Introduction
Ovarian cancer is the most lethal of all the
gynecological malignancies. Initial treatment is associated with a
70% response rate, but the majority of patients eventually relapse
due to chemoresistance (1). The
identification of novel molecular markers that target
chemoresistant disease would, therefore, assist in improving the
outcome of ovarian cancer therapy.
Cancer stem cells (CSCs) are known to represent a
small sub-population of highly malignant cells (2). These cells have an increased
resistance to apoptosis and DNA damage, and are therefore more
likely to be resistant to chemotherapy and be associated with
relapse (3). A previous study in
acute myelogenous leukemia has provided compelling evidence for the
existence of CSCs (4). Other
studies have characterized CSCs in other types of tumors, including
brain, breast, colon, pancreas, prostate and ovarian cancer
(5–8).
Self-renewal and lineage capacity are hallmarks of
all stem cells (5). Various methods
have been developed to capitalize on these characteristics in order
to obtain cancer stem cells. Evaluating the capacity of cancer
cells to grow as multi-cellular spheroids in stem-condition culture
systems is one such method (6).
Using this method, ovarian CSCs (OCSCs) have been obtained from
patients with ascites (7). The most
common use of cell surface markers to identify OCSCs in ovarian
cancer involves the use of cluster of differentiation
(CD)133+ cell populations (8).
Forkhead box M1 (FOXM1) regulates transcriptional
genes that are necessary for cell cycle progression and cell
survival (9,10). The FOXM1 transcription factor is
upregulated in the majority of human cancers, indicating that it
may participate in the initiation of human carcinogenesis (11). A previous study has shown that FOXM1
expands the pool of human epithelial stem/progenitor cells
(11). It has also been reported
that the overexpression of FOXM1 leads to epithelial-mesenchymal
transition (EMT) and a CSC phenotype in pancreatic cancer cells
(12). FOXM1 may therefore be a
novel target for therapeutic agents that target CSCs (11,12).
Genistein (4′,5,7-trihydroxyisoflavone; GEN) has a
similar chemical structure to that of estrogen, but more extensive
biological activities (13,14). GEN has been shown to inhibit
tyrosine kinase and reduce cancer cell proliferation in vivo
and in vitro without causing toxicity to non-cancerous cells
(15). A study by Wang et al
has previously shown that FOXM1 activation is inhibited by GEN in
pancreatic cancer cells, resulting in apoptotic cell death
(16). The low absorption of GEN in
the intestine and its rapid metabolic elimination resulting from
the hydroxyl groups at the C-5, C-7 and C-4′ positions allows GEN
to bind to glucuronic and sulfuric acid. This reduces its
bioavailability and bioactivity in vivo and restricts its
clinical usefulness (17). Studies
performed at the Laboratory of Medicine Engineering, Medical
College, Hunan Normal University (Changsha, China) have
demonstrated that a novel synthetic GEN analogue,
7-difluoromethoxyl-5,4′-di-n-octylgenistein (DFOG), induces cell
apoptotic death in ovarian and gastric cancer cells by inactivating
FOXM1 (17,18). GEN also has the potential to
attenuate FOXM1-mediated cell growth, migration and invasion, the
acquisition of an EMT phenotype, and CSC self-renewal capacity in
pancreatic cancer cells (12). The
present study investigated whether DFOG attenuates the
characteristics of OCSCs by inactivating FOXM1.
Materials and methods
Cell lines and sphere culture
Human ovarian cell lines, SKOV3 and A2780, were
obtained from the Cell Bank of the Chinese Academy of Sciences
(Shanghai, China). The cells were maintained as a monolayer in high
glucose Dulbecco’s modified Eagle’s medium (DMEM) supplemented with
10% fetal bovine serum, 100 IU/ml penicillin G and 100 μg/ml
streptomycin (Life Technologies, Shanghai, China) at 37°C in a
humidified 5% CO2 incubator.
For the sphere-forming culture, the cells were
collected and washed to remove the serum, prior to being suspended
in serum-free DMEM/F12 supplemented with 100 IU/ml penicillin, 100
μg/ml streptomycin, 20 ng/ml human recombinant epidermal growth
factor, 10 ng/ml human recombinant basic fibroblast growth factor,
2% B27 supplement without vitamin A and 1% N2 supplement
(Invitrogen, Carlsbad, CA, USA). The cells were subsequently
cultured in ultra-low attachment 6-well plates (Corning Inc.,
Corning, NY, USA) at a density of ≤2,000 cells/well.
Sphere passage and sphere formation
assay
The spheres were collected by gentle centrifugation
(500 × g for 5 min), dissociated with trypsin-EDTA and mechanically
disrupted with a pipette. The resulting single cells were
centrifuged (500 × g for 5 min) to remove the enzyme, and
re-suspended in serum-free medium where they were allowed to
re-form spheres. The spheres were passaged every 8 days until they
reached a diameter of 100 μm. Dissociated single sphere-forming
cells (SFCs) were diluted to a density of 500 cells/ml. The diluted
cell suspension was plated onto ultra-low attachment 96-well plates
at 2 μl/well (Corning Inc.). Serum-free medium (150 μl) was then
added. Wells with only one cell were marked and observed every
day.
In vivo tumorigenicity experiments
Four-week-old BALB/c-nu male mice (Shanghai
Laboratory Animal Center, Chinese Academy of Sciences, Shanghai,
China) were housed and maintained in accordance with the
Institutional Guidelines of Hunan Normal University (Changsha,
Hunan, China). The study was approved by the ethics committee of
Hunan Normal University. The SKOV3 parental cells and the third
passages of the SFCs were used in the tumorigenicity experiments.
Trypan blue staining was used to assess cell viability. Various
numbers of viable single cells in serum-free DMEM/Matrigel (1:1; BD
Biosciences, Shanghai, China) were subcutaneously injected into the
mice using a 100-μl microsyringe [Sangon Biotech (Shanghai) Co.,
Ltd., Shanghai, China]. The mice were humanely sacrificed 8 weeks
after the injection, and the tumors were harvested for further
examination.
MTT assay
The SFCs from the SKOV3 cell line and the parental
cells were seeded in 96-well plates (precoated with Matrigel) at a
density of 5,000 cells per well. The cells were exposed to
increasing concentrations of DFOG. After 48 h, MTT reagent
(Sigma-Aldrich, St. Louis, MO, USA) was added to each well
according to the manufacturer’s instructions. Absorbance was
measured at 570 nm.
Plasmids and transfection
The FOXM1 cDNA plasmid was purchased from OriGene
Technologies Inc. (Rockville, MD, USA). The SFCs derived from the
SKOV3 cell line were transfected with cDNA using Lipofectamine 2000
(Life Technologies), as previously described (17).
Western blot analysis
Western blot analysis was carried out, as previously
described (18). Monoclonal mouse
anti-FOXM1, monoclonal mouse anti-CD133 and monoclonal mouse
anti-CD44 (Cell Signaling Technology, Inc., Danvers, MA, USA), as
well as monoclonal mouse anti-aldehyde dehydrogenase 1 (ALDH1) and
monoclonal mouse anti-β-actin (Santa Cruz Biotechnology, Inc.,
Santa Cruz, CA, USA) antibodies were used as primary antibodies.
The cells were lysed by being incubated in lysis buffer for 20 min
at 4°C. The protein concentration was determined using the Bio-Rad
assay system (Bio-Rad, Hercules, CA, USA). Total proteins were
fractionated using SDS-PAGE and transferred onto a polyvinylidene
fluoride membrane (Millipore, Billerica, MA, USA). Signals were
detected using an Enhanced Chemiluminescence Advance western blot
analysis system (Amersham Pharmacia Biotech Inc., Piscataway, NJ,
USA).
Statistical analysis
Statistical analysis and database management was
undertaken using SPSS version 15.0 software (SPSS, Inc., Chicago,
IL, USA). Data are represented as the mean ± standard deviation.
Multiple group comparisons were made using one-way analysis of
variance, and pairwise comparisons were performed using the least
squares difference method. A two-tailed t-test was used when
appropriate. Values of P<0.05 were considered to indicate a
statistically significant difference.
Results
Characteristics of ovarian cancer
stem-like cells (OCSLCs) in the SKOV3 cell line
Ovarian cells were plated in stem cell-conditioned
culture medium in 6-well plates at a density of 2,000 cells/well,
which allowed the formation of discrete colonies. Under these
conditions, the cells grew as non-adherent, three-dimensional
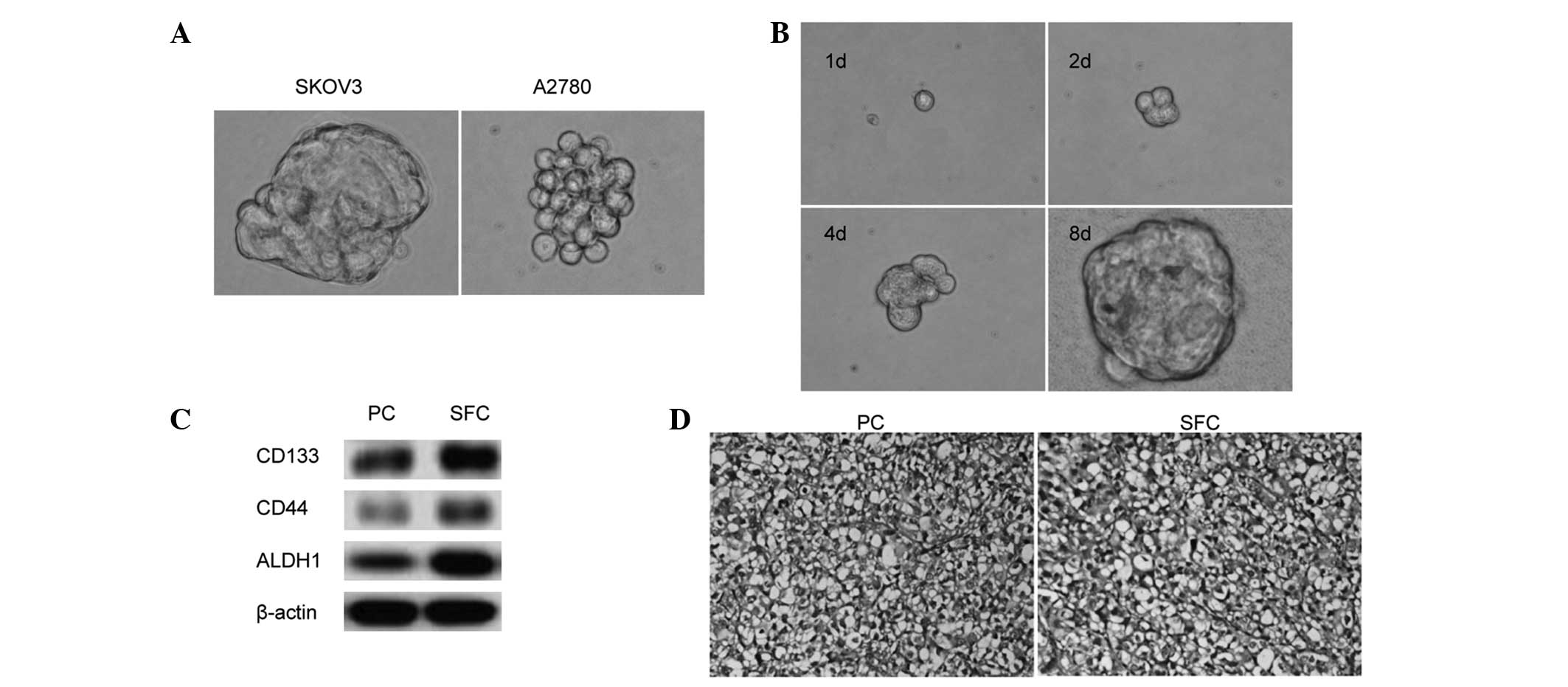
sphere clusters. Fig. 1A shows the
anchorage-independent spheres that formed in the SKOV3 and A2780
cells. The spheres were passaged after 8 days, when they had
reached ~50 μm in diameter. The SKOV3 and A2780 spheres were
serially passaged for >12 generations, indicating their
self-renewal capability in vitro. To corroborate the finding
that a sphere could be generated from a single cell, single SKOV3
cells were plated on a 96-well plate and the wells with one cell
were visualized everyday. Fig. 1B
shows the process by which a single SKOV3 cell formed a sphere.
Next, the ovarian CSC markers, CD133, CD44 and
ALDH1, were evaluated using western blot analysis; the results
showed enrichment of the CD133+, CD44+ and
ALDH-high populations in the SFCs derived from the SKOV3 cells
compared with the parental cells (Fig.
1C).
To confirm that the SFCs from the SKOV3 cells
exhibited enhanced tumor-initiating capability, BALB/c-nu mice were
transplanted with varying numbers of SKOV3 SFCs. SKOV3 parental
cells were used as controls. The results indicated that as few as
1,000 SFCs were sufficient for tumor development, whereas, at least
2×105 parental cells were necessary to consistently
generate a tumor in the same model over a longer period of time
(Table I). The tumor nodules formed
by the SFCs of the SKOV3 cell line displayed similar histology to
that observed with the parental cells (Fig. 1D). These findings indicated that the
tumorigenic efficacy of the SFCs was higher than that of the
parental cells, and that non-adherent tumor spheres from the
ovarian cancer SKOV3 cell line cultured in stem cell-conditioned
medium possess OCSLC properties.
 | Table ITumorigenicity experiments with the
SFCs derived from the SKOV3 cell line and the parental cells in
BALB/c-nu mice. |
Table I
Tumorigenicity experiments with the
SFCs derived from the SKOV3 cell line and the parental cells in
BALB/c-nu mice.
| Cell type | Cell numbers | Incidence, n | Latency, days |
|---|
| Parental cells | 5×104 | 0/4 | - |
| 1×105 | 0/4 | - |
| 2×105 | 3/4 | 35 |
| 5×105 | 4/4 | 29 |
| 1×106 | 4/4 | 12 |
| CD133+
cells | 5×102 | 0/4 | - |
| 1×103 | 4/4 | 25 |
| 5×103 | 4/4 | 13 |
| 1×104 | 4/4 | 9 |
| 5×104 | 4/4 | 6 |
DFOG inhibits the proliferation and
self-renewal of OCSLCs derived from the SKOV3 cell line
It has been reported that CSCs have the
characteristics of extensive proliferation, and GEN has been shown
to inhibit the proliferative activity of pancreatic cancer stem
cells (11). In the present study,
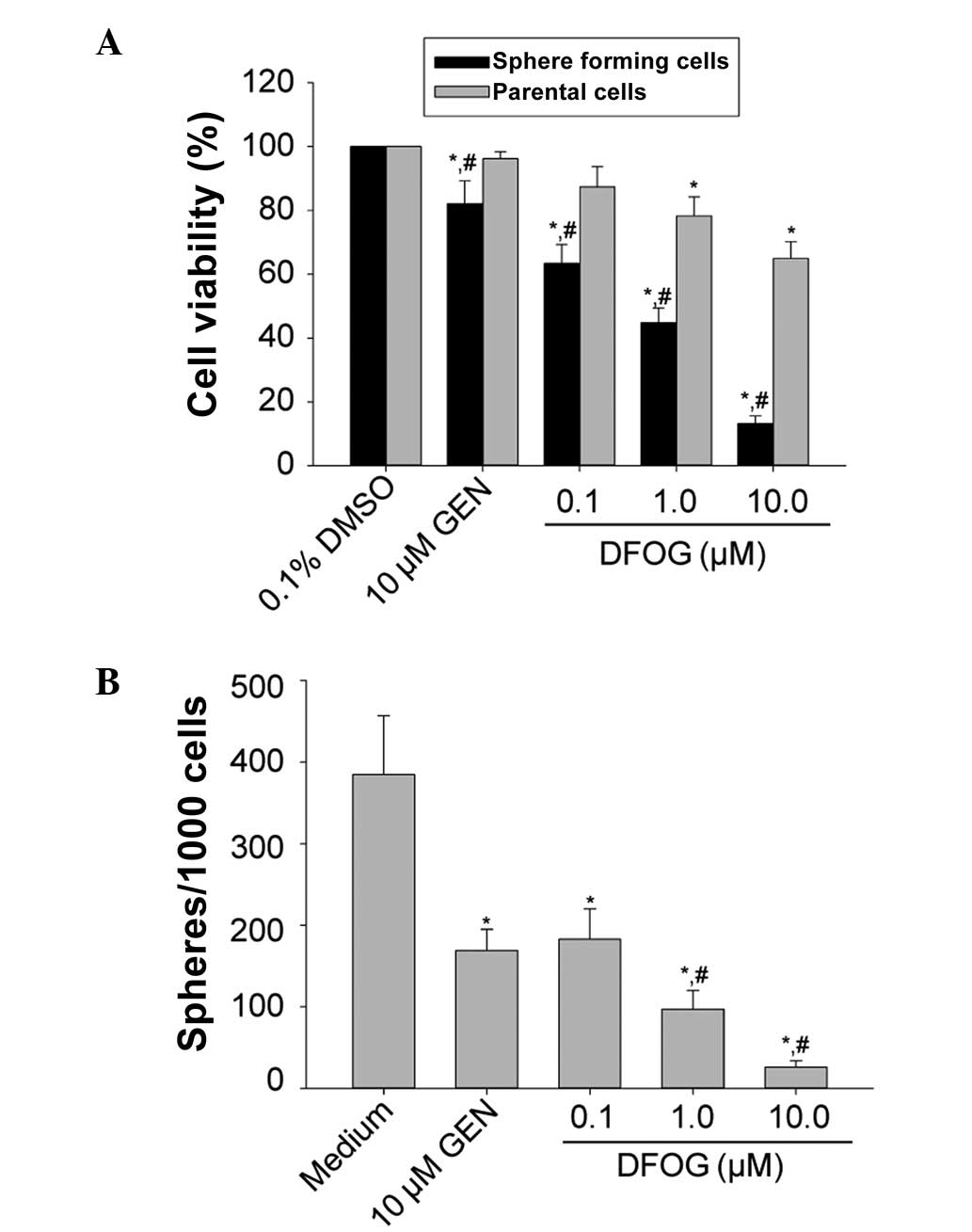
the MTT results showed that DFOG (0.1, 1.0 and 10.0 μmol/l) and GEN
(10.0 μmol/l) preferentially inhibited the proliferation of the
SFCs derived from the SKOV3 cells (P=0.030; Fig. 2A), indicating that DFOG is able to
preferentially suppress the proliferative ability of OCSLCs.
DFOG (0.1, 1.0 and 10.0 μmol/l) reduced the number
of spheroids formed in the SFCs from the SKOV3 cells in a
concentration-dependent manner (P=0.022; Fig. 2B). These results indicate that DFOG
can suppress the self-renewal of OCSLCs.
DFOG downregulates the expression of
FOXM1 and CSC markers in OCSLCs derived from the SKOV3 cell
line
We previously demonstrated the molecular role of
FOXM1 in mediating the biological effects of GEN and DFOG in human
ovarian cancer cell lines, including the SKOV3 cell line (17). A previous study reported that the
overexpression of FOXM1 leads to EMT and the formation of a cancer
stem cell phenotype in pancreatic cancer cells (12). Based on these findings, the present
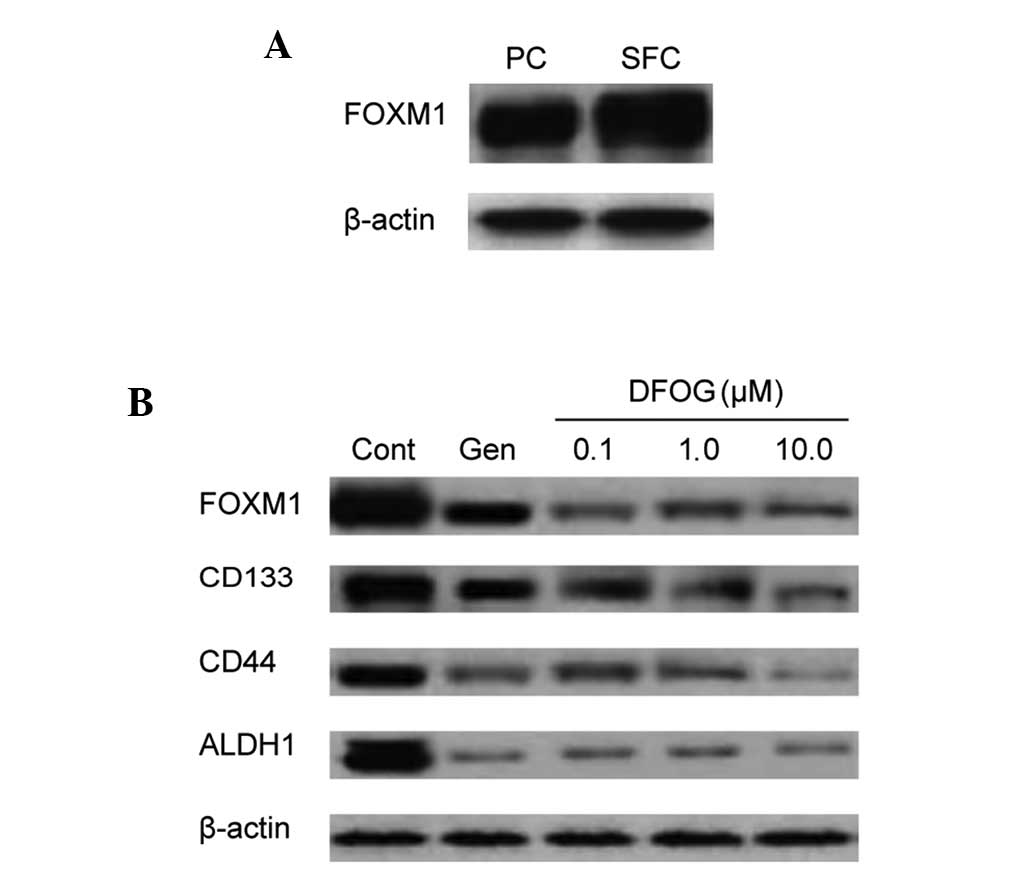
study next sought to compare the status of FOXM1 protein expression
in the parental cells and SFCs. The results showed that FOXM1
expression was higher in the SFCs compared with the parental cells
(Fig. 3A). In addition, FOXM1
expression in the SFCs was downregulated by DFOG (Fig. 3B).
In pancreatic cancer stem cells, the overexpression
of FOXM1 has been shown to result in increased sphere-forming
capacity and the increased expression of the CSC surface marker,
CD44 (12). In the present study,
DFOG inhibited the protein expression of CD133, CD44 and ALDH1 in
the SFCs (Fig. 3B).
Overexpression of FOXM1 attenuates the
inhibitory effects of DFOG on the expression of CSC markers and the
self-renewal in OCSLCs derived from the SKOV3 cell line
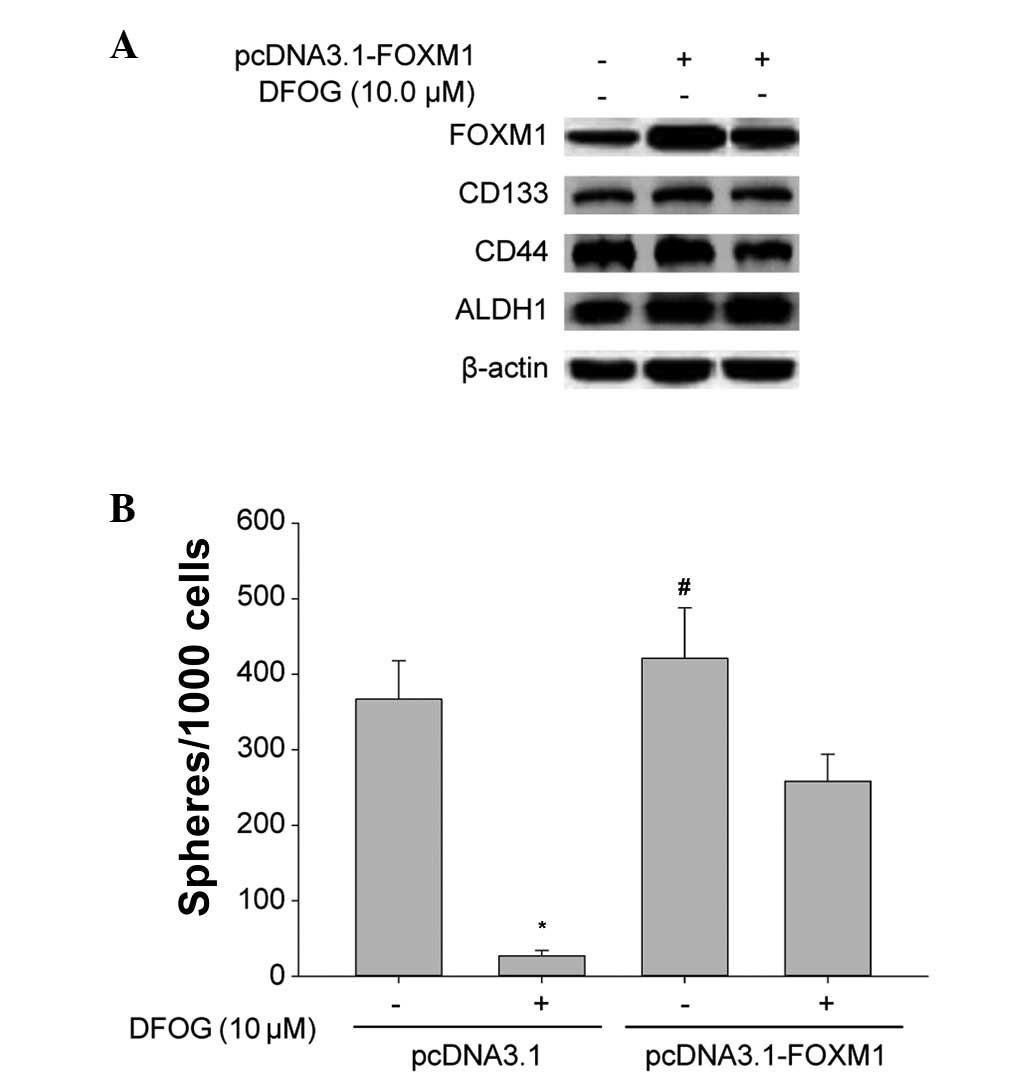
Western blot analysis showed that the upregulation
of FOXM1 by pcDNA3.1-FOXM1 transfection resulted in the
overexpression of FOXM1, CD133, CD44 and ALDH1 proteins in the SFCs
from the SKOV3 cells (Fig. 4A). The
overexpression of FOXM1 reversed the DFOG-induced downregulation of
FOXM1, CD133, CD44 and ALDH1 protein expression levels (Fig. 4A), and reduced the inhibition of
self-renewal to a certain extent (Fig.
4B). These results provide mechanistic evidence, indicating
that DFOG-inhibited self-renewal is in part due to the inactivation
of FOXM1 in OCSLCs derived from SKOV3 cells.
Discussion
In 1996, undifferentiated multipotent neural cells
were first grown and maintained in suspension using a neurosphere
assay (19). Following this
discovery, anchorage-independent sphere cultures of stem cells then
became instrumental in the study of stem cells, including nerve,
prostate and mammary stem cells (20). More recently, the functional
approach of using sphere formation has been utilized for enriching
potential CSC subpopulations when specific CSC markers have not
been defined (21). In the present
study, it was confirmed that SFCs derived from the SKOV3 cell line
possess self-renewal capacity in vitro and have greater
tumor-initiating capability in vivo than the parent cells.
It was also found that CD133+, CD44+ and
ALDH-high populations were enriched in tumor spheroids from the
SKOV3 cells. These findings indicate that SFCs from SKOV3 cells
exhibit the characteristics of OCSCs and are therefore OCSLCs.
CSCs have been identified in many malignant tumor
tissues, including ovarian cancer tissues (22–24).
CSCs are believed to play critical roles in drug resistance and
cancer metastasis, indicating that targeting CSC self-renewal
capacity would eliminate the essential cause of tumor recurrence
(25). Previous studies at the
Laboratory of Medicine Engineering, Medical College, Hunan Normal
University have demonstrated that DFOG, a novel synthetic GEN
analogue, induces cell apoptotic death in ovarian and gastric
cancer cells (17,18). Significantly, the present study
shows for the first time that DFOG significantly inhibits the
proliferative activity and self-renewal capability of OCSLCs
derived from SKOV3 cells.
Numerous studies have demonstrated that GEN inhibits
the growth of breast, prostate and pancreatic cancer cells in
vitro and in vivo (17,26).
Genes that are critical for the control of cell proliferation,
apoptosis, the cell cycle, oncogenesis, transcription regulation
and cell signal transduction pathways have been found to be
differentially regulated by GEN. These findings are consistent with
the apoptosis-inducing effects of GEN being mediated through
inactivation of nuclear factor-κB and Akt signaling pathways
(26). In our previous studies, it
was shown that DFOG-induced cell apoptotic death was mediated by
the inactivation of FOXM1 in ovarian and gastric cancer cells
(17,18). The present study showed for the
first time that the forced overexpression of FOXM1 led to an
increased self-renewal capacity of SFCs derived from SKOV3 cells.
This was consistent with the increased expression of the CSC cell
surface markers, CD133, CD44 and ALDH1, which could be attenuated
by exposure to DOFG.
In summary, the present results demonstrated that
the forced overexpression of FOXM1 leads to an increased CSC
self-renewal capacity, which is inhibited by DFOG and GEN.
Therefore, DFOG and GEN may represent a useful way to inhibit CSC
activity, making them potentially significant agents for the
prevention of recurrence and/or the treatment of human ovarian
cancer.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant no. 81301894), Guangdong
Province Science and Technique Department Item (grant no.
2012B031800271) of China and the Guangzhou Science and Information
Bureau Item (grant no. 201300000151) of China.
References
|
1
|
Jelovac D and Armstrong DK: Role of
farletuzumab in epithelial ovarian carcinoma. Curr Pharm Des.
18:3812–3815. 2012.
|
|
2
|
Wang Y, Yu Y, Tsuyada A, Ren X, Wu X,
Stubblefield K, Rankin-Gee EK and Wang SE: Transforming growth
factor-β regulates the sphere-initiating stem cell-like feature in
breast cancer through miRNA-181 and ATM. Oncogene. 30:1470–1480.
2011.
|
|
3
|
Bhola NE, Balko JM, Dugger TC, Kuba MG,
Sanchez V, Sanders M, Stanford J, Cook RS and Arteaga CL: TGF-β
inhibition enhances chemotherapy action against triple-negative
breast cancer. J Clin Invest. 123:1348–1358. 2013.
|
|
4
|
Somervaille TC, Matheny CJ, Spencer GJ,
Iwasaki M, Rinn JL, Witten DM, Chang HY, Shurtleff SA, Downing JR
and Cleary ML: Hierarchical maintenance of MLL myeloid leukemia
stem cells employs a transcriptional program shared with embryonic
rather than adult stem cells. Cell Stem Cell. 4:129–140. 2009.
|
|
5
|
Achilleos A and Trainor PA: Neural crest
stem cells: discovery, properties and potential for therapy. Cell
Res. 22:288–304. 2012.
|
|
6
|
Huang FT, Zhuan-Sun YX, Zhuang YY, Wei SL,
Tang J, Chen WB and Zhang SN: Inhibition of hedgehog signaling
depresses self-renewal of pancreatic cancer stem cells and reverses
chemoresistance. Int J Oncol. 41:1707–1714. 2012.
|
|
7
|
Latifi A, Luwor RB, Bilandzic M,
Nazaretian S, Stenvers K, Pyman J, Zhu H, Thompson EW, Quinn MA,
Findlay JK and Ahmed N: Isolation and characterization of tumor
cells from the ascites of ovarian cancer patients: molecular
phenotype of chemoresistant ovarian tumors. PLoS One.
7:e468582012.
|
|
8
|
Ponnusamy MP, Seshacharyulu P, Vaz A, Dey
P and Batra SK: MUC4 stabilizes HER2 expression and maintains the
cancer stem cell population in ovarian cancer cells. J Ovarian Res.
4:72011.
|
|
9
|
Myatt SS and Lam EW: The emerging roles of
forkhead box (Fox) proteins in cancer. Nat Rev Cancer. 7:847–859.
2007.
|
|
10
|
Wierstra I and Alves J: FOXM1, a typical
proliferation-associated transcription factor. Biol Chem.
388:1257–1274. 2007.
|
|
11
|
Gemenetzidis E, Elena-Costea D, Parkinson
EK, Waseem A, Wan H and Teh MT: Induction of human epithelial
stem/progenitor expansion by FOXM1. Cancer Res. 70:9515–9526.
2010.
|
|
12
|
Bao B, Wang Z, Ali S, Kong D, Banerjee S,
Ahmad A, Li Y, Azmi AS, Miele L and Sarkar FH: Over-expression of
FoxM1 leads to epithelial-mesenchymal transition and cancer stem
cell phenotype in pancreatic cancer cells. J Cell Biochem.
112:2296–2306. 2011.
|
|
13
|
Akiyama T, Ishida J, Nakagawa S, Ogawara
H, Watanabe S, Itoh N, Shibuya M and Fukami Y: Genistein, a
specific inhibitor of tyrosine-specific protein kinases. J Biol
Chem. 262:5592–5595. 1987.
|
|
14
|
Hillman GG, Wang Y, Kucuk O, Che M, Doerge
DR, Yudelev M, Joiner MC, Marples B, Forman JD and Sarkar FH:
Genistein potentiates inhibition of tumor growth by radiation in a
prostate cancer orthotopic model. Mol Cancer Ther. 3:1271–1279.
2004.
|
|
15
|
Barnes S: Effect of genistein on in vitro
and in vivo models of cancer. J Nutr. 125(3 Suppl): 777S–783S.
1995.
|
|
16
|
Wang Z, Ahmad A, Banerjee S, Azmi A, Kong
D, Li Y and Sarkar FH: FoxM1 is a novel target of a natural agent
in pancreatic cancer. Pharm Res. 27:1159–1168. 2010.
|
|
17
|
Ning Y, Li Q, Xiang H, Liu F and Cao J:
Apoptosis induced by 7-difluoromethoxyl-5,4′-di-n-octyl genistein
via the inactivation of FoxM1 in ovarian cancer cells. Oncol Rep.
27:1857–1864. 2012.
|
|
18
|
Xiang HL, Liu F, Quan MF, Cao JG and Lv Y:
7-difluoromethoxyl-5,4′-di-n-octylgenistein inhibits growth of
gastric cancer cells through downregulating forkhead box M1. World
J Gastroenterol. 18:4618–4626. 2012.
|
|
19
|
Reynolds BA and Weiss S: Clonal and
population analyses demonstrate that an EGF-responsive mammalian
embryonic CNS precursor is a stem cell. Dev Biol. 175:1–13.
1996.
|
|
20
|
Shi X, Gipp J and Bushman W:
Anchorage-independent culture maintains prostate stem cells. Dev
Biol. 312:396–406. 2007.
|
|
21
|
Zhang S, Balch C, Chan MW, Lai HC, Matei
D, Schilder JM, Yan PS, Huang TH and Nephew KP: Identification and
characterization of ovarian cancer-initiating cells from primary
human tumors. Cancer Res. 68:4311–4320. 2008.
|
|
22
|
Van den Broeck A, Gremeaux L, Topal B and
Vankelecom H: Human pancreatic adenocarcinoma contains a side
population resistant to gemcitabine. BMC Cancer. 12:3542012.
|
|
23
|
Penumatsa K, Edassery SL, Barua A,
Bradaric MJ and Luborsky JL: Differential expression of aldehyde
dehydrogenase 1a1 (ALDH1) in normal ovary and serous ovarian
tumors. J Ovarian Res. 3:282010.
|
|
24
|
Creighton CJ, Chang JC and Rosen JM:
Epithelial-mesenchymal transition (EMT) in tumor-initiating cells
and its clinical implications in breast cancer. J Mammary Gland
Biol Neoplasia. 15:253–260. 2010.
|
|
25
|
Wang Z, Ahmad A, Li Y, Banerjee S, Kong D
and Sarkar FH: Forkhead box M1 transcription factor: a novel target
for cancer therapy. Cancer Treat Rev. 36:151–156. 2010.
|
|
26
|
Wang Z, Li Y, Ahmad A, Banerjee S, Azmi
AS, Kong D, Wojewoda C, Miele L and Sarkar FH: Down-regulation of
Notch-1 is associated with Akt and FoxM1 in inducing cell growth
inhibition and apoptosis in prostate cancer cells. J Cell Biochem.
112:78–88. 2011.
|