the Notch
extracellular domain, required for subsequent recognition and
cleavage by the γ-secretase complex. γ-secretase cleavage of Notch
liberates the intracellular domain, which translocates to the
nucleus, enabling gene transcription of Notch downstream targets
(7).
Deregulated Notch signaling has been implicated in a
number of tumor types, including hematological cancers and solid
tumors (8–11). For RCC, it has been previously
reported that the Notch signaling cascade is constitutively active
in human RCC cell lines (12), and
high expression of Notch has been associated with increased risk of
metastasis (13). However, contrast
theories remain that the expression of Notch receptors is
downregulated and Notch signaling may function as a tumor
suppressor in the progress of RCC (14).
Our previous study reported that Jagged1 is
expressed at an elevated level in RCC and its overexpression may
predict poor outcome in RCC patients (15). In order to further confirm the role
of Notch singling in RCC, the present study detected the expression
of Notch1 and Jagged1, as well as the effects of Notch pathway
inhibition on the proliferation and apoptosis of renal carcinoma
cells. The present study indicated that Notch plays a role in the
tumorigenesis of RCC and highlights the potential use of
γ-secretase inhibitor as a novel treatment for RCC.
Materials and methods
Reagents and antibodies
The antibodies used against Notch1 (polyclonal
rabbit anti-human Notch1; ab27526) and Jagged1 (polyclonal goat
anti-human Jagged1; sc-6011) were purchased from Abcam (Cambridge,
UK) and Santa Cruz Biotechnology, Inc. (Santa Cruz, CA, USA),
respectively. The peroxidase-conjugated mouse anti-goat IgG
antibody was purchased from Shanghai Changdao Biologic Technology
Co., Ltd. (Shanghai, China). Glyceraldehyde-3-phosphate
dehydrogenase (GAPDH) was purchased from Santa Cruz Biotechnology,
Inc. and the γ-secretase inhibitor,
N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl
ester (DAPT), was purchased from Merck Biosciences (Darmstadt,
Germany). Tissue culture media and fetal bovine serum (FBS) were
purchased from Gibco (Fullerton, CA, USA). The Annexin
V-fluorescein isothiocyanate (FITC) apoptosis detection kit was
purchased from Beckman Coulter (Fullerton, CA, USA). Human renal
carcinoma cell lines, 786-0, 769-p and Caki, were obtained from the
Shanghai Institute of Cell Biology, Chinese Academy of Sciences
(Shanghai, China).
Patients and tissue samples
The present study was approved by the ethical
committee of Zhongshan Hospital, Fudan University (no.2008-98;
Shanghai, China). Each patient was involved after providing
informed written consent. For western blot analysis, fresh tumor
tissues (later verified as clear cell RCC) and normal (non-tumor)
kidney tissues were obtained intraoperatively from eight patients
who underwent radical nephrectomy at the Department of Urology,
Zhongshan Hospital. The tissue samples were then snap-frozen in
liquid nitrogen and stored at −80°C prior to analysis. For
immunostaining, a total of 129 patients with pathologically
verified clear cell RCC were enrolled consecutively. All patients
underwent nephrectomy (partial or radical) performed at the
Department of Urology, Zhongshan Hospital, between 2003 and
2008.
Western blot analysis
The eight paired samples of RCC and normal renal
tissues were solubilized in a lysis buffer (SDS) on ice. All
lysates were centrifuged at 4°C at 10,000 × g for 10 min. The
protein concentration was determined using the Bradford protein
assay (Bio-Rad, Hercules, CA, USA). In total, 100 μg protein
content from each sample was electrophoresed in 8% SDS-PAGE
(Shanghai Changdao Biologic Technology Co., Ltd., Shanghai,
China)and blotted on a nitrocellulose membrane (Bio-Rad). The
membrane was blocked with 5% bovine serum albumin in 1×
Tris-buffered saline (TBS) buffer at room temperature for 2 h and
incubated with Notch1 (1:200) or Jagged1 (1:500) antibodies at 4°C
overnight. Following three washes for 15 min in TBS, the membrane
was incubated with the peroxidase-conjugated mouse anti-goat IgG
antibody for 2 h at room temperature. Immunoreactive proteins were
visualized by an enhanced chemiluminescence system (Immobilon,
Millipore, Billerica, MA, USA) and GAPDH was used as the control
for protein loading.
Immunohistochemistry
Immunohistochemistry was performed using standard
techniques with 129 cases of pathologically verified clear cell
RCC. Briefly, 4-mm paraffin-embedded tissue sections were dewaxed
in xylene and rehydrated in graded alcohols. Endogenous peroxidase
was blocked using 3% hydrogen peroxide. Antigen retrieval was
accomplished by boiling tissue sections in 10 mM citrate buffer (pH
6.0) for 10 min. Non-specific protein binding was performed by
30-min incubations with goat serum. These treatments were
alternated with rinses in phosphate-buffered saline (PBS). The
slides were then treated with Notch1 (1:200) or Jagged1 (1:100)
antibodies for 1 h at room temperature. Next, the slides were
rinsed with PBS and incubated with horse radish
peroxidase-conjugated secondary antibody, followed by a rinse in
PBS, incubation with 3,3′-diaminobenzidine staining and
counterstaining with hematoxylin blue. Negative controls were
performed by substituting the primary antibody with a non-immune
serum. Control sections were treated in parallel with the
samples.
Evaluation of staining
All stained sections were evaluated by three
independent investigators in a blind manner. The scoring was based
on color intensity and extensity as previously described (16). Briefly, the proportion score was
determined semi-quantitatively by assessing the whole tumor section
at low magnification and each sample was scored on the following
scale of 0–3: 0, no positive cells; 1, 1–20% of positive cells; 2,
21–60% of positive cells; and 3, 61–100% of positive cells. The
intensity score was determined at high magnification as follows: 0,
negative staining; 1, weakly positive staining; 2, moderately
positive staining; and 3, markedly positive staining. Then, the
total score of each section was calculated by sum of the two
parameters.
Cell culture
Human renal carcinoma cell lines, 786-0, 769-p and
Caki, were cultured in Dulbecco’s modified Eagle’s medium
(Invitrogen Life Technologies, Carlsbad, CA, USA) supplemented with
10% FBS, 2 mM L-glutamine, 100 U/ml penicillin and 0.1 mg/ml
streptomycin in a humidified atmosphere with 5% CO2
incubator at 37°C. Cells were seeded in six-well plates at a
density of 5×104/well and allowed to adhere overnight.
The medium was replaced with medium containing the inhibitor
diluted in dimethyl sulphoxide (DMSO). For
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
and apoptosis detection, as well as cell circle analysis by flow
cytometry, at least three independent experiments were
performed.
Cell viability assay
The antiproliferative effect of DAPT against various
groups of cells was determined using the MTT (Sigma-Aldrich, St.
Louis, MO, USA) assay. Briefly, cells were seeded in 96-well plates
at a density of 1.0×104 cells per well. Following
overnight incubation, the cells were treated with DAPT (1, 2, 5, 10
and 20 μM) for 48 h. Following DAPT treatment, the medium was
removed and 20 μl MTT (5 mg/ml in PBS) was added to each well.
Following incubation for 4 h at 37°C, the supernatant was removed
and the formazan crystals were solubilized by adding 150 μl DMSO.
Viable cells were detected by measuring absorbance at 490 nm using
MRX II absorbance reader (Dynex Technologies, Chantill, VA, USA).
The reduction in viability of DAPT-treated cells was expressed as a
percentage compared with non-DAPT-treated control cells. Control
cells were considered to be 100% viable.
Detection of apoptotic cells by flow
cytometry
Cells were plated in six-well plates (2 ml/well) at
a density of 5×105 cells/ml and incubated overnight.
DAPT of various concentrations (1, 2, 5 and 10 μM) was then added
into each well and incubated for 48 h. The cells were collected and
washed with PBS, followed by resuspension in binding buffer at a
concentration of 1×106 cells/ml. A total of 100 ml
(1×105 cells) of the solution was removed and mixed with
Annexin V-FITC and propidium iodide (PI) according to the
manufacturer’s instructions. The mixed solution was incubated in
the dark at room temperature for 15 min, 400 μl dilution buffer was
then added to each tube and cell apoptosis analysis was performed
using the Beckman Coulter FC500 Flow Cytometry system (Beckman
Coulter) within 1 h.
Analysis of cell cycle distribution
Cell cycle analysis was performed using the Coulter
DNA Prep™ Reagents kit (Beckman Coulter). Cells were prepared as
previously described. The cells were then exposed to various
concentrations of DAPT (1, 2, 5 and 10 μM) for 48 h at 37°C. Cells
were harvested, washed with cold PBS, fixed with 70% ethanol and
stored at 4°C for subsequent cell cycle analysis. For detecting DNA
content, cells were incubated in the dark at room temperature with
0.5 ml RNase A for 20 min and with 1 ml PI for 20 min. The DNA
content of the cells was measured using the Beckman Coulter FC500
Flow Cytometry system. The percentage of cells in G1, S and G2/M
phases was calculated.
Statistical analysis
Difference of immunostaining between neoplastic and
normal kidney tissues was detected by the χ2 test, as
well as the correlation between protein expression and clinical and
pathological characteristics. Statistical analyses were performed
using a statistical software package (SPSS, version 16.0; SPSS,
Inc., Chicago, IL, USA). P<0.05 was considered to indicate a
statistically significant difference and all P-values were
two-sided.
Results
Western blot analysis
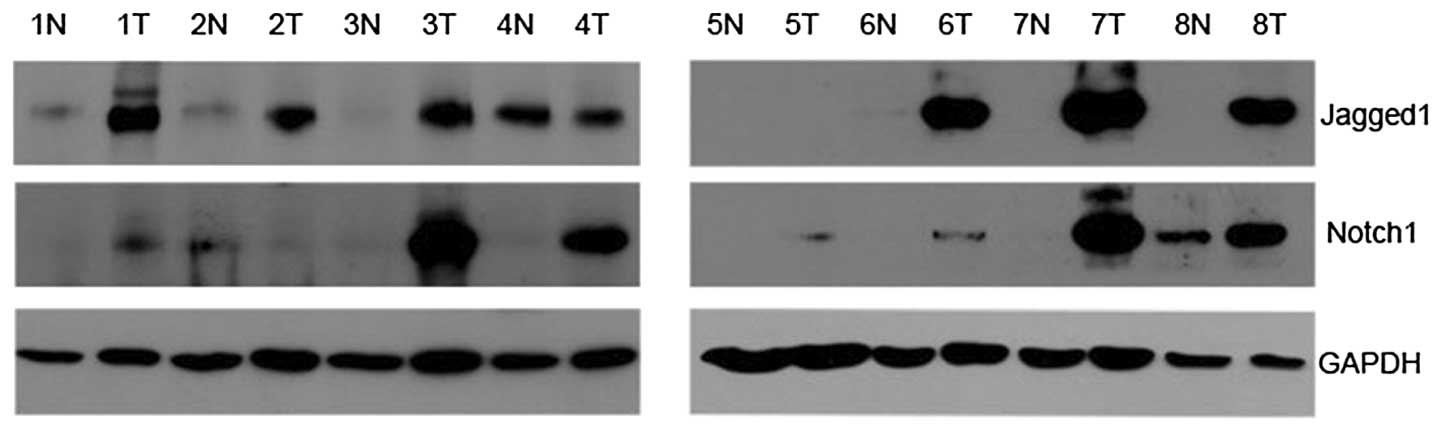
As Fig. 1 shows, the
protein of Notch1 was expressed in adjacent non-neoplastic tissues
and RCC tissues, with specific bands at 80 kDa. Notch1 was found to
be upregulated in seven cases of RCC tissues (7/8; 87.5%) compared
with paired non-neoplastic tissues. Similarly, Jagged1 was detected
at 150 kDa. The expression of Jagged1 was higher in six tumor
tissues (6/8, 75.0%) than in paired non-neoplastic tissues.
Clinical and pathological
characteristics
In total, 129 cases with clear cell RCC were
enrolled for immunostaining of Jagged1. In total, eight cases were
collected for western blot analysis. The clinical and pathological
characteristics of the patients are listed in Table I.
 | Table IClinicopathological characteristics of
clear cell RCC cases. |
Table I
Clinicopathological characteristics of
clear cell RCC cases.
| Characteristics | IHC (n=129) | Western blotting
(n=8) |
|---|
| Gender, n (%) |
| Male | 84 (65.1) | 6 (75.0) |
| Female | 45 (34.9) | 2 (25.0) |
| Age, years |
| Mean | 55.5 | 61.5 |
| Range | 27–83 | 55–76 |
| Surgery, n (%) |
| Radical
nephrectomy | 122 (94.6) | 7 (87.5) |
| Partial
nephrectomy | 7 (5.4) | 1 (12.5) |
| Tumor size, cm |
| Mean | 5.3 | 4.3 |
| Range | 1.5–15 | 2.5–12 |
| TNM stage, n (%) |
| I | 81 (62.8) | 2 (25.0) |
| II | 17 (13.2) | 3 (37.5) |
| III | 24 (18.6) | 2 (25.0) |
| IV | 7 (5.4) | 1 (12.5) |
| Fuhrman grade, n
(%) |
| 1 | 53 (41.1) | 3 (37.5) |
| 2 | 54 (41.9) | 3 (37.5) |
| 3 | 18 (14) | 1 (12.5) |
| 4 | 4 (3.0) | 1 (12.5) |
| Relapse, n (%) |
| Yes | 37 (28.7) | 1 (12.5) |
| No | 92 (71.3) | 7 (87.5) |
Immunohistochemistry
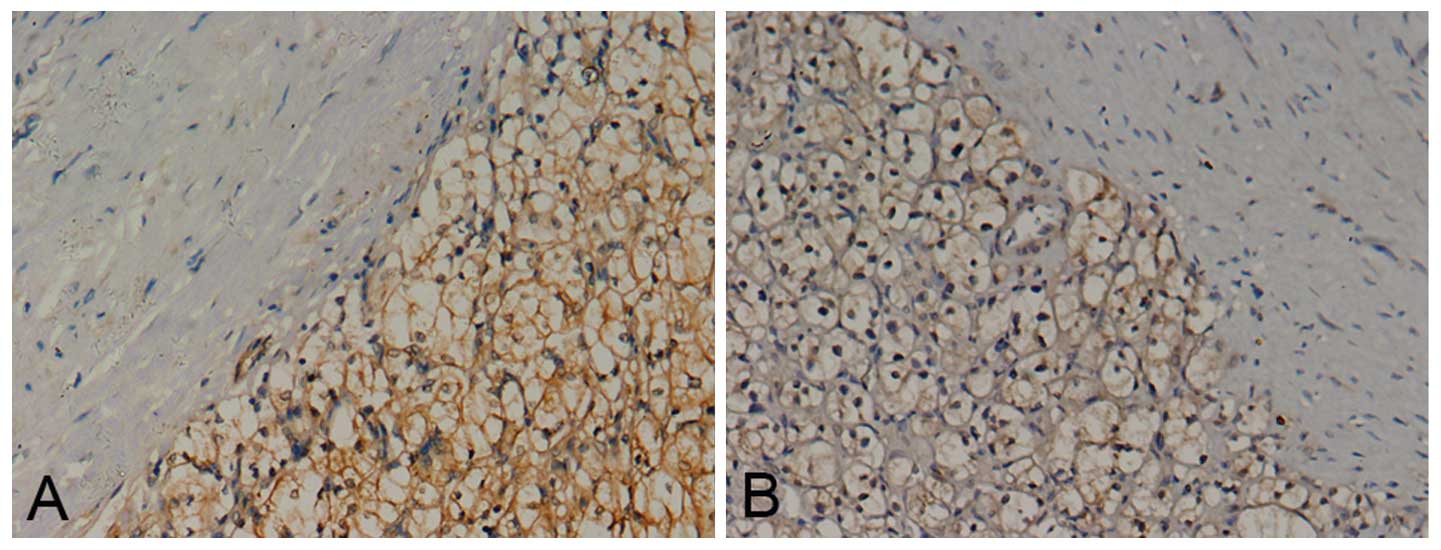
Notch1 and Jagged1 staining was present mainly in
the cell membrane and/or cytoplasm (Fig. 2). The positive staining rate of
Notch1 in RCC tissues was 95.3% (123/129), compared with 36.4%
(12/33) in normal kidney tissues (P<0.05; χ2=65.8).
The positive staining rate of Jagged1 in RCC tissues was 93.0%
(120/129), while that in normal kidney tissues was 42.4% (14/33)
(P<0.05; χ2=47.1). Notch1 and Jagged1 exhibited a
significantly higher expression in RCC tissues than in normal
kidney tissues.
Low expression was designated as a total score of
0–3, while high expression was designated as a total score of 4–6.
Tumors were subdivided according to protein expression level into
various groups. The expression level of Notch1 was found to
statistically correlate with nuclear grade (P=0.025), TNM stage
(P=0.037) and tumor size (P=0.002). The expression level of Jagged1
was also found to statistically correlate with nuclear grade
(P=0.001), TNM stage (P=0.002) and tumor size (P=0.016), which has
been mentioned in our previous study (15). In particular, cases with higher
Notch1 or Jagged1 expression showed higher rates of disease
relapse, with P=0.024 and P<0.001, respectively (Tables II and III).
 | Table IICorrelation between Notch1 and
clinicopathological characteristics. |
Table II
Correlation between Notch1 and
clinicopathological characteristics.
| Age, years | Gender | Tumor size, cm | TNM stage | Fuhrman grade | Relapse |
|---|
|
|
|
|
|
|
|
|---|
| Notch1 | <55 | ≥55 | Male | Female | <5.0 | ≥5.0 | I+II | III+IV | 1+2 | 3+4 | Yes | No |
|---|
| Low expression,
n | 37 | 32 | 44 | 25 | 41 | 28 | 57 | 12 | 62 | 7 | 14 | 55 |
| High expression,
n | 29 | 31 | 40 | 20 | 19 | 41 | 40 | 20 | 45 | 15 | 23 | 37 |
| χ2 | 0.359 | 0.119 | 9.936 | 4.373 | 5.006 | 5.108 |
| P-value | 0.549 | 0.730 | 0.002 | 0.037 | 0.025 | 0.024 |
 | Table IIICorrelation between Jagged1 and
clinicopathological characteristics. |
Table III
Correlation between Jagged1 and
clinicopathological characteristics.
| Age, years | Gender | Tumor size, cm | TNM stage | Fuhrman grade | Relapse |
|---|
|
|
|
|
|
|
|
|---|
| Jagged1 | <55 | ≥55 | Male | Female | <5.0 | ≥5.0 | I+II | III+IV | 1+2 | 3+4 | Yes | No |
|---|
| Low expression,
n | 40 | 27 | 46 | 21 | 38 | 29 | 58 | 9 | 63 | 4 | 9 | 58 |
| High expression,
n | 26 | 36 | 38 | 24 | 22 | 40 | 39 | 23 | 44 | 18 | 28 | 34 |
| χ2 | 3.867 | 0.769 | 5.835 | 9.667 | 12.107 | 15.848 |
| P-value | 0.053 | 0.380 | 0.016 | 0.002 | 0.001 | <0.001 |
DAPT inhibits renal carcinoma cell
growth
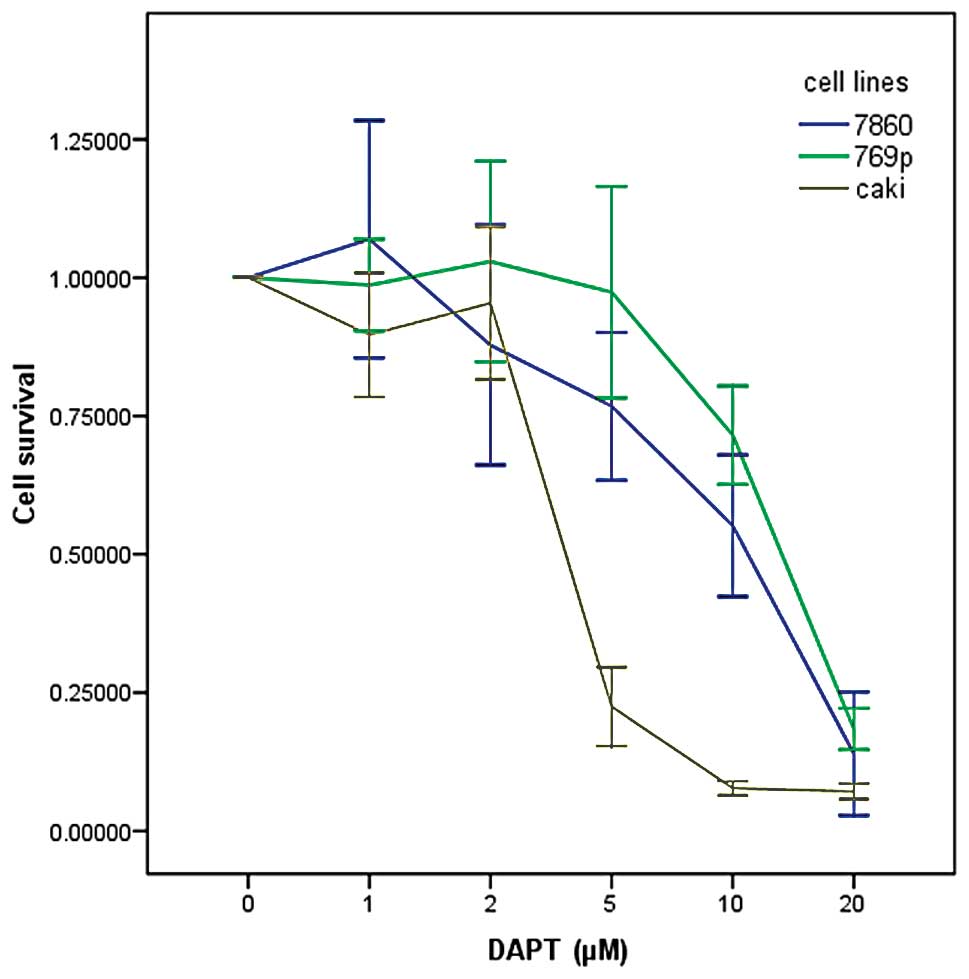
In order to investigate the potential effects of
DAPT on the growth and viability of human renal carcinoma cells,
various cell lines (786-0, 769-p and Caki) were treated with DAPT
at various concentrations (1, 2, 5, 10 and 20 μM) by MTT assay. As
shown in Fig. 3, inhibition of cell
proliferation by DAPT was generally in a dose-dependent manner. The
IC50 dose of DAPT for the proliferation of renal
carcinoma cells was ~12.8, 11.4 and 4.9 μM for 786-0, 769-p and
Caki renal carcinoma cell lines, respectively.
DAPT induces G2/M phase cell cycle
arrest
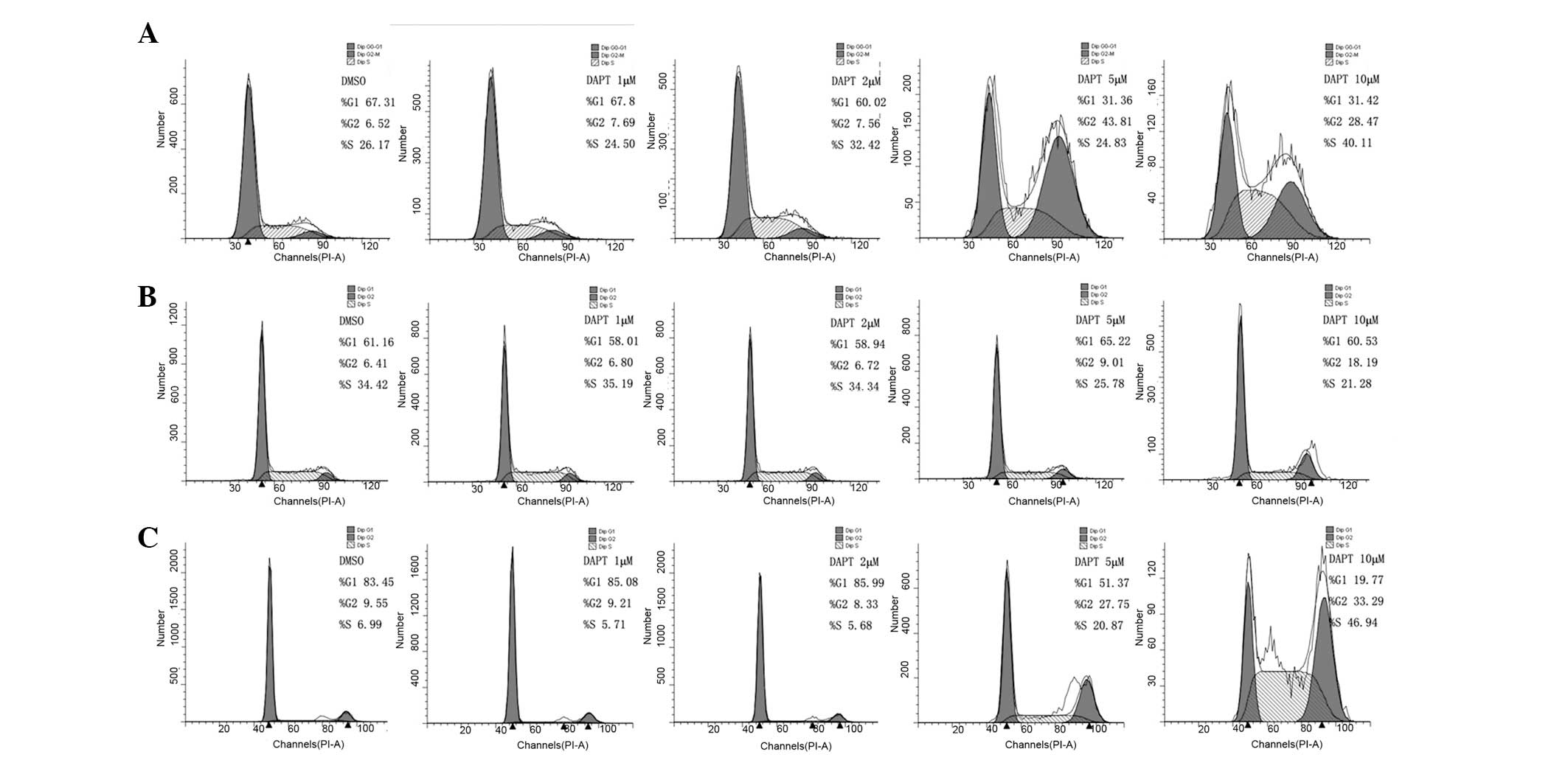
Based on the growth inhibitory response of DAPT
treatment in cells, its effect on cell cycle distribution was next
examined. Renal carcinoma cells were treated with various
concentrations of DAPT for 48 h and analyzed by flow cytometry. As
shown in Fig. 4, the level of
G2/M-phase arrest was observed. Following treatment with 1, 2, 5
and 10 μM DAPT for 48 h, the rate of G2/M phase for 786-0 cells was
increased by 7.69, 7.56, 43.81 and 28.47%, respectively. While for
769-p cells, the rate was 6.80, 6.72, 9.01 and 18.19%,
respectively, and for Caki cells, the rate was 9.21, 8.33, 27.75
and 33.29%, respectively. These results suggested that DAPT induces
G2/M phase cell cycle arrest in renal carcinoma cells.
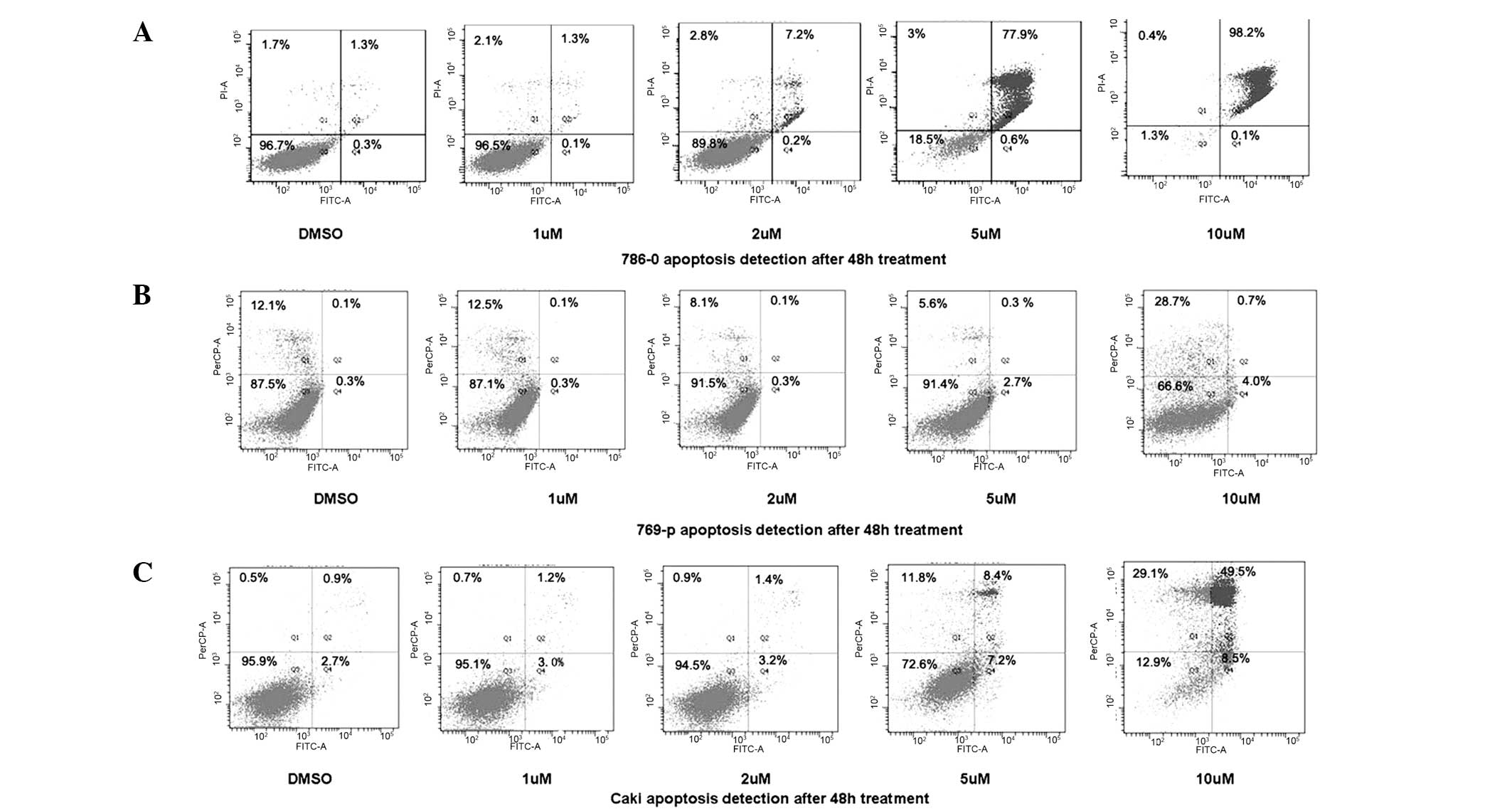
DAPT induces apoptosis in renal carcinoma
cells
To determine whether the DAPT-induced growth
inhibition was mediated by apoptosis, flow cytometry was further
used to identify the cell death types. As shown in Fig. 5, 786-0, 769-p and Caki RCC cell
lines treated with DAPT showed a dose-dependent increase in the
levels of apoptosis.
Discussion
The Notch pathway is critical in the determination
of cell fates by regulating cell growth, differentiation and
apoptosis (6). It plays an
oncogenic or a tumor suppressive role, depending on the cancer
type, the other signaling pathways involved and activation of the
Notch receptor (17).
Previously, the aberrant regulation of Notch
signaling has been implicated in tumorigenesis; however,
conflicting theories concerning the role of the Notch pathway in
RCC exist (12,14,15).
In the current study, the expression of Notch1 and Jagged1 was
detected and an elevated level was shown in neoplastic tissue as
compared with that in normal kidney tissue, which was also
confirmed by western blot analysis. In addition, the expression
levels of Notch1 and Jagged1 were found to markedly correlate with
tumor size, grade and TNM stage, as well as disease relapse,
suggesting that the Notch pathway may be associated with the
oncogenesis process of RCC.
When the γ-secretase inhibitor (DAPT) was applied to
renal carcinoma cell lines, the proliferation was decreased. The
suppression by DAPT was associated with induced G2/M-phase cell
cycle arrest, as well as cell apoptosis. The present study
indicated the oncogenic role of Notch signaling in the development
of RCC. Notably, the 769-p cells appeared to be less sensitive to
γ-secretase treatment than the other two cell lines. The mechanism
by which these cells partially escaped inhibition of γ-secretase
cleavage remains to be determined. It must be noted that specific
T-cell acute lymphoblastic leukemia (T-ALL) cells harboring Notch1
activating mutations were refractory to γ-secretase treatment
(18). It is important to clarify
whether mutations in the Notch pathway are present in the subset of
RCC.
The mechanism involved with the oncogenic role of
Notch may be multiple. Firstly, several previous studies have shown
that Notch signaling is pivotal for tumor angiogenesis (10,19,20).
Secondly, the regulatory effect of Notch signaling has been
reported to be associated with the suppression of p21Cip1 and
p27Kip1, two cyclin-dependent kinase inhibitory proteins of pivotal
importance in cell cycle control (12). This result is consistent with the
results of the present study that inhibition of the Notch pathway
leads to considerable inhibition of cell cycle progression.
Finally, according to our previous study, the phosphatidylinositide
3-kinases (PI3K)/protein kinase B (Akt) pathway is regulated by
Notch1 activation and elevated Notch1 signaling activity may exert
its growth-promoting effects via the PI3K/Akt pathway (21).
γ-secretase is a protease complex and is composed of
a catalytic subunit (presenilin-1 or −2) and accessory subunits
(presenilin enhancer 2, anterior pharynx-defective 1 and nicastrin)
(22). Since γ-secretase inhibitors
are able to prevent Notch receptor activation, the γ-secretase
complex may be a potential therapeutic target in a wide array of
carcinomas. Inhibition of Notch signaling by a γ-secretase
inhibitor, PF-03084014, resulted in suppression of tumor cell
proliferation and induction of apoptosis in T-ALL (23). In breast cancer, Rasul et al
showed that inhibition of γ-secretase activity in breast cancer
cell lines induced G2/M arrest and downregulated antiapoptotic
proteins leading to cell death (24).
Although, for clear cell RCC, several kinase
inhibitors, including sorafenib and sunitinib, show effects on the
progression-free survival rate for specific patients. However, the
side effects of kinase inhibitors must not be underestimated
(25). The efficacy of these drugs
is likely to be associated with their capacity to inhibit
hypoxia-inducible factor-mediated autocrine growth factor signaling
and proangiogenic effects. Notably, loss of von Hippel-Lindau is
associated with good prognosis in clear cell RCC (26,27).
The therapeutic effect of γ-secretase inhibition on clear cell RCC
tumor growth indicates that the inhibition of Notch signaling
presents at least a complementary therapeutic approach for
treatment of clear cell RCC. In the present study, inhibition of
clear cell RCC cells with DAPT led to a considerable decrease of
cell proliferation and increased apoptosis. The results support the
therapeutic effect of DAPT for clear cell RCC. Considering the
limitation of kinase inhibitors, a comprehensive evaluation of the
optimal administration regime of γ-secretase inhibitors is of
priority for clear cell RCC.
Deficiencies remain in the current study. Firstly,
γ-secretase inhibitor blocked the Notch pathway without specifying
the individual contributions of the respective receptors or
ligands. We supposed that targeting each receptor or ligand using
siRNA is necessary to elucidate their respective contribution to
proliferation. Secondly, since tumorigenesis is a complicated and
comprehensive pathway, the underlying detailed mechanism of this
difference also requires further study.
In conclusion, the current study indicated that
Notch signaling is important in the tumorigenesis of RCC. The
γ-secretase inhibitor (DAPT) has the potential of being a novel
therapeutic regimen towards RCC, although, further investigation is
required.
Acknowledgements
The current study was supported by a grant from the
Natural Science Foundation of Ningbo, China (2011A610054). The
authors would like to thank Le Xu for his contribution to the
figures and experimental design.
References
|
1
|
Zbar B, Klausner R and Linehan WM:
Studying cancer families to identify kidney cancer genes. Annu Rev
Med. 54:217–233. 2003.
|
|
2
|
Ljungberg B, Hanbury DC, Kuczyk MA, et al:
Renal cell carcinoma guideline. Eur Urol. 51:1502–1510. 2007.
|
|
3
|
Escudier B, Eisen T, Stadler WM, et al:
Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J
Med. 356:125–134. 2007.
|
|
4
|
Motzer RJ, Hutson TE, Tomczak P, et al:
Sunitinib versus interferon alfa in metastatic renal-cell
carcinoma. N Engl J Med. 356:115–124. 2007.
|
|
5
|
Kim WY and Kaelin WG: Role of VHL gene
mutation in human cancer. J Clin Oncol. 22:4991–5004. 2004.
|
|
6
|
Artavanis-Tsakonas S, Rand MD and Lake RJ:
Notch signaling: cell fate control and signal integration in
development. Science. 284:770–776. 1999.
|
|
7
|
Bettenhausen B, Hrabĕ de Angelis M, Simon
D, Guenet JL and Gossler A: Transient and restricted expression
during mouse embryogenesis of Dll1, a murine gene closely related
to Drosophila Delta. Development. 121:2407–2418. 1995.
|
|
8
|
Ellisen LW, Bird J, West DC, et al: TAN-1,
the human homolog of the Drosophila notch gene, is broken by
chromosomal translocations in T lymphoblastic neoplasms. Cell.
66:649–661. 1991.
|
|
9
|
Miele L, Golde T and Osborne B: Notch
signaling in cancer. Curr Mol Med. 6:905–918. 2006.
|
|
10
|
Qi R, An H, Yu Y, et al: Notch1 signaling
inhibits growth of human hepatocellular carcinoma through induction
of cell cycle arrest and apoptosis. Cancer Res. 63:8323–8329.
2003.
|
|
11
|
Shi W and Harris AL: Notch signaling in
breast cancer and tumor angiogenesis: cross-talk and therapeutic
potentials. J Mammary Gland Biol Neoplasia. 11:41–52. 2006.
|
|
12
|
Sjölund J, Johansson M, Manna S, et al:
Suppression of renal cell carcinoma growth by inhibition of Notch
signaling in vitro and in vivo. J Clin Invest. 118:217–228.
2008.
|
|
13
|
Ai Q, Ma X, Huang Q, et al: High-level
expression of Notch1 increased the risk of metastasis in T1 stage
clear cell renal cell carcinoma. PLoS One. 7:e350222012.
|
|
14
|
Sun S, Du R, Gao J, et al: Expression and
clinical significance of Notch receptors in human renal cell
carcinoma. Pathology. 41:335–341. 2009.
|
|
15
|
Wu K, Xu L, Zhang L, Lin Z and Hou J: High
Jagged1 expression predicts poor outcome in clear cell renal cell
carcinoma. Jpn J Clin Oncol. 41:411–416. 2010.
|
|
16
|
Massi D, Tarantini F, Franchi A, et al:
Evidence for differential expression of Notch receptors and their
ligands in melanocytic nevi and cutaneous malignant melanoma. Mod
Pathol. 19:246–254. 2006.
|
|
17
|
Weng AP and Aster JC: Multiple niches for
Notch in cancer: context is everything. Curr Opin Genet Dev.
14:48–54. 2004.
|
|
18
|
Weng AP, Ferrando AA, Lee W, et al:
Activating mutations of NOTCH1 in human T cell acute lymphoblastic
leukemia. Science. 306:269–271. 2004.
|
|
19
|
Ridgway J, Zhang G, Wu Y, et al:
Inhibition of Dll4 signalling inhibits tumour growth by
deregulating angiogenesis. Nature. 444:1083–1087. 2006.
|
|
20
|
Zeng Q, Li S, Chepeha DB, et al: Crosstalk
between tumor and endothelial cells promotes tumor angiogenesis by
MAPK activation of Notch signaling. Cancer Cell. 8:13–23. 2005.
|
|
21
|
Xu L, Zhu Y, Xu J, et al: Notch1
activation promotes renal cell carcinoma growth via PI3K/Akt
signaling. Cancer Sci. 103:1253–1258. 2012.
|
|
22
|
Lazarov VK, Fraering PC, Ye W, Wolfe MS,
Selkoe DJ and Li H: Electron microscopic structure of purified,
active gamma-secretase reveals an aqueous intramembrane chamber and
two pores. Proc Natl Acad Sci USA. 103:6889–6894. 2006.
|
|
23
|
Wei P, Walls M, Qiu M, et al: Evaluation
of selective gamma-secretase inhibitor PF-03084014 for its
antitumor efficacy and gastrointestinal safety to guide optimal
clinical trial design. Mol Cancer Ther. 9:1618–1628. 2010.
|
|
24
|
Rasul S, Balasubramanian R, Filipović A,
Slade MJ, Yagüe E and Coombes RC: Inhibition of gamma-secretase
induces G2/M arrest and triggers apoptosis in breast cancer cells.
Br J Cancer. 100:1879–1888. 2009.
|
|
25
|
Park SJ, Lee JL, Park I, et al:
Comparative efficacy of sunitinib versus sorafenib as first-line
treatment for patients with metastatic renal cell carcinoma.
Chemotherapy. 58:468–474. 2012.
|
|
26
|
Banks RE, Tirukonda P, Taylor C, et al:
Genetic and epigenetic analysis of von Hippel-Lindau (VHL) gene
alterations and relationship with clinical variables in sporadic
renal cancer. Cancer Res. 66:2000–2011. 2006.
|
|
27
|
Yao M, Yoshida M, Kishida T, et al: VHL
tumor suppressor gene alterations associated with good prognosis in
sporadic clear-cell renal carcinoma. J Natl Cancer Inst.
94:1569–1575. 2002.
|