Introduction
Malignant transformation of fibrous dysplasia (FD)
is rare (1–4). It can occur in monostotic and
polyostotic FD, with a frequency of <1% among all FD (2). The most common type of malignant tumor
arising from FD is osteosarcoma (~70%), followed by fibrosarcoma
(~20%), and chondrosarcoma (~10%), with malignant fibrous
histiocytoma (~4%) occurring less commonly (2).
Activating missense mutations in the guanine
nucleotide-binding protein α-subunit (GNAS) gene, which
encodes the stimulatory α subunit of the G-protein
(Gsα), resulting in a change at the Arg 201 codon from
arginine to cysteine (Arg-to-Cys, R201C) or arginine to histidine
(Arg-to-His, R201H) have been identified in both the monostotic and
polyostotic forms of FD, as well as in McCune-Albright syndrome
(5–7). These mutations are central to the
pathogenesis of FD; however, it remains unknown whether the
Gsα mutations are retained following malignant
transformation of FD. In addition, to the best of our knowledge, no
studies have been performed on chromosomal alterations that occur
in FD with malignant transformation. The present study reveals the
chromosomal analysis, as well as the status of the Gsα
mutations, of a patient with an osteosarcoma arising in polyostotic
FD. Patient provided written informed consent.
Case report
Case summary
A 72-year-old male presented to the Ojiya General
Hospital (Ojiya, Japan) with right knee pain of 3 months in
duration, with no history of previous trauma. This patient had a
history of colon cancer that had been resected 2 years prior to
presentation. There was no recurrence or metastasis. Initial workup
roentgenograms, which were performed at the Nagaoka Red Cross
Hospital (Nagaoka, Japan) showed a ground glass appearance and a
well-defined lucency with sclerotic margins in the right femur and
tibia. Although these features are consistent with polyostotic FD,
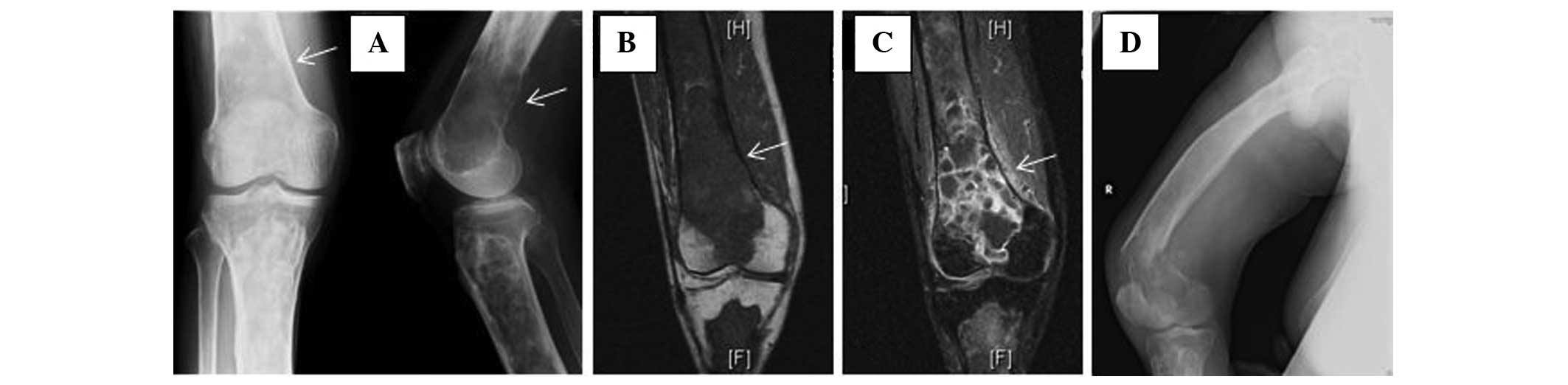
an ill-defined osteolytic lesion, 4×3 cm in size, was superimposed
on the changes of FD in the inferior part of the femur (Fig. 1A). Magnetic resonance imaging (MRI)
showed the extraosseous extent of the tumor from the lesion in the
distal part of the femur (Fig. 1B).
The lesion was intensively enhanced by injection of gadopentetate
dimeglumine (Fig. 1C), which is
suggestive of malignant transformation of FD. The patient did not
show cutaneous pigmentation, endocrine disturbances, or soft tissue
lesions, as can be seen in McCune-Albright and Mazabraud’s
syndromes. No other members of the family had a history of bone
tumor. Blood chemistry data showed that the alkaline phosphatase
and C-reactive protein levels were elevated to 960 IU/l (normal
level, 120–325 IU/l) and 7.39 mg/dl (normal level, <0.3 mg/dl),
respectively. Other values, including those of serum calcium (9.4
mg/dl; normal level, 8.7–11.0 mg/dl), phosphorus (4.2 mg/dl; normal
level, 2.6–4.4mg/dl), aspartate aminotransferase (24 U/l; normal
level, 12–34 U/l), alanine aminotransferase (12 U/l; normal level,
7–36 U/l) and total bilirubin (0.7 mg/dl; normal level, 0.2–1.2
mg/dl), were within acceptable limits. Open biopsy revealed an
osteosarcoma with an adjacent area of FD. The patient was referred
to the Niigata Cancer Center Hospital (Niigata, Japan) for further
treatment of this lesion. Since a pathological fracture through the
lesion of the distal femur (see Fig.
1D) had become evident after admission to our hospital, the
patient underwent thigh amputation.
Pathological analysis
Gross specimen analysis showed that in the distal
femur, the tumor had destroyed part of the cortex and had extended
to the surrounding soft tissue. Microscopic examination of this
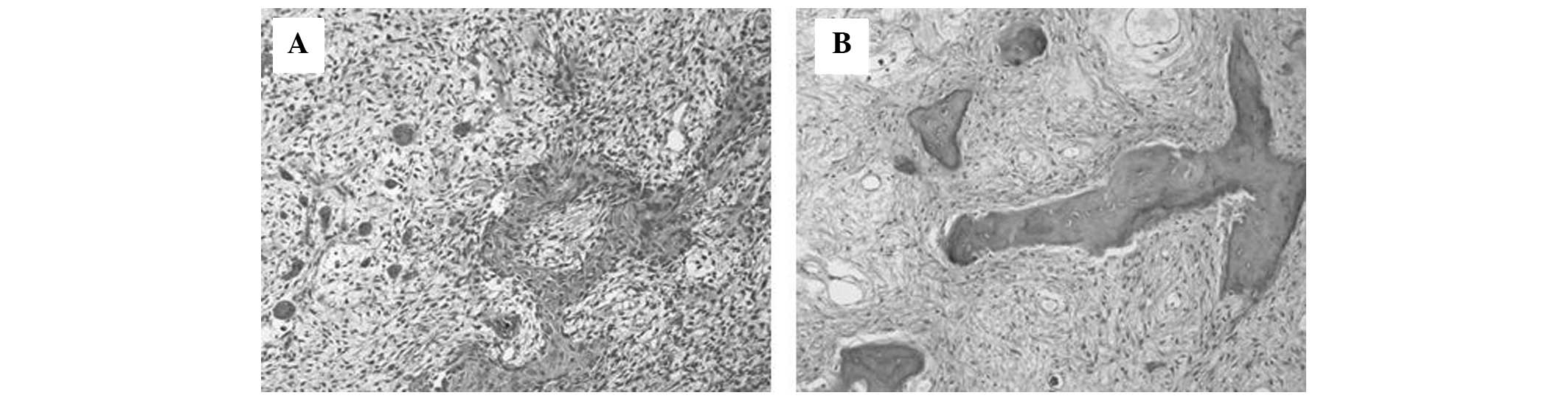
area showed highly pleomorphic, spindle-shaped tumor cells,
producing various forms of osteoid (Fig. 2A). The tumor was densely cellular
with a high mitotic rate, including atypical figures. The
histological features of this area confirmed the diagnosis of
osteosarcoma. The adjacent intramedullary part of the tumor in the
femur and the tibia showed a solid yellow-white appearance.
Microscopic examination of these lesions showed features consistent
with FD; small trabeculae of woven bone of various sizes and
shapes, scattered within a fibrous tissue without evidence of
osteoblastic activity (Fig. 2B).
Thus, the case was diagnosed as secondary osteosarcoma arising in
pre-existing FD.
Chromosomal analysis and reverse
transcription-polymerase chain reaction (RT-PCR)
Chromosomal analysis by G-banded karyotyping showed
44,X,-Y, add(4)(p11), add(5)(p15), der(11)add(11)(p15)t(1;11)(q21;q23),add(12)(q11), -13, der(22)t(12;22)(q11;p12) in
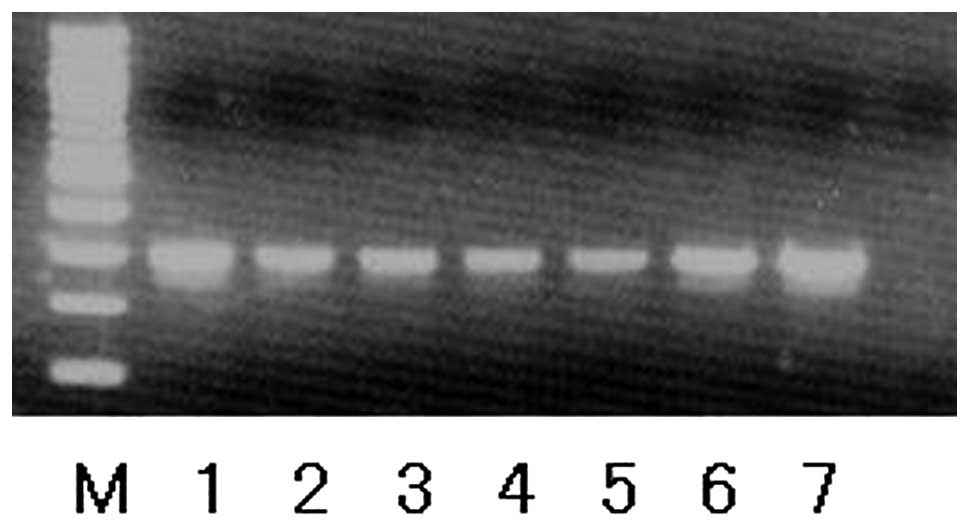
nine out of 10 metaphases from the osteosarcoma lesion. RT-PCR
analysis for Gsα mutations, which was performed as
previously described (8),
demonstrated the presence of a Gsα mutation at the Arg
201 codon in both the primary tumor cells and secondary
osteosarcoma cells (Fig. 3).
Follow-up
In terms of systemic treatment, adjuvant
chemotherapy was not administrated to the patient due to advanced
age. At 4 years of clinical follow-up, the patient was well without
local recurrence or metastatic disease.
Discussion
FD is a common benign fibro-osseous lesion; it
occurs in 5–7% of all benign bone tumors (9,10). The
etiology of FD is linked to activating missense mutations in the
GNAS gene, which encodes Gsα and is located at
20q13 (5). Gsα mutations
have been found in tumors from both the monostotic and polyostotic
form of FD, as well as in the McCune-Albright syndrome, a disorder
that combines polyostotic FD, skin pigmentation and one or several
endocrinopathies (9,10). Among fibro-osseous lesions of bone,
Gsα mutations are specific to FD (6–8).
Malignant transformation of FD is very rare
(1–4). Thus, the status of Gsα
mutations in osteosarcoma arising from FD has not been reported in
the English literature. In the current case, the same
Gsα mutation was detected in both the region of FD and
the region of malignant transformation. In addition, chromosomal
analysis of the osteosarcoma cells did not show any alterations in
chromosome 20, which harbors the GNAS gene. From the current
study, it is not clear whether the Gsα mutation itself
was directly responsible for the pathogenesis of the malignant
transformation of FD. However, the fact that the Gsα mutation did
not change through the process of malignant transformation leads us
to believe that this mutation has the potential to at least be a
clinical marker for distinguishing de novo osteosarcoma
(primary osteosarcoma) from secondary osteosarcoma arising from
FD.
Tumorigenesis in osteosarcoma may involve a complex
interplay of chromosomal alternations, with loss of tumor
suppressor genes, altered expression of oncogenes and increased
levels of certain growth factors (11–13).
Although no characteristic chromosome translocations have been
identified in osteosarcomas, several chromosomal regions appear to
be altered non-randomly (14,15).
Bridge et al examined 111 chromosomally abnormal
osteosarcoma specimens and found that chromosomal regions 1p11–13,
1q10–12, 1q21–22, 11p15, 12p13, 17p12–13, 19q13 and 22q11–13 were
most frequently rearranged, and that the most common numerical
abnormalities were +1, −9, −10, −13, and −17 (14). Among these, the most thoroughly
investigated deletion hotspots are those at 13q14 and 17p13, which
correspond with the RB1 and TP53 tumor suppressor genes,
respectively. Mutations in TP53 have been shown to result in
impaired DNA repair mechanisms and disrupted antiangiogenesis
activity (12,15). The RB1 gene is critical to
cell-cycle control, and inherited mutations in the RB1 gene cause
retinoblastoma syndrome, a condition that predisposes a patient to
multiple malignancies (12,15). Mutations or dysfunction in both the
TP53 and RB1 genes have also been shown to be involved in
osteosarcoma pathogenesis (11,12,15).
Of the abovementioned common chromosomal
alterations, loss of chromosome 13 and rearranged chromosomal
regions 1q21–22 and 11p15 were found in the current case. The loss
of chromosome 13 in this case would have resulted in the
inactivation of RB1, which correlated with the malignant
transformation of FD. Although the definitive roles of the other
chromosomal alterations involved are not known, given the fact that
the majority of common chromosomal abnormalities in primary
osteosarcoma were also found in secondary osteosarcoma, it seems
reasonable to assume that these chromosomal abnormalities also play
important roles in tumorigenesis in osteosarcoma.
In summary, the present study reports the presence
of a Gsα mutation and chromosomal alterations in
secondary osteosarcoma arising from polyostotic FD. Further
investigation is required to elucidate the mechanism and impact of
these alterations in the malignant transformation of FD.
References
|
1
|
Yabut SM Jr, Kenan S, Sissons HA and Lewis
MM: Malignant transformation of fibrous dysplasia. A case report
and review of the literature. Clin Orthop Relat Res. (228):
281–289. 1988.
|
|
2
|
Ruggieri P, Sim FH, Bond JR and Unni KK:
Malignancies in fibrous dysplasia. Cancer. 73:1411–1424. 1994.
|
|
3
|
Hoshi M, Matsumoto S, Manabe J, Tanizawa
T, Shigemitsu T, Izawa N, Takeuchi K and Kawaguchi N: Malignant
change secondary to fibrous dysplasia. Int J Clin Oncol.
11:229–235. 2006.
|
|
4
|
Doganavsargil B, Argin M, Kececi B, Sezak
M, Sanli UA and Oztop F: Secondary osteosarcoma arising in fibrous
dysplasia, case report. Arch Orthop Trauma Surg. 129:439–444.
2009.
|
|
5
|
Riminucci M, Robey PG, Saggio I and Bianco
P: Skeletal progenitors and the GNAS gene: fibrous dysplasia of
bone read through stem cells. J Mol Endocrinol. 45:355–364.
2010.
|
|
6
|
Liang Q, Wei M, Hodge L, Fanburg-Smith JC,
Nelson A, Miettinen M, Foss RD and Wang G: Quantitative analysis of
activating alpha subunit of the G protein (Gsα) mutation by
pyrosequencing in fibrous dysplasia and other bone lesions. J Mol
Diagn. 13:137–142. 2011.
|
|
7
|
Tabareau-Delalande F, Collin C,
Gomez-Brouchet A, Decouvelaere AV, Bouvier C, Larousserie F, Marie
B, Delfour C, Aubert S, Rosset P, de Muret A, Pagès JC and de
Pinieux G: Diagnostic value of investigating GNAS mutations in
fibro-osseous lesions: a retrospective study of 91 cases of fibrous
dysplasia and 40 other fibro-osseous lesions. Mod Pathol.
26:911–921. 2013.
|
|
8
|
Gu W, Ogose A, Matsuba A, Kawashima H,
Hotta T, Kudo N, Hoshino M, Kondo N, Mera H and Endo N: Activating
Gs a mutation rarely occurs in musculoskeletal tumors other than
fibrous dysplasia. Anticancer Res. 26(2B): 1611–1614. 2006.
|
|
9
|
DiCaprio MR and Enneking WF: Fibrous
dysplasia. Pathophysiology, evaluation, and treatment. J Bone Joint
Surg Am. 87:1848–1864. 2005.
|
|
10
|
Siegal GP, Bianco P and Dal Cin P: Fibrous
dysplasia. WHO Classification of Tumours of Soft Tissue and Bone.
Fletcher CDM, Bridge JA, Hogendoorn PCW and Mertens F: 4th edition.
International Agency for Research on Cancer; Lyon, France: pp.
352–353. 2013
|
|
11
|
Gorlick R and Khanna C: Osteosarcoma. J
Bone Miner Res. 25:683–691. 2010.
|
|
12
|
Broadhead ML, Clark JC, Myers DE, Dass CR
and Choong PF: The molecular pathogenesis of osteosarcoma: a
review. Sarcoma 2011. 2011:9592482011.
|
|
13
|
Rosenberg AE, Cleton-Jansen A-M, de
Pinieux G, Deyup AT, Hauben E and Squire J: Conventional
osteosarcoma. WHO Classification of Tumours of Soft Tissue and
Bone. Fletcher CDM, Bridge JA, Hogendoorn PCW and Mertens F: 4th
edition. International Agency for Research on Cancer; Lyon, France:
pp. 282–288. 2013
|
|
14
|
Bridge JA, Nelson M, McComb E, McGuire MH,
Rosenthal H, Vergara G, Maale GE, Spanier S and Neff JR:
Cytogenetic findings in 73 osteosarcoma specimens and a review of
the literature. Cancer Genet Cytogenet. 95:74–87. 1997.
|
|
15
|
Martin JW, Squire JA and Zielenska M: The
genetics of osteosarcoma. Sarcoma. 2012:6272542012.
|