Introduction
Prostate cancer is one of the most common malignant
diseases and the second leading cause of cancer mortality among
males in the USA (1). Sulindac is a
non-steroidal anti-inflammatory agent, which has shown significant
activity in inhibiting gastrointestinal tumor formation in mouse
models of colorectal cancers, as well as in inhibiting colorectal
cancer cell proliferation and inducing cell apoptosis (2,3).
However, these activities require p21, not p27 (4,5), and
have been associated with c-Jun NH2-terminal kinase (JNK) 1
activation via phosphorylation in vitro and in vivo
(6–8). The current study identified that
sulindac exerts anticancer activities on prostate cancer cells via
the inhibition of cell proliferation and induction of apoptosis by
targeting the JNK1/β-catenin signaling pathway.
The JNKs have been identified as members of the
mitogen-activated protein kinase family, and phosphorylate and
activate various transcriptional factors, including c-Jun,
activating transcription factor 2, activation protein-1 and p53
(9–13). Our previous studies demonstrated
that JNK1 is critical in intestinal tumorigenesis, which was
identified to be associated with p21 expression in a JNK1 gene
knockout mouse model as well as colorectal cancers (14). It is also well known that the JNK
signaling transduction pathway is significant in a variety of
cellular processes, including cell proliferation, differentiation
and apoptosis (15,16), particularly in regulating apoptosis
(17–19).
To elucidate the bioactivities of sulindac and the
underlying mechanism, the current study analyzed the efficacy of
sulindac with regard to dosage as well as the involvement and roles
of JNK1/β-catenin signaling in prostate cancer.
Materials and methods
Human prostate cancer cell culture
Human prostate cancer cell lines, PC-3 and LNCaP,
were purchased from the American Type Culture Collection (Manassas,
VA, USA) and maintained in RPMI-1640 media supplemented with 10%
fetal bovine serum (FBS), 1× antibiotic/antimycotic (100 U/ml
streptomycin, 100 U/ml penicillin and 0.25 μg/ml amphotericin B),
100 μM non-essential amino acids and 10 mM HEPES buffer solution
(all Invitrogen Life Technologies, Carlsbad, CA, USA). All cells
were cultured at 37°C in a humidified atmosphere of 5%
CO2. Sulindac (Sigma-Aldrich, St. Louis, MO, USA) was
dissolved in dimethyl sulfoxide (DMSO) and diluted in a serials
concentration.
Apoptosis analysis
The treated cells were harvested at different time
points, washed in cold phosphate-buffered saline (PBS) and stained
with Annexin V and propidium iodide according to the manufacturer’s
instructions for the Alexa Fluor 488 Annexin V/Dead Cell Apoptosis
kit (Invitrogen Life Technologies). The cells were analyzed using a
CyAn ADP three channel flow cytometer and Summit3 software (both
Beckman Coulter, Miami, FL, USA). The reactions were performed in
triplicate and the data are representative of three independent
experiments.
Cell proliferation assay
A total of 1×104 cells in 100 μl
RPMI-1640 medium supplemented with 10% FBS was seeded in 96-well
plates one day prior to the assay. After 18–20 h, the medium was
removed and 100 μl complete assay medium was added to each well and
simultaneously, sulindac was added to the medium to reach final
concentrations of sulindac; 0, 0.4 and 0.8 mM. Next, 100 μl full
medium with an equal volume of DMSO was added to each well as a
control. All of the groups of cells were cultured in triplicate.
The plates were incubated at 37°C for 24 h and the cell
proliferation was determined by 3-(4,5-dimethyl
thiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay
(CellTiter 96 Non-Radioactive Cell Proliferation Assay kit; Promega
Corporation, Madison, WI, USA). Briefly, 15 μl MTT (Promega
Corporation) was added to each well and the plate was incubated at
7°C for 4 h in a humidified atmosphere of 5% CO2. Next,
100 μl stop solution was added to each well and incubated for 1 h.
Finally, the absorbance was measured at 570 nm using a microplate
reader (Synergy 2; BioTek Instruments, Inc., Winooski, VT,
USA).
TOP/FOP-Flash transfection and luciferase
assay
To examine the effect of sulindac on
β-catenin/T-cell factor (TCF) signaling, cells were seeded in 24
well-plates at a cell density of 5,000 cells/well in minimum
essential media (Invitrogen Life Technologies) without antibiotics.
The cells were transiently cotransfected with a TOP- or FOP-Flash
plasmid (Upstate Biotechnology, Inc., Lake Placid, NY, USA) and the
Renilla luciferase expression vector served as a control for
transfection efficiency using Lipofectamine 2000 (Invitrogen Life
Technologies). At 6 h after transfection, the medium was removed
and the cells were supplied with fresh medium supplemented with 0.8
mM sulindac for 48 h and DMSO served as a control. The cells were
washed with cold PBS, lysed with passive lysis buffer and
luciferase activity was measured using the Dual Luciferase Report
Assay System (Promega Corporation). The lysate firefly luciferase
values were normalized to Renilla luciferase activity and all
experiments were independently performed in triplicate.
Immunoblotting analysis
Total protein was isolated from sulindac-treated
cells, quantified by Bradford analysis and measured at 595 nm with
a microplate reader using the Bio-Rad Protein Assay kit (Bio-Rad,
Hercules, CA, USA). Next, 30 μg protein/lane was resolved by 10%
SDS-PAGE and transferred to polyvinylidene fluoride membranes
(Millipore, Bedford, MA, USA). The immunoblot was incubated
overnight at 4°C with the primary antibodies, anti-JNK1 and
-phosphorylated-JNK1 (p-JNK) purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). β-catenin was obtained from
Sigma-Aldrich and horseradish peroxidase-conjugated affinipure goat
anti-mouse IgG (Promega Corporation) secondary antibodies were
used. Electrochemiluminescence western blotting detection reagents
(Amersham Pharmacia Biotech, Piscataway, NJ, USA) were used as the
protein signal and β-actin (Sigma-Aldrich) served as a loading
control.
Results
Sulindac induces human prostate cancer
apoptosis
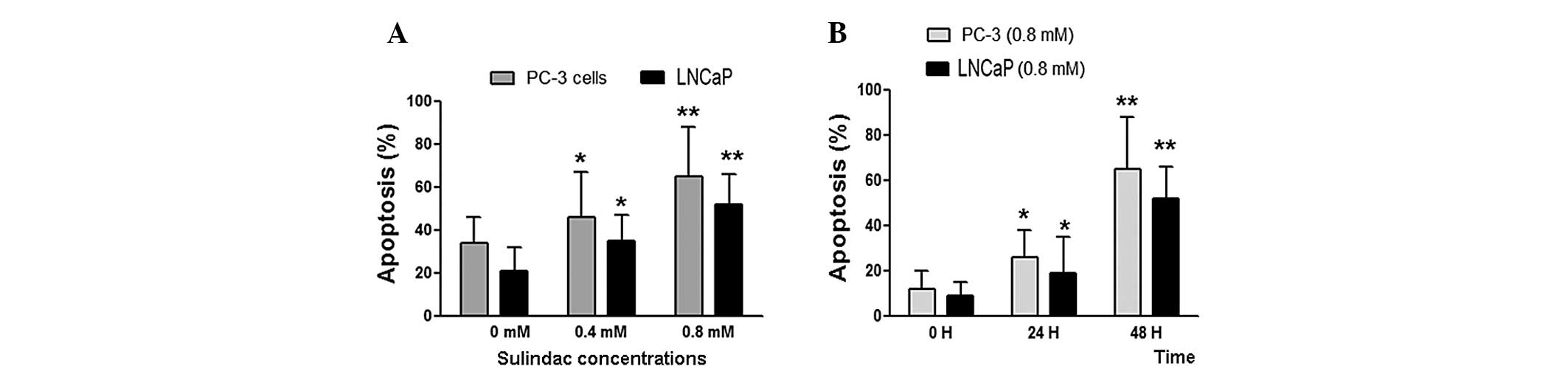
To determine the effects of sulindac on prostate
cancer cell apoptosis, PC-3 and LNCaP cells were treated with
various concentrations of sulindac (0, 0.4 and 0.8 mM) for 48 h.
Sulindac was found to significantly induce cell apoptosis in the
two cell lines (Fig. 1A; P<0.05
at 0.4 mM and P<0.01 at 0.8 mM) compared with the untreated
groups. To determine if the apoptosis was induced in a
time-dependent manner, the cells were treated with 0.8 mM sulindac
for 24 and 48 h. As shown in Fig.
1B, following 24 h of treatment, sulindac was found to promote
apoptosis in the PC-3 and LNCaP cells (P<0.05), however,
following 48 h of treatment, the induction of apoptosis was more
significant (P<0.01) compared with the untreated groups. These
results indicated that sulindac induces apoptosis in a dose- and
time-dependent manner.
Sulindac inhibits human prostate cancer
cell proliferation
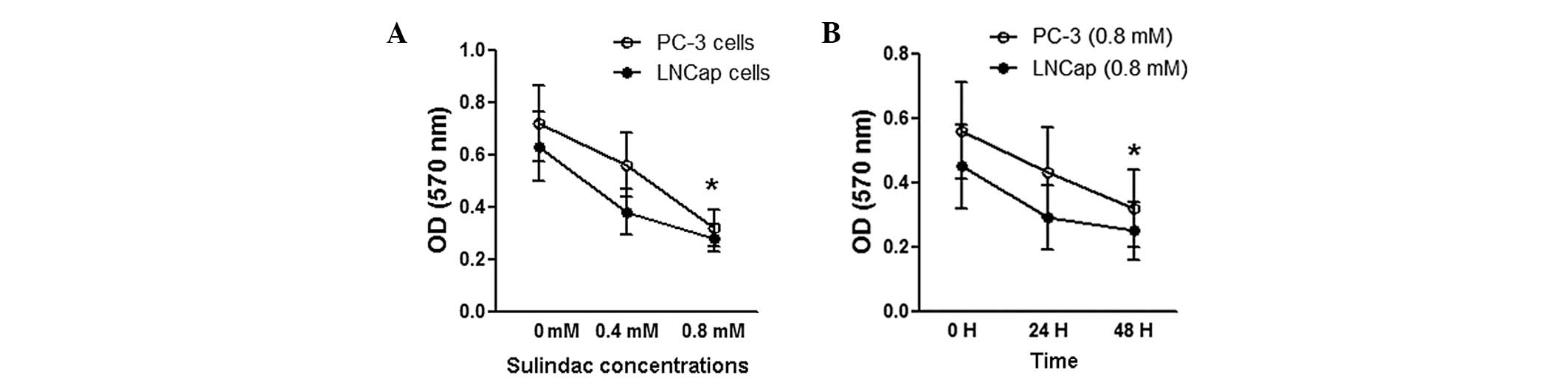
Next, the effects of sulindac on prostate cancer
cell proliferation were determined. As shown in Fig. 2A, 0.8 mM sulindac was found to
significantly inhibit cell proliferation in the PC-3 and LNCaP cell
lines (P<0.05). However, 0.4 mM sulindac also inhibited cell
proliferation in the two cell lines (P>0.05) in comparison with
the untreated groups as determined by MTT. In addition, 0.8 mM
sulindac was found to significantly inhibit cell proliferation
following 48 h treatment (P<0.05) compared with the untreated
groups (Fig. 2B). These results
indicated that cell proliferation inhibition by sulindac occurred
in a dose- and time-dependent manner.
Sulindac inhibits β-catenin/TCF signaling
in human prostate cancer cells
Our previous studies demonstrated that sulindac
inhibits colorectal cancer cell proliferation via Wnt-β-catenin/TCF
signaling (6). In the current
study, it was determined that sulindac exhibits similar mechanisms
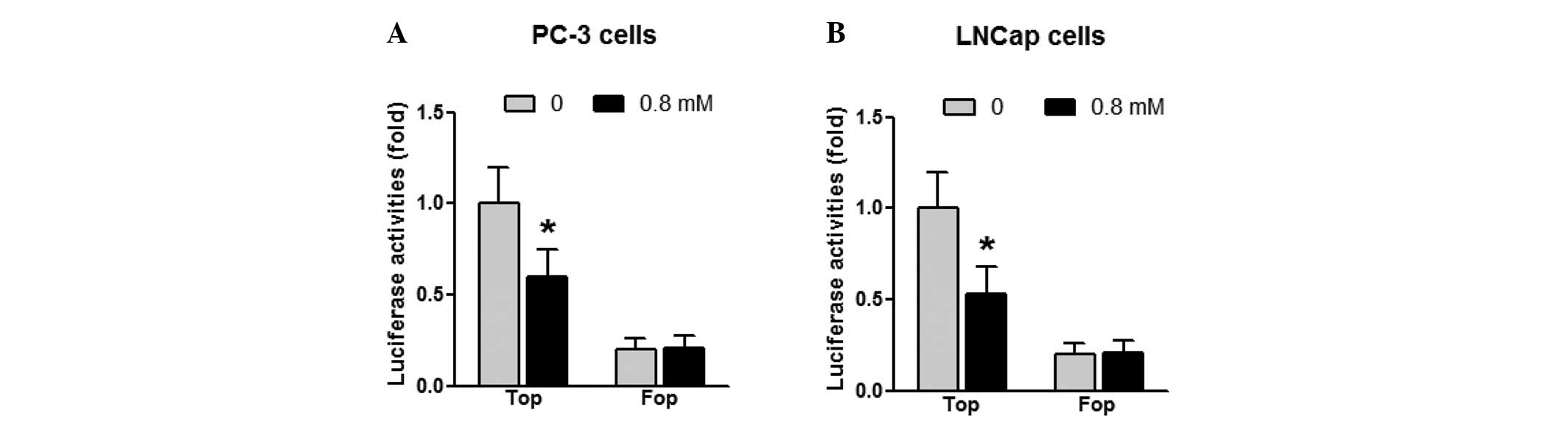
within prostate cancer cells. Cells were transiently transfected
with a β-catenin-TCF luciferase reporter construct, TOP-Flash,
which contains multiple optimal TCF/lymphocyte enhancing factor
(LEF) binding sites that induce transcription of a luciferase
reporter gene when activated by β-catenin, or a negative control
FOP-Flash, which contains mutant and inactivated TCF/LEF binding
sites. As shown in Fig. 3, sulindac
was found to inhibit β-catenin-TCF luciferase reporter activities
by ~40% in PC-3 cells and ~50% in LNCaP cells (P<0.05) compared
with the untreated cells.
Sulindac suppresses β-catenin expression
and induces JNK1 phosphorylation in human prostate cancer
cells
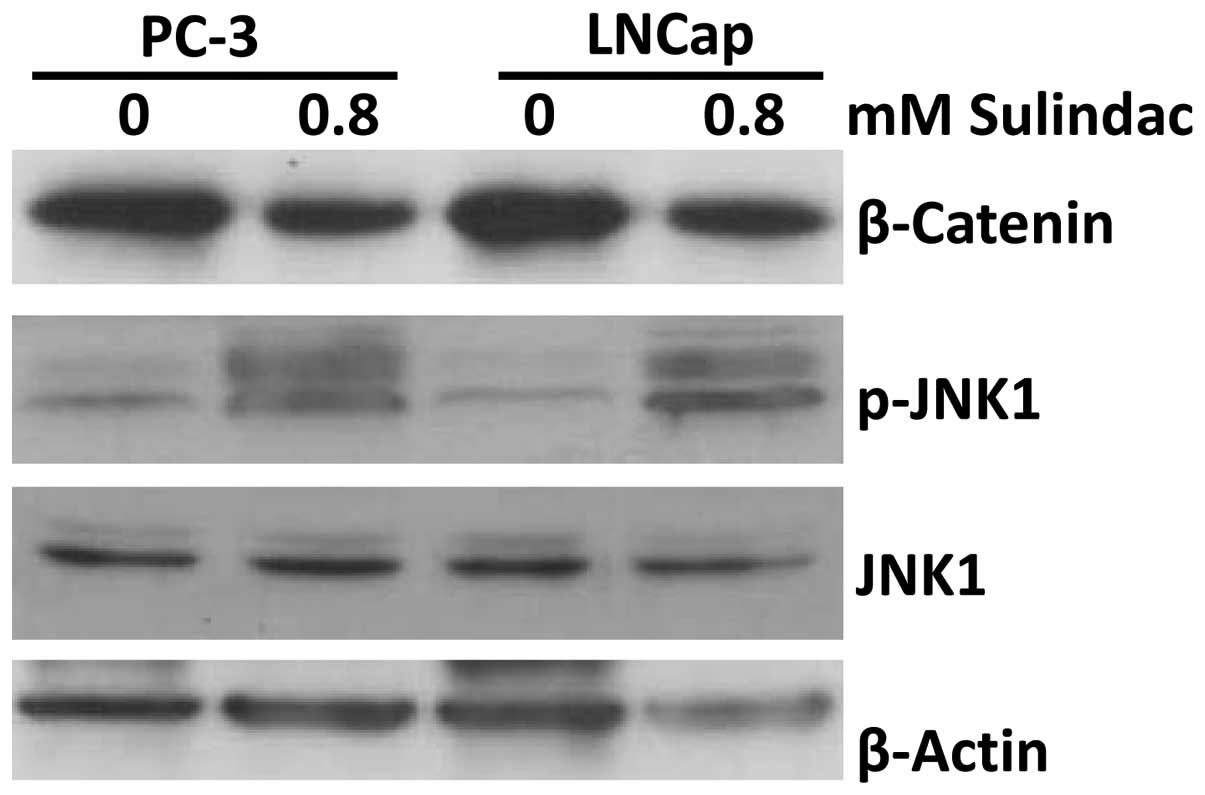
To determine the mechanism of the sulindac-mediated
induction of apoptosis and inhibition of cell proliferation in
prostate cancer cells, the changes in β-catenin and JNK1
phosphorylation were investigated. Following 48 h of treatment, 0.8
mM sulindac was found to suppress β-catenin expression and induce
JNK1 phosphorylation (an activated form of JNK1) in the PC-3 and
LNCaP cells (Fig. 4).
Discussion
Previous studies have shown the cancer preventive
activities of sulindac on gastrointestinal cancers (4,5). The
current study demonstrated that sulindac also exhibits an
anticancer function within human prostate cancer cells via the
promotion of cancer cell apoptosis and inhibition of cell
proliferation, which was associated with the suppression of
β-catenin/TCF signaling and increased JNK1 phosphorylation.
In addition to the preventive effect of sulindac on
colorectal cancer, sulindac also exerts tumor inhibition on human
lung and breast cancer cells (6).
The present study provides evidence that sulindac influences cancer
inhibition in prostate cancer cells. Similar to colorectal, lung
and breast cancer, in prostate cancer cells, β-catenin is highly
expressed and may present a therapeutic target for sulindac.
Furthermore, sulindac was found to significantly suppress β-catenin
expression at the translational and transcriptional levels, as
determined by the inhibition of TOP-Flash, a vector containing
multiple optimal TCF/LEF binding sites that induce transcription of
a luciferase reporter gene when β-catenin is activated.
Our previous studies demonstrated that β-catenin is
negatively regulated by p-JNK1 in colorectal cancers (20,21).
The current study also showed that the suppression of β-catenin by
sulindac is associated with increased levels of p-JNK1, although
total JNK1 levels were not changed, which provides increased
evidence of the involvement of sulindac in tumor inhibition by
targeting the JNK1/β-catenin signaling pathway.
JNK1 has multiple functions in cell processing,
particularly in response to stress, and mediates cell apoptosis and
regulates cell maturation in the gastrointestinal tract (14). Based on the induction of cell
apoptosis and inhibition of cell proliferation by sulindac, we
hypothesize that these functions of sulindac may be associated with
increased JNK1 phosphorylation and suppression of β-catenin in
human prostate cancer cells.
In conclusion, sulindac exhibits anticancer
activities in human prostate cancer cells by promoting apoptosis
and inhibiting cell proliferation by targeting the JNK1/β-catenin
signaling pathway. These findings indicate that sulindac may be a
potential agent for prostate cancer prevention or therapy.
Acknowledgements
This study was supported in part by the Doctoral
Startup Fund (grant no. 505011) from the Xinxiang Medical
University (Xinxiang, China).
Abbreviations:
|
JNK1
|
c-Jun NH2-terminal kinase 1
|
References
|
1
|
Siegel R, Ward E, Brawley O and Jemal A:
Cancer statistics, 2011: the impact of eliminating socioeconomic
and racial disparities on premature cancer deaths. CA Cancer J
Clin. 61:212–236. 2011.
|
|
2
|
Clevers H: Colon cancer - understanding
how NSAIDs work. N Engl J Med. 354:761–763. 2006.
|
|
3
|
Kelloff GJ, Lippman SM, Dannenberg AJ,
Sigman CC, Pearce HL, Reid BJ, Szabo E, Jordan VC, Spitz MR, Mills
GB, et al; AACR Task Force on Cancer Prevention. Progress in
chemoprevention drug development: the promise of molecular
biomarkers for prevention of intraepithelial neoplasia and cancer -
a plan to move forward. Clin Cancer Res. 12:3661–3697. 2006.
|
|
4
|
Yang W, Bancroft L and Augenlicht LH:
Methylation in the p21WAF1/cip1 promoter of Apc+/−, p21+/− mice and
lack of response to sulindac. Oncogene. 24:2104–2109. 2005.
|
|
5
|
Yang W, Bancroft L, Liang J, Zhuang M and
Augenlicht LH: p27kip1 in intestinal tumorigenesis and
chemoprevention in the mouse. Cancer Res. 65:9363–9368. 2005.
|
|
6
|
Han A, Song Z, Tong C, Hu D, Bi X,
Augenlicht LH and Yang W: Sulindac suppresses β-catenin expression
in human cancer cells. Eur J Pharmacol. 583:26–31. 2008.
|
|
7
|
Song Z, Tong C, Liang J, Dockendorff A,
Huang C, Augenlicht LH and Yang W: JNK1 is required for
sulindac-mediated inhibition of cell proliferation and induction of
apoptosis in vitro and in vivo. Eur J Pharmacol. 560:95–100.
2007.
|
|
8
|
Bi X, Pohl N, Dong H and Yang W: Selenium
and sulindac are synergistic to inhibit intestinal tumorigenesis in
Apc/p21 mice. J Hematol Oncol. 6:82013.
|
|
9
|
Chang L and Karin M: Mammalian MAP kinase
signalling cascades. Nature. 410:37–40. 2001.
|
|
10
|
Bode AM and Dong Z: The functional
contrariety of JNK. Mol Carcinog. 46:591–598. 2007.
|
|
11
|
Davis RJ: Signal transduction by the JNK
group of MAP kinases. Cell. 103:239–252. 2000.
|
|
12
|
Weston CR and Davis RJ: The JNK signal
transduction pathway. Curr Opin Cell Biol. 19:142–149. 2007.
|
|
13
|
Weston CR, Lambright DG and Davis RJ:
Signal transduction. MAP kinase signaling specificity. Science.
296:2345–2347. 2002.
|
|
14
|
Tong C, Yin Z, Song Z, Dockendorff A,
Huang C, Mariadason J, Flavell RA, Davis RJ, Augenlicht LH and Yang
W: c-Jun NH2-terminal kinase 1 plays a critical role in intestinal
homeostasis and tumor suppression. Am J Pathol. 171:297–303.
2007.
|
|
15
|
Bode AM and Dong Z: Signal transduction
pathways in cancer development and as targets for cancer
prevention. Prog Nucleic Acid Res Mol Biol. 79:237–297. 2005.
|
|
16
|
Liu J and Lin A: Role of JNK activation in
apoptosis: a double-edged sword. Cell Res. 15:36–42. 2005.
|
|
17
|
Dong C, Yang DD, Wysk M, Whitmarsh AJ,
Davis RJ and Flavell RA: Defective T cell differentiation in the
absence of Jnk1. Science. 282:2092–2095. 1998.
|
|
18
|
Kuan CY, Yang DD, Samanta Roy DR, Davis
RJ, Rakic P and Flavell RA: The Jnk1 and Jnk2 protein kinases are
required for regional specific apoptosis during early brain
development. Neuron. 22:667–676. 1999.
|
|
19
|
Liu J, Minemoto Y and Lin A: c-Jun
N-terminal protein kinase 1 (JNK1), but not JNK2, is essential for
tumor necrosis factor alpha-induced c-Jun kinase activation and
apoptosis. Mol Cell Biol. 24:10844–10856. 2004.
|
|
20
|
Hu D, Bi X, Fang W, Han A and Yang W:
GSK3beta is involved in JNK2-mediated beta-catenin inhibition. PLoS
One. 4:e66402009.
|
|
21
|
Hu D, Fang W, Han A, Gallagher L, Davis
RJ, Xiong B and Yang W: c-Jun N-terminal kinase 1 interacts with
and negatively regulates Wnt/beta-catenin signaling through
GSK3beta pathway. Carcinogenesis. 29:2317–2324. 2008.
|