Introduction
Giant cell tumor (GCT) of bone is locally aggressive
and generally occurs in the meta-epiphyseal region of long bones.
In the USA, GCT accounts for ~20% of all primary bone lesions, with
a similar occurence in Asia (1–3). The
presenting symptom of GCT is pain accompanied by deformity,
swelling and limited joint function at the affected extremity.
Occasionally, symptoms from nerve compression and pathological
fracture are also identified. Shi et al (4) reported that the 5-, 10- and 15-year
survival rates were 97, 93 and 81%, respectively, following
radiotherapy treatment. The treatment of GCT is often complicated
with local recurrence. Intralesional curettage is the standard of
treatment for primary GCTs. Due to the high incidence of recurrence
and metastasis associated with GCT, local adjuvant therapies, such
as phenol or liquid nitrogen zoledronic acid, have been recommended
(5–7). However, at present, there are no
effective methods to prevent local recurrence and metastasis.
Ultrasonic scalpels may be used to cut tissue and simultaneously
avoid bleeding. Therefore, these instruments have been widely used
in laparoscopic surgery. Based on the unique effect of the
ultrasonic scalpel, it has been utilized to treat bone tumors
(8). In the past five years, we
have experienced successful treatment of GCT of long bones using
this technique (9). Therefore, the
present study aimed to investigate the advantages and long-term
outcomes of ultrasonic scalpel in the treatment of GCT of long
bones.
Patients and methods
Patients
This study retrospectively analyzed 32 patients with
GCT of long bones, including 24 male cases and 8 female cases, who
presented at the Beijing Ditan Hospital, Capital Medical University
(Beijing, China) between February 2004 and February 2007. The age
ranged from 8 to 34 years old (mean age, 23.5 years old), and the
32 cases of GCT were randomly divided into observation group (n=10)
and control group (n=22). The 10 cases of the observation group
included eight males and two females, with an age range of 8–28
years old (mean age, 22 years old). Among these 10 cases, the tumor
occurrence sites were as follows: Four cases in the distal femur,
two in the proximal femur, three in the proximal tibia and one in
the proximal humerus. Additionally, one case with proximal femur
GCT and one case with proximal humerus GCT presented with
pathological fracture. The 22 cases of the control group included
16 males and six females, with an age range of 10–34 years old
(mean age, 24.2 years old); The tumor occurrence sites of the
control group may be broken down as follows: Eight cases in the
distal femur, six cases in the proximal femur, seven cases in the
proximal tibia and one case in the proximal humerus.
Plain radiographs, chest X-ray, computed tomography
(CT) and/or magnetic resonance imaging (MRI) were performed on more
than one plane in all patients. In addition, all patients received
fine needle aspiration cytology and/or open biopsy. The thickness
of the subchondral bone at the adjacent articular surface was
measured, and clinical and radiographic examinations were performed
regularly in the follow-up study.
The two GCT groups received intralesional curettage
followed by local methotrexate treatment and bone grafting. While
the observation group underwent ultrasonic scalpel for
intralesional curettage.
Routine postoperative follow-up examinations were
performed at 1, 3 and/or 6 months and thereafter every 6 months for
3 years. Following this, no further follow-up examination was
routinely scheduled. Patients who did not experience recurrence
were censored at the last follow-up study, and the mean duration of
follow-up was 78 months (range, 60–96 months). Routine follow-up
study included clinical examination and conventional radiography at
the operative site. CT and MRI were used for further investigation
when radiography demonstrated a suspected relapse (such as graft or
bone resorption, expansile change and local soft tissue swelling or
mass formation) or when clinical symptoms and signs showed
recurrence despite negative radiography. In addition, a plain
radiograph or CT of the chest was performed to exclude metastasis.
Informed consent was obtained from all patients.
Ultrasonic scalpel
The Exploiter™ ultrasonic scalpel (UOSS-II) was
purchased from Beijing Beyonder Technologies Co., Ltd. (Beijing,
China) and consists of three parts: The main engine, the hand shank
and burr and the cooling system. The signal generator is controlled
by the ultrasonic frequency electrical signal from the computer.
Following amplification by the power amplifier, the electrical
signal drives the ultrasonic transducer. Subsequently, the
ultrasonic transducer produces a vibratory motion. The ultrasonic
amplitude transformer amplifies the amplitude and drives the cutter
to function. The operational frequency is 40±2 kHz. In the present
study, real-time automatic frequency tracking was performed and the
amplitude of the cutter was <300 μm. Additionally, 3- and 2-mm
burrs were equipped with cutting teeth and notches, respectively,
which were suited to the different requirements of burring. The
ultrasonic energy output was set to 30% and the handle was equipped
with a cooling system. Cutting tools could take the clockwise or
anticlockwise and reciprocal rotation, alternately, to increase the
burring ability (8).
Surgical procedures
According to the patient’s condition, they were
anesthetized by local anesthesia or general anesthesia, as
appropriate. The preferred treatment of primary GCTs was
intralesional curettage with high-speed ultrasonic scalpel of the
tumor cavity, to improve the thoroughness of tumor removal,
combined with local methotrexate gelfoam adjuvant treatment and
filling of the cavity with allograft and/or homograft bone. This
procedure began with sufficient fenestration as well as repeatedly
scraping the inner wall of the tumor until the tumor tissue was
completely invisible to the naked eye. The normal bone and
epiphysial bone lamella were carefully reserved. Following this,
the surgical area was rinsed repeatedly with physiological saline
and then methotrexate regional chemotherapy was applied with a
gelatin sponge fixed with methotrexate. For the bone
transplantation, the size of the bone cavity was measured and
autogenous iliac bone was harvested. If the bone cavity was too
large for this, allogeneic freeze-dried bone (Osteolink Biomaterial
Co., Ltd., Hubei, China) was used. One case with proximal femur GCT
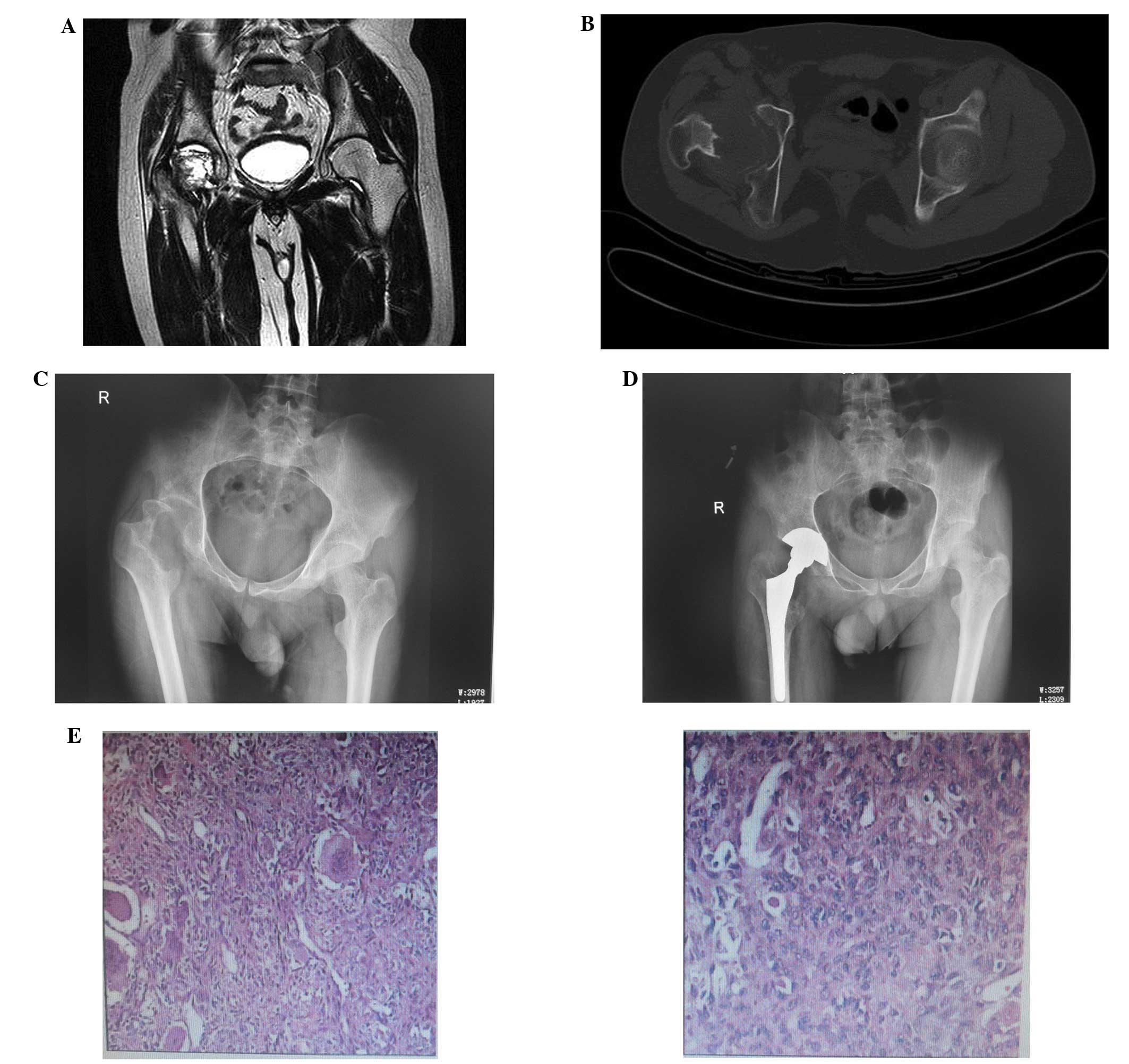
exhibited a pathological fracture; tumor resection and artificial
total hip replacement were conducted for this patient. Furthermore,
one case of proximal humerus GCT exhibited a pathological fracture,
for which external fixation was employed. The control group
underwent the same procedure, however rather than using the
ultrasonic scalpel to scrape the inner wall of the tumor, this was
undertaken using curettes.
Statistical analysis
The Statistical Package for the Social Sciences,
version 13.0 (SPSS, Inc., Chicago, IL, USA) was used for
statistical calculations. All data are presented as the mean ±
standard deviation. Student’s t-test was used to compare the means
between the two groups, and P<0.05 was considered to indicate a
statistically significant difference.
Results
Operation method
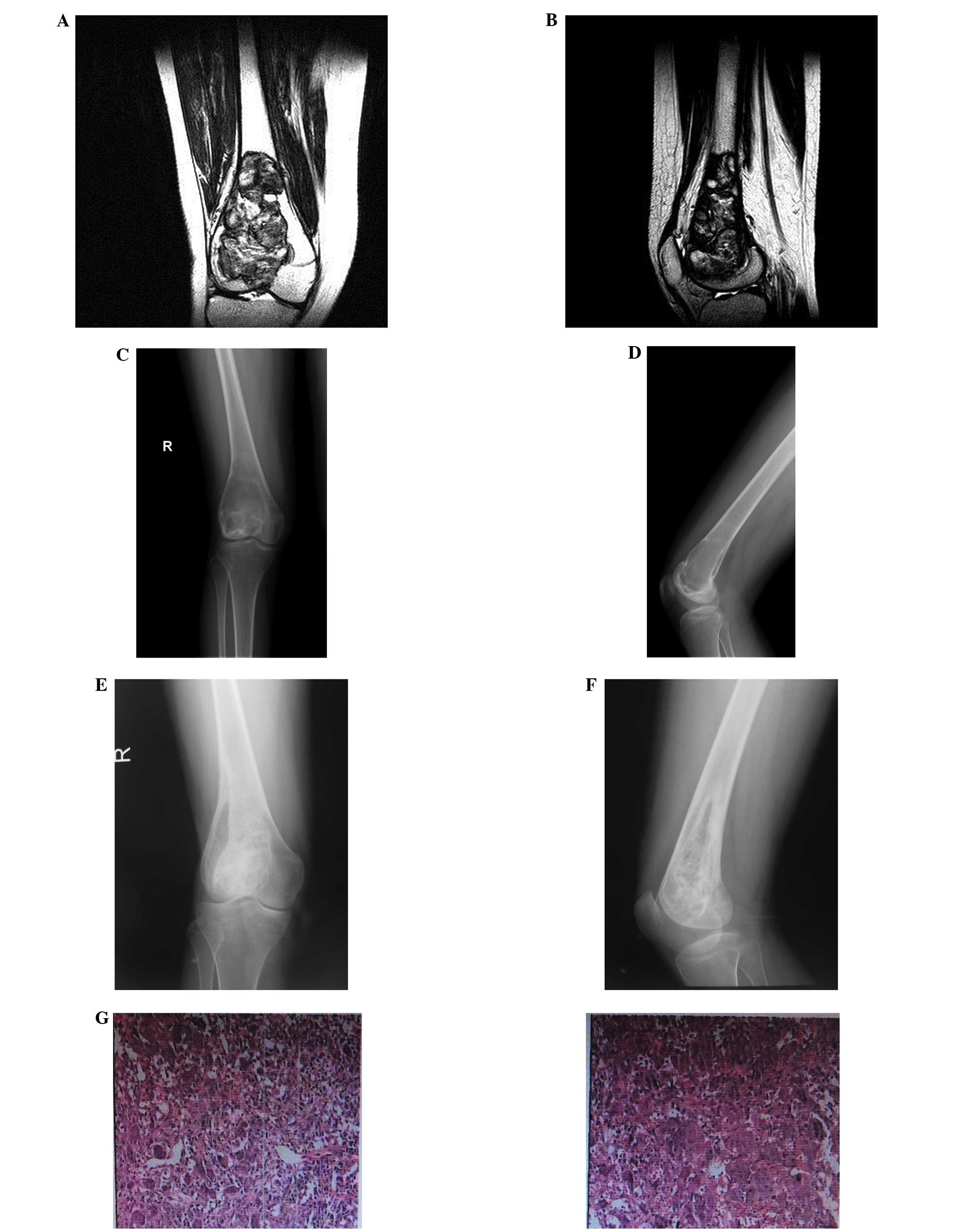
In total, 10 patients with GCT of the long bones
received ultrasonic scalpel treatment of the tumor cavity, to
improve the thoroughness of tumor removal, followed by local
methotrexate gelfoam adjuvant treatment and filling of the cavity
with allograft and/or homograft bone. The average bone cavity
volume was 25.5 ml in observation group.
The procedure used for the observation group was
successful. The time required for the procedure was shorter in the
observation group (mean, 15 min) compared with that of the control
group (mean, 30 min) due to the use of curettes in the control
group. In the control group the field of view was unclear due to a
high level of bleeding, which led to incomplete tumor removal and
slight damage to the normal tissue.
Bone healing
No rejection reaction and bone resorption phenomenon
were observed in the autogenous iliac bone and allogeneic
freeze-dried bone mix filling. In addition, the allograft
reconstruction was successful. One case of GCT of the proximal
femur received a total hip replacement, while another case of GCT
in the proximal humerus received external fixation. The two cases
achieved primary healing.
Recurrence
Following surgery, tumor local recurrence and
distant metastasis were not identified during the 5–8 years of
follow-up among patients in the observation group; however, six
cases of the control group showed recurrence following surgery,
however, no distant metastasis was idetnified (P<0.05).
All 10 cases in the observation group demonstrated
good bone repair and no physical deformities, partial collapse,
fracture, obvious functional issues or rejection were observed
(Figs. 1 and 2).
Discussion
Giant cell tumor (GCT) of bone is a rare benign
tumor that predominantly occurs in the meta-epiphyseal region of
the long bones. GCT results in disability and may be associated
with a relatively high local recurrence rate (10). Chemicals (phenol and alcohol) and
thermal procedures (cryotherapy and bone cement filling) have been
used as adjuvants to eliminate tumor remnants. Surgical treatment
options for GCT include intralesional curettage and segmental
resection (7). The rate of
recurrence following wide resection of bone GCTs is 6.25% (11). The overall recurrence rate of
intralesional curettage was 32%. Implantation of
polymethylmethacrylate instead of bone grafting has been
demonstrated to be associated with a lower risk of subsequent
recurrence in intralesional procedures (14 versus 50%; age range
between 18.5 and 40 years) (7).
However, it is not suitable for younger patients (<18.5 years
old). Curettage combined with adjuvant treatment has been shown to
reduce the recurrence rate to ~10%. At present, local adjuvant
treatment including hyperthermia (microwave or electricity),
cryotherapy (liquid nitrogen), chemical reagent daub or soaking
(phenol, liquid nitrogen, carbolic acid, alcohol, 50% zinc
chloride, hydrogen peroxide or zoledronic acid), high-speed
abrasive drilling and pulse-rinsing can clean the tumor tissue well
(12–18). The ultrasonic scalpel has developed
rapidly in recent years, and owing to its selective fragmentation,
low injury rate, high accuracy and the unique advantage of avoiding
bleeding, it has been applied in orthopedics (9,19).
The functions of the ultrasonic scalpel in the human
body include heating and cavitation, mechanical, thixotropic,
dispersion, fragmentation and hemostatic effects (20,21).
Three of these functions in particular, fragmentation, cavitation
effect and homeostatic effect, are widely used by surgeons.
Ultrasonic cutting capacity varies according to the type of tissue
found in different organizational structures and their different
water contents. Generally speaking, for hard or fibrous tissue, the
ultrasonic burring function mainly exerts a fracturing effect,
whereas for soft tissue or tissues with a high water content, it
mainly exerts a cavitation effect.
During the process of fragmentation, the ultrasound
propagating to the tissue causes elastic vibration (22). When the vibration acceleration
reaches the cutting threshold of 50,000 × g, the biological tissue
is broken due to the sharp vibration and is stripped from the
surrounding tissue. The cutting threshold of 20 KHz must be reached
prior to using the scalpel, and the amplitude must be >40 μm.
Fragmentation plays a leading role in surgical procedures such as
craniotomy and spinal decompression.
In soft tissue, such as brain and liver tumors,
which has a higher water content, a large amount of bubbles are
produced by ultrasound. The inner and outer pressure difference of
these bubbles can reach several kilobars (1 bar=106
dyne/cm2). When these bubbles burst, the tissue is
emulsified, which is known as the cavitation effect. The cavitation
effect is closely associated with water content, and so the effect
is tissue-selective (23). Owing to
this feature, peripheral nerves and blood vessels cannot be
incidentally damaged whilst cutting tissues such as liver and brain
tumors (24,25). This feature of the ultrasonic
scalpel renders it superior to other surgical instruments in
use.
The present retrospective analysis indicates that
the most efficient way to avoid multiple recurrences of GCT of long
bone is by ultrasonic scalpel treatment of the tumor cavity,
combined with local methotrexate gelfoam adjuvant treatment and
filling with allograft and/or homograft bone. Thus, this procedure
may be a suitable choice to minimize the risk of multiple
recurrences and pulmonary metastases.
The current study identified that the ultrasonic
scalpel can reduce the difficulty of the surgical procedure and
shorten the operating time. The effect of burring and damaging the
tumor tissue was more effective, and the ultrasonic scalpel makes
the surgery safer. The working temperature of the scalpel is
70–80°C, which is sufficient to destroy the tumor cells (26,27).
In addition, the surface of the wound and the bone graft were found
to heal at a normal rate in the current study. When the ultrasonic
scalpel is in operation, its working temperature can promote the
solidification of hemoglobin, rendering simultaneous homeostasis.
Compared with electric cutting and coagulation, there is less
smoke, an absence of eschars and a clearer surgical field. The
ultrasonic scalpel has a unique property, which is that the
separation, hemostasis and cutting can work together in one machine
(28,29). The device can damage and remove the
tumor more completely than intralesional curettage without any
damage of the normal tissue. Ultrasonic scalpel has a good
application prospect due to its safety, easy control and good
application effect (30).
We think that the advantages of using ultrasonic
scalpel in the treatment of GCT were mainly due to its
fragmentation and cavitation effects. These two functions can
thoroughly clean the tumor cavity tissue even in the depth of
normal bone, completely remove the source of the tumor and create a
good bone graft bed. In the present patient cohort, the bone healed
rapidly and there was no tumor recurrence or metastasis.
Additionally, ultrasonic scalpel avoids the disadvantages of
traditional treatment methods, including the fact that the tumor
tissue cannot be removed thoroughly, the normal bone can undergo
necrosis and the normal bone healing is delayed. The 10 cases
treated with ultrasonic scalpel in the present 5- to 8-year
follow-up study had no recurrence, which was an improved outcome
compared with that of traditional surgery. As the sample size was
small and the follow-up time was short, further study is required
to determine the clinical significance of the present study
findings.
References
|
1
|
Chakarun CJ, Forrester DM, Gottesgen CJ,
et al: Giant cell tumor of bone: review, mimics, and new
developments in treatment. Radiographics. 33:197–211. 2013.
|
|
2
|
Thomas DM and Skubitz KM: Giant cell
tumour of bone. Curr Opin Oncol. 21:338–344. 2009.
|
|
3
|
Sung HW, Kuo DP, Shu WP, et al: Giant-cell
tumor of bone: analysis of two hundred and eight cases in Chinese
patients. J Bone Joint Surg Am. 64:755–761. 1982.
|
|
4
|
Shi W, Indelicato DJ, Reith J, Smith KB,
Morris CG, Scarborough MT, Gibbs CP Jr, Mendenhall WM and Zlotecki
RA: Radiotherapy in the management of giant cell tumor of bone. Am
J Clin Oncol. 36:505–508. 2013.
|
|
5
|
Nishisho T, Hanaoka N, Endo K, Takahashi M
and Yasui N: Locally administered zoledronic Acid therapy for giant
cell tumor of bone. Orthopedics. 34:e312–e315. 2011.
|
|
6
|
Kafchitsas K, Habermann B, Proschek D,
Kurth A and Eberhardt C: Functional results after giant cell tumor
operation near knee joint and the cement radiolucent zone as
indicator of recurrence. Anticancer Res. 30:3795–3799. 2010.
|
|
7
|
Klenke FM, Wenger DE, Inwards CY, Rose PS
and Sim FH: Recurrent giant cell tumor of long bones: analysis of
surgical management. Clin Orthop Relat Res. 469:1181–1187.
2011.
|
|
8
|
Amaral JF: Laparoscopic cholecystectomy in
200 consecutive patients using an ultrasonically activated scalpel.
Surg Laparosc Endosc. 5:255–262. 1995.
|
|
9
|
Zhang Q, Zou DW, Ma HS and Hai Y:
Application of ultrasonic scalpel in treatment of giant cell tumor
of the long bones. Zhongguo Jiao Xing Wai Ke Za Zhi. 15:1181–1183.
2007.
|
|
10
|
Arbeitsgemeinschaft Knochentumoren. Becker
WT, Dohle J, et al: Local recurrence of giant cell tumor of bone
after intralesional treatment with and without adjuvant therapy. J
Bone Joint Surg Am. 90:1060–1067. 2008.
|
|
11
|
Liu HS and Wang JW: Treatment of giant
cell tumor of bone: a comparison of local curettage and wide
resection. Changgeng Yi Xue Za Zhi. 1:37–43. 1998.
|
|
12
|
Zhen W, Yaotian H, Songjian L, Ge L and
Qingliang W: Giant cell tumour of bone: The long term results of
treatment by curettage and bone graft. J Bone Joint Surg Br.
86:212–216. 2004.
|
|
13
|
Hoch B, Inwards C, Sundaram M and
Rosenberg AE: Multicentric giant cell tumor of bone.
Clinicopathologic analysis of thirty cases. J Bone Joint Surg Am.
88:1998–2008. 2006.
|
|
14
|
Vult von Steyern F, Bauer HC, Trovik C, et
al: Treatment of local recurrences of giant cell tumour in long
bones after curettage and cementing. A Scandinavian Sarcoma Group
study. J Bone Joint Surg Br. 88:531–535. 2006.
|
|
15
|
Turcotte RE: Giant cell tumor of bone.
Orthop Clin North Am. 37:35–51. 2006.
|
|
16
|
Fraquet N, Faizon G, Rosset P, Phillipeau
JM, Waast D and Gouin F: Long bones giant cells tumors: treatment
by curretage and cavity filling cementation. Orthop Traumatol Surg
Res. 95:402–406. 2009.
|
|
17
|
Campanacci DA, Scoccianti G, Beltrami G,
Mugnaini M and Capanna R: Ankle arthrodesis with bone graft after
distal tibia resection for bone tumors. Foot Ankle Int.
29:1031–1037. 2008.
|
|
18
|
Sakayama K, Sugawara Y, Kidani T, et al:
Diagnostic and therapeutic problems of giant cell tumor in the
proximal femur. Arch Orthop Trauma Surg. 127:867–872. 2007.
|
|
19
|
Al-Mahfoudh R, Qattan E, Ellenbogen JR, et
al: Applications of the ultrasonic bone cutter in spinal surgery -
our preliminary experience. Br J Neurosurg. 28:56–60. 2014.
|
|
20
|
Ying C, Zhaoying Z and Ganghua Z: Effects
of different tissue loads on high power ultrasonic surgery scalpel.
Ultrasound Med Biol. 32:415–420. 2006.
|
|
21
|
Chen Y, Luo XN, Shi WY and Zhou ZY: The
application and development of ultrasonic scalpel. J Biomed Eng.
22:377–380. 2005.
|
|
22
|
Yilmaz KB, Dogan L, Nalbant H, et al:
Comparing scalpel, electrocautery and ultrasonic dissector effects:
the impact on wound complications and pro-inflammatory cytokine
levels in wound fluid from mastectomy patients. J Breast Cancer.
14:58–63. 2011.
|
|
23
|
Hodgson WJ: The ultrasonic scalpel. Bull N
Y Acad Med. 55:908–915. 1979.
|
|
24
|
Abbasoglu O and Sayek I: Parenchymal
transection with ultrasonic scalpel in liver resection. HPB
(Oxford). 5:167–169. 2003.
|
|
25
|
Kanzaki J, Inoue Y, Kurashima K and
Shiobara R: Use of the ultrasonically activated scalpel in acoustic
neuroma surgery: preliminary report. Skull Base Surg. 10:71–74.
2000.
|
|
26
|
Homayounfar K, Meis J, Jung K, et al:
Ultrasonic scalpel causes greater depth of soft tissue necrosis
compared to monopolar electrocautery at standard power level
settings in a pig model. BMC Surg. 12:32012.
|
|
27
|
Yuan CH, Xiu DR, Jia YM, Xiong JW and
Zhang TL: Laparoscopic liver tumor resection of clinical experience
in 126 patients. Zhonghua Wai Ke Za Zhi. 51:776–779. 2013.(In
Chinese).
|
|
28
|
Fette A, Schleef J, Haberlik A and
Seebacher U: Circumcision in paediatric surgery using an ultrasound
dissection scalpel. Technol Health Care. 8:75–79. 2000.
|
|
29
|
Bensaha T: A new approach for the surgical
exposure of impacted canines by ultrasonic surgery through soft
tissue. Int J Oral Maxillofac Surg. 42:1557–1561. 2013.
|
|
30
|
Higami T: Ultrasonic scalpel. Kyobu Geka.
62:612–616. 2009.(In Japanese).
|