Introduction
Cisplatin [cis-diamminedichloroplatinum (II); DDP]
is a platinum-based drug that is frequently used in the treatment
of various cancers, including non-Hodgkin’s lymphoma and breast,
testicular, ovarian, head and neck, esophageal and bladder cancer
(1–3). Despite its therapeutic use, the
intrinsic resistance that is acquired following continuous
treatment limits the benefit of cisplatin in cancer therapy.
Therefore, the identification of new anticancer drugs is required
to optimize the performance of DDP. Sphingosine-1-phosphate (S1P)
is a bioactive lipid that has been implicated in regulating a
variety of cell biological responses, including cell proliferation,
survival, differentiation and migration (4). There is a great deal of interest in
the roles that S1P and other sphingolipids play in cell signaling
and the response to stress (5–8).
Furthermore, numerous studies have linked S1P to various aspects of
cell motility, chemotaxis and metastasis in mammalian and
SGC7901/DDP cells (9–11).
The enzyme sphingosine kinase (SphK) is an oncogene
that is tightly regulated by a number of growth factors and protein
kinases. SphK is able to increase the level of intracellular S1P.
4-[4-(4-chloro-phenyl)-thiazol-2-ylamino]-phenol (SKI-II) is mainly
used to inhibit SphK activity. SKI-II is able to inhibit S1P
formation in cells that express drug transport proteins, including
P-glycoprotein (P-gp), multidrug resistance (MDR)-associated
protein-1 (MRP1) and glutathione S-transferase (GST). The majority
of antitumor activities correlate with the concentrations of the
proteins that are present in the blood and tumors (12). The results provide further
validation for SphK as a cancer therapeutic target, as well as
small molecule inhibitors of SphK. The present study analyzed the
effects and explored the mechanisms of the combination of SKI-II
and DDP in SGC7901/DDP cells, which may aid the further clinical
application of this combination in chemotherapy.
Materials and methods
Reagents
The following reagents and detection kits were used:
SKI-II (Sigma-Aldrich Co., St Louis, MO, USA). SKI-II dissolved in
dimethyl sulfoxide (DMSO); RMPI-1640 medium (Beyotime Institute of
Biotechnology, Haimen, Jiangsu, China); fetal bovine serum (FBS;
Tianjin Hao Yang Biological Manufacture Co., Ltd., Tianjin, China);
3-(4, 5-dimethylthazol-2-yl)-2, 5-diphenyl tetrazolium bromide
(MTT; Amresco, USA); glutathione (GSH); a GST detection kit
(Nanjing Jiancheng Bioengineering Institute, Nanjing, Jiangsu,
China) and a total RNA Extraction kit (Sangong Biotechnology,
Shanghai, China).
Monoclonal antibodies (mAb) against P-gp, MRP1,
phosphorylated extracellular-signal-regulated kinase (p-ERK) and
phosphorylated c-Jun N-terminal kinase (p-JNK; Santa Cruz
Biotechnology Institute, Shanghai, China) were also used.
Cell line and cell culture
The human gastric carcinoma SGC7901/DDP cell line
was purchased from the Nanjing KeyGen Biological Co., Ltd.
(Nanjing, Jiangsu, China). The cells were cultured in RMPI-1640
medium (pH 7.2–7.4) containing 100 ml/l fetal calf serum (FCS) and
10 ml/l penicillin and streptomycin at 37°C in a humidified (95%)
incubator containing 95% air and 50 ml/l 5% CO2. The
SGC7901/DDP cells were cultured for 24 h and then incubated with
various concentrations of SKI-II (μmol/l) + DDP (mg/l) for 48
h.
Cytotoxicity and MDR reversal assay
The cells were seeded into 96-well plates at
2×104 cells/well. Various concentrations of the compound
SKI-II (μmol/l) + DDP (mg/l; 0+0, 0+0.625, 0+1.25, 0+2.5, 0+5,
1.25+0, 1.25+0.625, 1.25+1.25, 1.25+2.5 and 1.25+5) were
subsequently added and incubated for 48 h. The IC50
value of DDP in the absence or presence of 1.25 μmol/l SKI-II for
48 h was calculated from the plotted results using the untreated
cells as 100% by probit analysis using SPSS 16.0 (SPSS, Inc.,
Chicago, IL, USA). The reversion fold (RF) values, as the potency
of reversal, were calculated using the formula RF = IC50
of DDP alone/IC50 of DDP incubated with the test
compounds (8,10). Triplicate experiments with
triplicate samples were performed.
Immunocytochemistry assay
The exponentially-growing cells were cultured in a
24-well plate at a density of 1×104 cells/well. Various
concentrations of the SKI-II (μmol/l) + DDP compound (mg/l; 0+0,
0+2.5, 1.25+0, 1.25+2.5, 5+0, 5+2.5, 10+0 and 10+2.5) were
subsequently added and incubated for 48 h. An IHC detection reagent
and DAB kit (Santa Cruz Biotechnology Institute) were used to
examine the expression of P-gp, MRP1, p-JNK and p-ERK. The treated
cells were rinsed three times with 0.01 M phosphate-buffered
solution (PBS) and fixed for 30 min with 4% paraformaldehyde at
4°C. Following this, the cells were incubated with 0.3% TritonX-100
for 20 min and 3% H2O2 for 10 min. The cells
were then blocked with 10% normal goat serum for 1 h at room
temperature. Primary antibodies were added and incubated at 4°C
overnight. Subsequent to the cells being rinsed three times with
cold PBS, the secondary antibody was added and incubated at room
temperature for 30 min. Finally, the cells were rinsed with 0.01 M
PBS three times and incubated with the DAB complexes for 20 min.
The cells were observed and images were captured using a phase
contrast microscope (Olympus DP-11, Tokyo, Japan). Triplicate
experiments with triplicate samples were performed.
Western blot analysis
The cells were plated on culture flasks at a density
of 1×104 cells/ml with various concentrations of SKI-II
(μmol/l) + DDP (mg/l; 0+0, 0+2.5, 1.25+0, 1.25+2.5, 5+0, 5+2.5,
10+0 and 10+2.5) for 48 h. Following each experiment, the cells
were rinsed with 0.01 mol/l PBS three times and 200 μl lysate
containing 150 ml/l protease inhibitor was added, followed by use
of an ice bath for 15 min. The cells were then scraped from the
flasks, harvested and centrifuged at 15,000 × g for 20 min for
supernatant collection of the total proteins. The protein contents
were determined by a Lowry assay. Sodium dodecyl sulfate
polyacrylamide gel electrophoresis (SDS-PAGE) gel sample buffer was
added to the lysates, which were heated to 100°C for 5 min.
Following this, 100 μg protein was loaded into each well of a 12.5%
SDS-PAGE gel for electrophoresis and the targeted protein was
transferred onto a nitrocellulose membrane. The membrane was
blocked using 5% skimmed milk for 2 h at room temperature,
incubated overnight at 4°C with primary antibodies directed against
P-gp, MRP1, p-ERK, p-JNK (all diluted 1:500 in primary antibody
dilution buffer) and β-actin (loading control, diluted 1:1000 in
primary antibody dilution buffer). The membrane was washed three
times for 5 min each in washing buffer and incubated with the
appropriate AP-conjugated secondary antibody (rabbit anti-P-gp,
MRP1, p-JNK, p-ERK and rabbit anti-β-actin diluted 1:1000 in
secondary antibody dilution buffer) for 2 h at room temperature.
The blotted protein bands were visualized using the BCIP/NBT
Alkaline Phosphatase Color Development kit (Beijing Biotech Co.,
Ltd., Beijing, China). The developed films were digitized using an
Epson Perfection 2480 scanner (Seiko Corp, Nagano, Japan). The
optical density (OD) values of the proteins were obtained using
Glyko Bandscan software (Glyko, Novato, CA, USA).
Intracellular GSH and GST
quantification
Subsequent to being incubated with various
concentrations of SKI-II (μmol/l) + DDP (mg/l; 0+0, 0+2.5, 1.25+0,
1.25+2.5, 5+0, 5+2.5, 10+0 and 10+2.5) for 48 h, 1×105
cells were collected and homogenized with 10 mM HCl. The homogenate
was placed in a 96-well microplate and measured using a
spectrophotometer (Shimadzu UV-1208; Shimadzu, Kyoto, Japan) at 412
nm. The reactions between 5,5-dithiobis(2-nitrobenzoic acid) (DNTB)
and intracellular GSH and GST were quantified by comparing the
absorbance at 412 nm for each sample with that of a GSH standard
solution (0–100 μM/l). The absorbance was measured 10 min after the
addition of DNTB.
RNA extraction and semi-quantitative
polymerase chain reaction (PCR)
The cells were plated on culture flasks at a density
of 1×104 cells/ml with various concentrations of SKI-II
(μmol/l) + DDP (mg/l; 0+0, 0+2.5, 1.25+0, 1.25+2.5, 5+0, 5+2.5,
10+0 and 10+2.5) for 48 h. Total RNA was isolated using a Total RNA
Extraction kit (Tiangen Biotech Co., Ltd., Beijing, China), and
DNase treatment was performed using the RNase-Free DNase Set
according to the manufacturer’s instructions. Reverse transcription
of ≥10 μg total RNA was performed in a total volume of 100 μl
containing 250 pmol oligo (dT)18, 80 U rRNasin ribonuclease
inhibitor and 500 U Moloney murine leukemia virus reverse
transcriptase in 50 mM Tris-HCl (pH 8.3), 75 mM KCl, 3 mM
MgCl2, 10 mM DTT and 0.5 mM dNTPs. The total RNA and
oligo (dT)18 solutions were initially heated at 70°C for 10 min and
then immediately chilled on ice. The other reagents were added and
incubated for 15 min at 30°C and then 60 min at 42°C.
The primers and TaqMan probes for MRP1 and GST were
designed using Primer Express software (Tiangen Biotech Co., Ltd.).
The primers and TaqMan probes for β-actin were purchased from
Tiangen Biotech Co., Ltd.. The evaluation of β-actin expression,
used as a control of the RNA amount, was performed using the
following primer sequences: forward, 5′-GTGGGGCGCCCCAGGCACCA-3′ and
reverse, 5′-CTC CTTAATGTCACGCACGATTT-3′, which yielded a 500-bp
product. The other primers sequences that were used were: MRP1
forward, 5′-CCCCATGAATCCAAGATACCTA-3′ and reverse,
5′-CCTTACCATTTGGAGATGAAGC, which yielded a 1808-bp product; GST
sequence forward, 5′-ACC TTCTTTGGTGGAACCTGTA-3′ and reverse,
5′-AAAGGC ATTAGGGTTGTTCTGA-3′, which yielded a 1016-bp product.
Quantification of the target cDNA (MRP1 and GST) and
an internal reference gene (β-actin) was conducted using a
fluorescence-based quantitative PCR method. PCR was performed in a
25-μl reaction volume containing cDNA equivalent to 1–10 ng total
RNA and 200 nM of each primer, 100 nM of probe and 12.5 U TaqMan
universal PCR Master Mix (containing 1XTaqMan buffer, 200 μM dATP,
dCTP and dGTP and 400 μM dUTP, 5 mM MgCl2, 1.25 U
AmpliTaqGold and 0.5 U AmpErase UNG). The thermal cycling
conditions were 50°C for 2 min and 95°C for 10 min, followed by 45
cycles at 95°C for 15 sec and 60°C for 1 min. The relative
quantification of gene expression was performed using the relative
standard curve method. The standard curve was created automatically
using the ABI PRISM 7700 Detection System software (Applied
Biosystems, Foster City, CA, USA) by plotting the threshold cycle
(CT) against each input amount of the control total RNA (16, 4, 1,
0.25, 0.063, 0.016 and 0.0039 ng total starting RNA), prepared from
the SGC7901/DDP human gastric carcinoma cells. The coefficient of
linear regression for each standard curve was >0.990. For each
unknown sample, the relative amount was calculated using a linear
regression analysis from the respective standard curve. A relative
target gene expression value was obtained by dividing the target
gene value by the β-actin value (internal reference gene).
Experimental animals and tumor-bearing
nude mouse model
A total of 24 female BALB/C nude mice, aged 4 weeks
and weighing 15–19 g, were obtained from the Experimental Animal
Center of Xuzhou Medical College. All experiments were carried out
in accordance with the National Institutes of Health Guide for the
Care and Use of Laboratory Animals. This study was approved by the
ethics committee of XuZhou Medical College.
Human gastric cancer SGC7901/DDP cells were
implanted under the skin on the right side in 16 BALB/C nude mice.
The inoculation volume was 0.1 ml (1×107 living
cells/ml). Following the inoculation, tumor nodes appeared at the
inoculated site, which were observed continuously for 1 week. The
tumors were formed from hard tumor nodes at the inoculated site and
increasing nodal enlargement. The mice were raised in an
appropriate animal house and fed with cool boiled water and fodder
sterilized with steam. The wood chips that were used for the
bedding were sterilized with steam and the mouse cages were
sterilized with a disinfectant solution. The water, fodder, wood
chips and cages were changed and cleaned once a day, and the
ambient temperature and humidity were kept at 22±1°C and 60±10%,
respectively, on a 12-h day/night cycle. The maximum tumor diameter
was measured using a vernier caliper.
All the animal experiments were performed in
compliance with the national laws relating to animal research. The
16 BALB/C tumor-bearing nude mice were randomized into four groups,
with four mice in each group. The normal group consisted of mice
that were treated with nothing. The DDP mouse group were treated
with a 2.5 mg/kg intraperitoneal perfusion (i.p.) of DDP for 48 h.
The SKI-II mouse group was treated with 0.3 mg/kg i.p. SKI-II for
48 h and the DDP + SKI-II mouse group were treated with 0.3 mg/kg
i.p. SKI-II and 2.5 mg/kg i.p. DDP for 48 h. The mice were then
sacrificed by cervical dislocation at 48 h subsequent to i.p.
administration. The tumors were removed to measure their weight.
Next, certain tumors were mounted on glass slides for
immunohistochemical (IHC) staining. Other tumors were collected and
stored at −80°C for western blotting and GSH and GST
quantification.
Immunohistochemical staining
The IHC detection reagent and DAB kit were used to
examine the expression of P-gp, MRP1, p-JNK and p-ERK. Briefly, the
deparaffinized sections were heated in a 700 W microwave oven for
12 min to retrieve the antigens and then cooled to room
temperature. Endogenous peroxide activity was blocked using 3%
H2O2 for 10 min. The sections were washed
with 0.01 M PBS, blocked with 10% rabbit serum for 15 min to reduce
non-specific antibody binding and incubated with the respective
primary antibody (against P-gp, MRP1, p-ERK or p-JNK) at 4°C
overnight. The other steps were similar to those described in the
IHC assay.
Western blotting
The tumors were collected over ice and mechanically
lysed in RIPA Lysis Buffer (P0013B; Beyotime Institute of
Biotechnology), which contained 50 mM Tris (pH 7.4), 150 mM NaCl,
1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, sodium
orthovanadate and 1 mM phenylmethylsulphonyl fluoride (PMSF). The
mucosa was homogenized for 30 sec and kept on ice for 20 min. The
homogenates were centrifuged at 12,000 × g for 30 min at 4°C, and
the supernatants that contained the cytoplasmic components were
retained and stored at −80°C and thawed once. The nuclear pellets
were dissolved in RIPA Lysis Buffer and PMSF, centrifuged at 12,000
× g for 20 min at 4°C and the supernatant extracts collected. The
nuclear extracts were aliquoted and stored at −80°C for western
blotting analysis of p65 protein activity. The protein
concentrations were determined using the bicinchoninic acid (BCA)
protein assay. Equal amounts of protein were resolved in
SDS-polyacrylamide gels and transferred electrophoretically onto a
nitrocellulose membrane (Amersham Biosciences, Amersham, UK). The
other steps were similar to those described in the western blot
analysis.
Tumor GSH and GST quantification
To detect the activity of GST and the contents of
GSH, the tumors were excised and subsequently homogenized in normal
saline at 4°C. The homogenate was centrifuged at 4,000 × g for 10
min and the supernatant was retained. The homogenate was placed in
a 96-well microplate and the absorbance was measured at 412 nm was
using a spectrophotometer (Schimadzu UV-1208). The other steps were
similar to those described in the intracellular GSH and GST
quantification.
Statistical analysis
The results are presented as the mean ± SD of three
replicated experiments. A one-way ANOVA was performed to determine
the differences among the groups. All the statistical analyses were
performed using GraphPad Prism 5 (GraphPad Software Inc., La Jolla,
CA, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
SKI-II reverses the resistance of cells
to DDP
SKI-II in combination with DDP had a greater effect
on the SGC-7901/DDP cells compared with DDP or SKI-II alone
(P<0.05). The resistance index of the SGC7901/DDP cells to DDP
was 6.3. Following the treatment with SKI-II, the IC50
of DDP to the SGC7901/DDP cells was reduced from 3.33 μmol/l to
2.062 μmol/l by 1.615-fold (Table
I).
 | Table IReversing effect of SKI-II on
SGC7901/DDP cells. |
Table I
Reversing effect of SKI-II on
SGC7901/DDP cells.
| Group, SKI-II
(μmol/l) + DDP (mg/l) | Inhibition rate
(%) | IC50 | RF |
|---|
| Pre-reversion |
| 0+0 | 0.00±0.000 | | 1.615 |
| 0+0.625 | 0.18±0.008 | | |
| 0+1.25 | 0.40±0.011 | 3.330 | |
| 0+2.5 | 1.75±0.063 | | |
| 0+5 | 4.08±0.024 | | |
| Post-reversion |
| 1.25+0 | 0.00±0.000 | | |
| 1.25+0.625 | 1.48±0.013 | | |
| 1.25+1.25 | 3.50±0.320 | | |
| 1.25+2.5 | 4.28±0.042 | 2.062 | |
| 1.25+5 | 12.15±0.175 | | |
Expression of P-gp, MRP-1, p-ERK and
p-JNK in immunocytochemistry assay
The immunocytochemistry assay in vitro
confirmed the expression of P-gp, MRP-1, p-ERK and p-JNK in the
human gastric carcinoma cells. The number of P-gp-, MRP-1-, p-ERK-
and p-JNK-positive cells in the SKI-II (μmol/l) + DDP (mg/lU; 5+0,
10+0, 5+2.5 and 10+2.5) groups were significantly decreased
(P<0.05). The immunocytochemistry assay in vivo confirmed
the expression of P-gp, MRP-1, p-ERK and p-JNK in the tumors. The
number of P-gp-, MRP-1-, p-ERK- and p-JNK-positive cells in
vitro and in vivo all decreased (P<0.05) following
the treatment with SKI-II alone and significantly decreased
following the treatment with the DDP + SKI-II group (P<0.05;
Tables II and III).
 | Table IIExpression of P-gp, MRP-1, p-ERK and
p-JNK in the immunocytochemistry assay in vitro. |
Table II
Expression of P-gp, MRP-1, p-ERK and
p-JNK in the immunocytochemistry assay in vitro.
| Groups, SKI-II
(μmol/l) + DDP (mg/l) | P-gp | MRP1 | p-JNK | p-ERK |
|---|
| 0+0 | 70.90±0.77 | 71.47±0.47 | 68.47±0.52 | 70.50±0.50 |
| 0+2.5 | 71.06±1.20 | 72.55±0.58 | 67.95±0.90 | 71.53±0.54 |
| 0+1.25 | 71.51±0.48 | 71.28±0.77 | 68.57±0.39 | 70.43±0.65 |
| 0+5 | 54.01±1.02abc | 53.37±0.61abc | 54.60±1.00abc | 52.86±1.00abc |
| 0+10 | 34.13±0.99abc | 39.55±0.50abc | 40.44±0.45abc | 39.73±0.36abc |
| 1.25+2.5 | 71.64±0.70 | 69.43±0.65 | 68.12±0.35 | 69.47±0.63 |
| 5+2.5 | 40.20±1.36abc | 44.31±0.90abc | 45.73±+0.26abc | 44.61±0.53abc |
| 10+2.5 | 28.64±0.85abc | 29.39±1.05abc | 30.57±0.70abc | 30.63±0.71abc |
 | Table IIIExpression of P-gp, MRP-1, p-ERK and
p-JNK in immunocytochemistry assay in vivo. |
Table III
Expression of P-gp, MRP-1, p-ERK and
p-JNK in immunocytochemistry assay in vivo.
| Protein expression
rate (%) |
|---|
|
|
|---|
| Group | P-gp | MRP1 | p-ERK | p-JNK |
|---|
| Control | 72.313±1.560 | 73.522±1.559 | 72.783±0.916 | 74.800±0.699 |
| DDP | 70.546±0.602 | 69.323±0.472 | 71.867±0.787 | 73.003±0.284 |
| SKI-II |
51.503±0.662ab |
50.624±0.673ab |
52.500±0.545ab |
52.173±0.982ab |
| DDP+SKI-II |
45.637±0.372abc |
42.321±0.403abc |
44.534±0.561abc |
46.040±0.830abc |
Expression of P-gp, MRP-1, p-ERK and
p-JNK in the western blot assay
The result of the western blot assay in vitro
demonstrated that P-gp, MRP-1, p-ERK and p-JNK were expressed in
the human gastric carcinoma cells. Compared with the DDP (2.5 mg/l)
group, the expression of P-gp, MRP-1, p-ERK and p-JNK decreased in
the SKI-II (μmol/l) + DDP (mg/l; 5+0, 10+0, 5+2.5 and 0+2.5;
P<0.05; Table IV). The result
of the western blot assay in vivo demonstrated that P-gp,
MRP-1, p-ERK and p-JNK were expressed in the tumors. Compared with
the DDP group, the expression of P-gp, MRP-1, p-ERK and p-JNK
decreased (P<0.05) in the tumors following the treatment with
SKI-II alone and significantly decreased following the treatment
with the DDP + SKI-II group (P<0.05; Fig. 1A, Table
V).
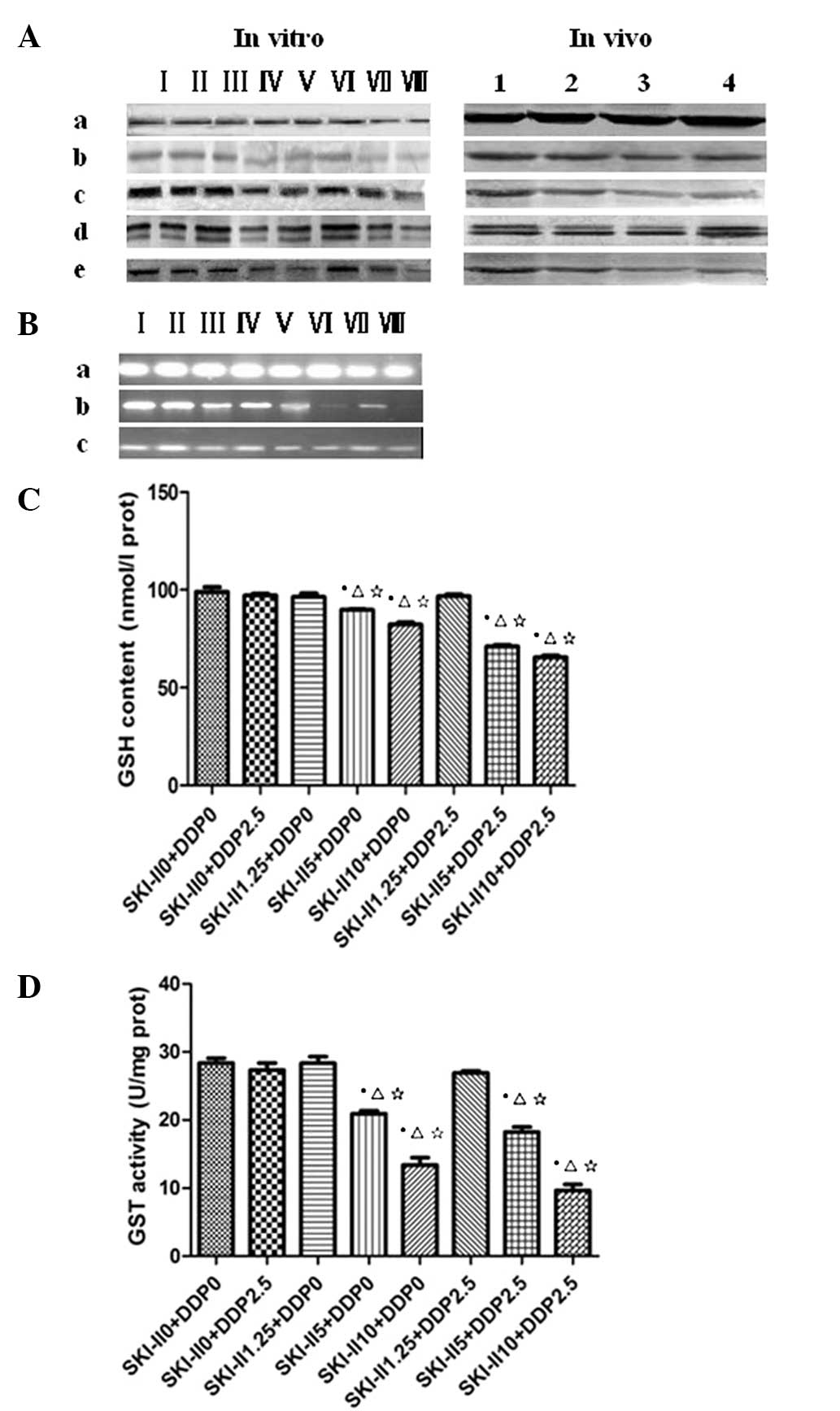 | Figure 1P-gp, MRP1, p-ERK and p-JNK expression
was downregulated by SKI-II and DDP, as determined by western
blotting. (A) a, β-actin; b, P-gp; c, MRP1; d, p-ERK; and e, p-JNK.
I, control; II, DDP 2.5; III, SKI-II 1.25; IV, SKI-II 5; V, SKI-II
10; VI, DDP 2.5 + SKI-II 1.25; VII, DDP 2.5 + SKI-II 5; and VIII,
DDP 2.5 + SKI-II 10. 1, Control; 2, DDP; 3, SKI-II and 4,
DDP+SKI-II. (B) MRP1, GSH and GST expression was downregulated
following treatment with SKI-II and DDP, as observed by RT-PCR. a,
β-actin; b, GST; and c, MRP1. I, control; II, DDP 2.5; III, SKI-II
1.25; IV, SKI-II 5; V, SKI-II 10; VI, DDP 2.5 + SKI-II 1.25; VII,
DDP 2.5 + SKI-II 5; and VIII, DDP 2.5 + SKI-II 10. The effects of
SKI-II+DDP on (C) GSH contents and (D) GST activity are shown
following the treatment. *P<0.05 vs. normal group;
ΔP<0.05 vs. DDP 2.5 group; ⋆P<0.05 vs.
SKI-II1.25 group. P-gp, P-glycoprotein; MRP1, multidrug
resistance-associated protein-1; p-ERK, phosphorylated
extracellular-signal-regulated kinase; p-JNK, phosphorylated c-Jun
N-terminal kinase; SKI-II,
4-[4-(4-chloro-phenyl)-thiazol-2-ylamino]-phenol; DDP,
cis-diamminedichloroplatinum (II); GSH, glutathione; GST,
glutathione S-transferase. |
 | Table IVOD value of P-gp, MRP1, p-JNK and
p-ERK in all the groups in vivo in the western blot
assay. |
Table IV
OD value of P-gp, MRP1, p-JNK and
p-ERK in all the groups in vivo in the western blot
assay.
| OD |
|---|
|
|
|---|
| Group | P-gp | MRP1 | p-JNK | p-ERK |
|---|
| Control | 1.250±0.002 | 1.231±0.002 | 1.044±0.035 | 1.089±0.004 |
| DDP 2.5 | 1.244±0.021 | 1.222±0.001 | 1.002±0.008 | 1.078±0.005 |
| SKI-II 1.25 | 1.231±0.004 | 1.224±0.003 | 1.009±0.009 | 1.086±0.004 |
| SKI-II 5 | 0.838±0.007abc | 0.844±0.004abc | 0.867±0.003abc | 0.864±0.00abc |
| SKI-II 10 | 0.744±0.005abc | 0.722±0.005abc | 0.728±0.002abc | 0.735±0.03abc |
| DDP 2.5+SKI-II
1.25 | 1.225±0.005 | 1.222±0.002 | 1.004±0.14 | 1.074±0.007 |
| DDP 2.5+SKI-II
5 | 0.695±0.005abc | 0.706±0.004abc | 0.745±0.006abc | 0.747±0.01abc |
| DDP 2.5+SKI-II
10 | 0.644±0.004abc | 0.669±0.003abc | 0.651±0.004abc | 0.668±0.00abc |
 | Table VOD value of P-gp, MRP1, p-JNK and
p-ERK in all the groups in vitro in the western blot
assay. |
Table V
OD value of P-gp, MRP1, p-JNK and
p-ERK in all the groups in vitro in the western blot
assay.
| OD |
|---|
|
|
|---|
| Group | P-gp | MRP1 | p-ERK | p-JNK |
|---|
| Control | 1.313±0.018 | 1.220±0.0265 | 1.093±0.203 | 1.083±0.040 |
| DDP | 1.300±0.017 | 1.220±0.0252 | 1.060±0.044 | 1.023±0.054 |
| SKI-II | 1.047±0.041ab | 0.997±0.068ab | 0.813±0.015ab | 0.817±0.026ab |
| DDP+SKI-II | 0.870±0.056abc | 0.810±0.026abc | 0.713±0.020abc | 0.680±0.023abc |
Expression levels of MRP-1, GST messenger
RNA (mRNA) in SGC7901/DDP cells
The expression levels of MRP-1 mRNA in the
SGC7901/DDP cells were calculated as: (Ratio of the amount of MRP-1
mRNA/amount of β-actin mRNA in the DDP-treated cells)/(ratio of the
amount of MRP1 mRNA/amount of β-actin mRNA in cells at 48 h
following SKI-II and/or DDP treatment). The MRP-1 levels peaked in
the SKI-II (μmol/l) + DDP (mg/l; 0+0;0+2.5) groups, but had
decreased in the SKI-II (μmol/l) + DDP (mg/l; 5+0; 10+0, 5+2.5 and
10+2.5) treatment groups after 48 h. In the SKI-II (μmol/l) + DDP
(mg/l; 5+0, 10+0, 5+2.5 and 10+2.5)-treated cells, the combination
of the two drugs resulted in a significantly lower level of MRP1
expression compared with the cells that were treated with SKI-II
alone at the 48-h time-point (P<0.05). The expression of GST
mRNA in the SGC7901/DDP cells was decreased by SKI-II + DDP (mg/l;
5+2.5 and 10+2.5) treatment after 48 h, exhibiting a significant
suppression of GST expression compared with SKI-II or DDP treatment
alone (P<0.05; Fig. 1B). This
suggested that SKI-II treatment enhanced DDP toxicity against the
SGC7901/DDP cells through the suppression of GST expression, which
is normally enhanced by DDP exposure in the SGC7901/DDP cells.
Contents of GSH and GST
In vitro, the expression of GSH decreased
significantly (P<0.05) in the cells subsequent to being treated
with SKI-II alone (5 μmol/l and 10 μmol/l), and significantly
decreased subsequent to being treated with SKI-II (μmol/l) + DDP
(mg/l; 5+2.5 and 10+2.5; P<0.05). The GST activity significantly
decreased in the SKI-II (μmol/l) + DDP (mg/l; 5+2.5 and 10+2.5)
groups when compared with SKI-II alone (5 umol/l and 10 umol/l;
P<0.05). In vivo, GSH decreased (P=0.027; P<0.05) in
the tumors subsequent to being treated with SKI-II alone and
significantly decreased following treatment with DDP + SKI-II
(P<0.05; Fig. 1C and D). The GST
activity significantly decreased in the DDP + SKI-II group when
compared with the SKI-II alone group (P<0.05).
Discussion
Gastric cancer is a common malignant tumor of the
alimentary tract and its incidence rate is among the three leading
kinds of neoplasm in various regions of China (13,14).
Conventional treatments for advanced gastric cancer include
extended resection, radiotherapy and chemotherapy, which have
little effect on the survival rate of affected patients. Cisplatin
is a platinum chemotherapeutic agent that is used in a variety of
malignancies. The major limitation in the clinical application of
cisplatin has been the development of cisplatin resistance by
tumors. A number of experimental strategies to overcome cisplatin
resistance are at the preclinical or clinical levels, including the
introduction of the bax gene, the inhibition of the JNK pathway,
the introduction of a functional p53 gene and the treatment of
tumors with aldose reductase inhibitors (15,16).
Of particular significance are the combinations of platinum drug
treatments with other drugs, radiation and the emerging gene
therapy regimens. The present study analyzed the effect of the
combination of SKI-II and DDP on the SGC7901/DDP cells and
identified that SKI-II in combination with DDP had an improved
effect compared with DDP or SKI-II alone. Following the treatment
with SKI-II, the IC50 of DDP to the SGC7901/DDP cells
was reduced. Furthermore, the present study explored the mechanisms
of SKI-II in vitro and in vivo.
MDR is an unfavorable factor that causes the failure
of treatments against cancer cells (17). MDR occurs when cancer cells acquire
a simultaneous resistance to various chemotherapeutic agents that
have no structural or functional similarities (18). The mechanisms that are involved in
MDR include the overexpression of mutispecific ATP-dependent drug
efflux pumps, including P-gp, MRP1 and BCRP (ABCG2), which reduce
the available concentration of the drug for the cancer cells
(19). The present study identified
that the combination of SKI-II and DDP was able to reverse the MDR
of the SGC7901/DDP cells via the downregulation of P-gp and MRP1,
which increased the concentrations of DDP in the SGC7901/DDP
cells.
Endogenous antioxidants, including reduced GSH, GSH
peroxidase (GSH-PX), superoxide dismutase (SOD) and catalase (CAT),
are compounds that act as free radical scavengers. These
antioxidants are electron donors and react with the free radicals
to form harmless products such as water. Therefore, antioxidants
protect against oxidative stress and prevent damage to cells
(20). Similarly, GST is a soluble
protein that is located in the cytosol and plays a significant role
in cell protection. The present study identified that the
combination of SKI-II and DDP decreased the levels of GSH and GST
in the SGC7901/DDP cells, which decreased the protection
possibility of GSH and GST to the cells. Therefore, the inhibition
rate of the SGC7901/DDP cells that were treated with SKI-II and DDP
increased significantly in vitro and in vivo.
MAPKs belong to a large family of proline-directed
serine-threonine protein kinases that are significant signaling
components in the conversion of extracellular signals into an
intracellular response through a series of phosphorylation cascades
(21). The ultimate effects of MAPK
activation and phosphorylation depend on the ability of the kinases
to induce the appropriate gene expression events. Three MAP kinase
pathways, ERK, JNK and p38, have been identified and well studied
(22,23). The expression and activation of the
JNK and ERK MAPK pathway correlate with prognosis and affects the
therapeutic outcome in several types of cancer (24,25).
The present study revealed that the combination of SKI-II and DDP
may decrease the MAPK pathway by decreasing the expression of ERK
and JNK in the SGC7901/DDP cells. Wasserman et al identified
that the activation of MAPK also induced the mRNA expression of
immediate early genes, including c-jun and c-fos, and
transcriptionally activated the antioxidant/electrophile response
element (ARE/EpRE) and the chloramphenicol acetyltransferase
reporter gene, which are present in a number of stress-response
genes, including GST, quinone reductase and heme oxygenase 1
(26–29). Furthermore, the present study
revealed that the combination of SKI-II and DDP may also decrease
the levels of GSH and GST in the SGC7901/DDP cells. This emphasizes
the complexity of MAPK signaling.
In summary, the results of the present study have
shown that SKI-II is able to reverse the chemoresistance of
SGC7901/DDP gastric cancer cells by decreasing the levels of P-gp
and MRP-1, which increases the concentrations of DDP in the
SGC7901/DDP cells. Furthermore, SKI-II decreased the levels of GSH
and GST in the cells, which decreased the protection possibility of
GSH and GST to the SGC7901/DDP cells. The present study
demonstrated that the combination of SKI-II and DDP was able to
regulate the MAPK pathways by decreasing the expression of ERK and
JNK in the SGC7901/DDP cells. The study has provided significant
insights into the signal transduction pathways that are induced by
the combination of SKI-II and DDP, which activate MAPK pathways,
leading to a decrease in GST for cellular protection signaling.
Therefore, further understanding of the regulatory system of MDR,
GSH and MAPK signaling is required for the application of SKI-II
and DDP in clinical settings.
Acknowledgements
This study was supported by the Jiangsu Provincial
Department of Health 2011 Annual Medical Research Project (no.
Z201016) and the Jiangsu Province Six Talents Peak Seventh Batches
of Funded projects (no. 032).
References
|
1
|
Lekakis L, Tryfonopoulos D, Pistamatzian
N, et al: Salvage chemotherapy with cisplatin and 5-fluorouracil in
metastatic breast cancer. Particular activity against liver
metastases. Anticancer Res. 32:1833–1837. 2012.
|
|
2
|
García-Velasco A, Durán I, García E, et
al: Biological markers of cisplatin resistance in advanced
testicular germ cell tumours. Clin Transl Oncol. 14:452–457.
2012.
|
|
3
|
Yanagawa M, Tatsumi M, Miyata H, et al:
Evaluation of response to neoadjuvant chemotherapy for esophageal
cancer: PET response criteria in solid tumors versus response
evaluation criteria in solid tumors. J Nucl Med. 53:872–880.
2012.
|
|
4
|
Kumar A and Saba JD: Lyase to live by:
sphingosine phosphate lyase as a therapeutic target. Expert Opin
Ther Targets. 13:1013–1025. 2009.
|
|
5
|
Hla T: Physiological and pathological
actions of sphingosine 1-phosphate. Semin Cell Dev Biol.
15:513–520. 2004.
|
|
6
|
Ogretmen B and Hannun YA: Biologically
active sphingolipids in cancer pathogenesis and treatment. Nat Rev
Cancer. 4:604–616. 2004.
|
|
7
|
Spiegel S and Milstien S: Sphingosine
1-phosphate, a key cell signaling molecule. J Biol Chem.
277:25851–25854. 2002.
|
|
8
|
Taha TA, Argraves KM and Obeid LM:
Sphingosine-1-phosphate receptors: receptor specificity versus
functional redundancy. Biochim Biophys Acta. 1682:48–55. 2004.
|
|
9
|
Kumar A, Wessels D, Daniels KJ, et al:
Sphingosine-1-phosphate plays a role in the suppression of lateral
pseudopod formation during Dictyostelium discoideum cell migration
and chemotaxis. Cell Motil Cytoskeleton. 59:227–241. 2004.
|
|
10
|
Oskouian B and Saba JD: Death and taxis:
what non-mammalian models tell us about sphingosine-1-phosphate.
Semin Cell Dev Biol. 15:529–540. 2004.
|
|
11
|
Spiegel S, English D and Milstien S:
Sphingosine 1-phosphate signaling: providing cells with a sense of
direction. Trends Cell Biol. 12:236–242. 2002.
|
|
12
|
French KJ, Upson JJ, Keller SN, et al:
Antitumor activity of sphingosine kinase inhibitors. J Pharmacol
Exp Ther. 318:596–603. 2006.
|
|
13
|
Roukos DH and Kappas AM: Perspectives in
the treatment of gastric cancer. Nat Clin Pract Oncol. 2:98–107.
2005.
|
|
14
|
Menges M, Schmidt C, Lindemann W, et al:
Low toxic neoadjuvant cisplatin, 5-fluorouracil and folinic acid in
locally advanced gastric cancer yields high R-0 resection rate. J
Cancer Res Clin Oncol. 129:423–429. 2003.
|
|
15
|
Zhao Z, Wang J, Tang J, et al: JNK-and
Akt-mediated Puma expression in the apoptosis of
cisplatin-resistant ovarian cancer cells. Biochem J. 444:291–301.
2012.
|
|
16
|
Hasegawa M, Ishiguro K, Ando T and Goto H:
Geranylgeranylacetone attenuates cisplatin-induced reductions in
cell viability by suppressing the elevation of intracellular p53
content without heat shock protein induction. Nagoya J Med Sci.
74:123–131. 2012.
|
|
17
|
Vasconcelos FC, Cavalcanti GB Jr, Silva
KL, et al: Constrasting features of MDR phenotype in leukemias by
using two fluorochromes: implications for clinical practice. Leuk
Res. 31:445–454. 2007.
|
|
18
|
Patel NH and Rothenberg ML: Multidrug
resistance in cancer chemotherapy. Invest New Drugs. 12:1–13.
1994.
|
|
19
|
Legrand O, Simonin G, Beauchamp-Nicoud A,
et al: Simultaneous activity of MRP1 and Pgp is correlated with in
vitro resistance to daunorubicin and with in vivo resistance in
adult acute myeloid leukemia. Blood. 94:1046–1056. 1999.
|
|
20
|
Valko M, Rhodes CJ, Moncol J, et al: Free
radicals, metals and antioxidants in oxidative stress-induced
cancer. Chem Biol Interact. 160:1–40. 2006.
|
|
21
|
Cobb MH and Goldsmith EJ: How MAP kinases
are regulated. J Biol Chem. 270:14843–14846. 1995.
|
|
22
|
Wagner EF and Nebreda AR: Signal
integration by JNK and p38 MAPK pathways in cancer development. Nat
Rev Cancer. 9:537–549. 2009.
|
|
23
|
Lee HG, Minematsu H, Kim KO, et al: Actin
and ERK1/2-CEBPβ signaling mediates phagocytosis-induced innate
immune response of osteoprogenitor cells. Biomaterials.
32:9197–9206. 2011.
|
|
24
|
McGlynn LM, Kirkegaard T, Edwards J, et
al: Ras/Raf-1/MAPK pathway mediates response to tamoxifen but not
chemotherapy in breast cancer patients. Clin Cancer Res.
15:1487–1495. 2009.
|
|
25
|
Atmaca A, Pauligk C, Steinmetz K, et al:
Prognostic impact of phosphorylated mitogen-activated protein
kinase expression in patients with metastatic gastric cancer.
Oncology. 80:130–134. 2011.
|
|
26
|
Wasserman WW and Fahl WE: Functional
antioxidant responsive elements. Proc Natl Acad Sci USA.
94:5361–5366. 1997.
|
|
27
|
Li Y and Jaiswal AK: Regulation of human
NAD(P)H: quinone oxidoreductase gene. Role of AP1 binding site
contained within human antioxidant response element. J Biol Chem.
267:15097–15104. 1992.
|
|
28
|
Rushmore TH and Pickett CB:
Transcriptional regulation of the rat glutathione S-transferase Ya
subunit gene. Characterization of a xenobiotic-responsive element
controlling inducible expression by phenolic antioxidants. J Biol
Chem. 265:14648–14653. 1990.
|
|
29
|
Prestera T, Holtzclaw WD, Zhang Y and
Talalay P: Chemical and molecular regulation of enzymes that
detoxify carcinogens. Proc Natl Acad Sci USA. 90:2965–2969.
1993.
|















