Introduction
Colorectal cancer (CRC) is the third most common
malignancy in the world and is one of the most frequent
malignancies in China (1). In
Shanghai, Beijing and other major cities, the increase in the
incidence of CRC has been much faster compared with Western
countries, and is associated with a corresponding rise in CRC
mortality. This is the result of changes in living standards and
diet coupled with the effect of genetic factors. Thus, the
development of CRC is the result of an interaction between genetic,
lifestyle and environmental factors.
Adenomatous polyposis coli (APC) has been identified
as a tumor suppressor gene, located on chromosome 5q21-22, with a
full-length of 11,025 bp. The cDNA sequence of APC is composed of
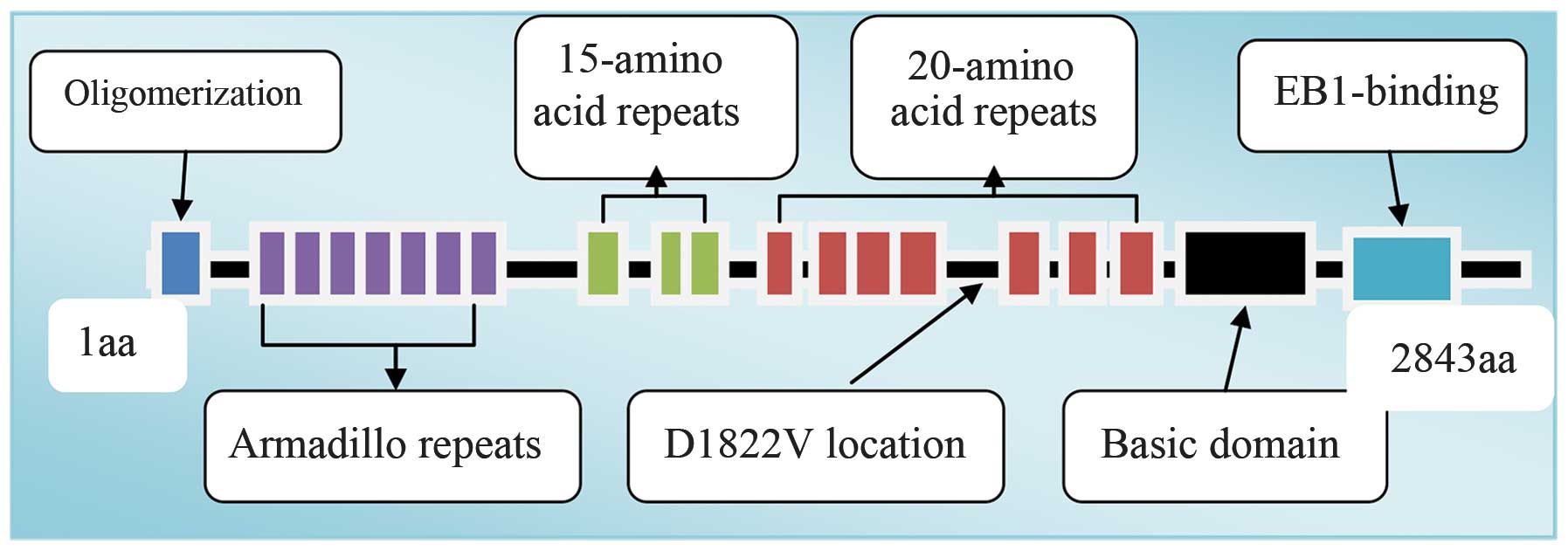
8,538 nucleotides that encode 2,843 amino acids (2). The APC protein has multiple functional
areas, including an oligomerization and armadillo area in the
N-terminal region, 15 and 20 AA nucleotide repeated sequences in
the intermediate region and a conserved area that connects
end-binding protein 1 and human disc-large protein in the
C-terminal region (3) (Fig. 1).
Gene polymorphisms define the predisposition to
human disease that arises from variations in genome sequences
(4,5). Polymorphisms also account for
different responses to medications and varied responses to
environmental factors (6,7). The most common and simple form of gene
polymorphisms are single nucleotide DNA sequence polymorphisms at
the level of the genome.
Mutations in the APC gene have been shown to be
responsible for the autosomal dominant inherited disease, familial
adenomatous polyposis (FAP). The types of mutation include missense
mutations, small insertions or deletions. These mutations result in
the early appearance of the stop codon, with a subsequent
generation of truncated APC protein (8), and aberrant cellular proliferation,
leading to the early stages of CRC. Recent reviews and
meta-analyses have indicated that APC is a candidate gene for the
susceptibility to colorectal neoplasia (9). Codon 1,822 of the APC gene is
identified by an A-to-T transversion, which causes a change in the
amino acid sequence from aspartate to valine.
These findings indicate that it is possible that a
single nucleotide polymorphism (SNP) may form the genetic basis for
different individuals with FAP, with similar lifestyles to each
other, having varied susceptibilities to CRC. D1822V is the most
common APC variant described in the literature (10). However, no studies have investigated
the association between this variant and the susceptibility of
sporadic CRC in the Chinese mainland population. The present study
was, therefore, undertaken to explore this correlation and to
determine whether D1822V variants are associated with CRC in the
population of Hubei, China.
Material and methods
Specimen collection
The present case-control study consisted of 196
patients with newly diagnosed and histopathologically confirmed
primary CRC who had not previously undergone radiotherapy or
chemotherapy. The patients were all admitted to the Zhong Nan
Hospital of Wuhan University (Wuhan, Hubei, China) between February
2010 and November 2011. The control group included 279
histopathologically-confirmed cancer-free subjects with no current
or previous diagnosis of cancer, who underwent health examinations
at the Zhong Nan Hospital during the same period. The cases and
controls were all residents of Hubei, and were matched for age and
gender frequency using a uniform questionnaire, which included
demographic characteristics, details of medical and family history
and other information.
The study was approved by the Investigation and
Ethics Committee of the Zhong Nan Hospital and was undertaken in
accordance with the Declaration of Helsinki 1975. All subjects
provided written informed consent prior to participating.
Genomic DNA preparation
Venous blood (20 ml) was obtained in the morning
from all the cases. Genomic DNA was isolated from whole blood using
a Blood Genome DNA Extraction kit (Tiangen Biotech (Beijing) Co.,
Ltd., Beijing, China). Ultraviolet spectrophotometry was used to
detect the content and concentration (A260/A280>1.8) of total
DNA. Agarose gel electrophoresis (Biowest Agarose G-10; Gene
Company Limited, Chi Wan, Hong Kong) was used to detect DNA
integrity. The DNA samples were diluted to 100 ng/ml in sterile
triple-distilled water and stored at 4°C.
Polymerase chain reaction (PCR) and
product determination
The upstream, 5′-ACCCAACAAAAATCAGTTAGATG-3′, and
downstream, 5′-GTGGCTGGTAACTTTAGCCTC-3′, primer were provided by
Invitrogen Life Technologies (Shanghai, China). PCR reactions were
performed using a 20-μl reaction system containing 2 μl DNA
template (100 ng/μl), 0.4 μl of each primer, 2 μl dNTPs, 0.2 μl
ExTaq enzyme, 2 μl 10× PCR Buffer and 13 μl ddH2O. The
PCR profile consisted of an initial 5-min denaturation step at
94°C, followed by 32 cycles of 30 sec at 94°C, 30 sec at 58°C and
extension for 45 sec at 72°C, and a final 10-min elongation step at
72°C. The final PCR product was 410 bp.
Agarose gel (1%) electrophoresis (Extraction kit,
Hangzhou Bioer Technology Co., Ltd., Hangzhou, Zhejiang, China) was
used to recover the PCR products. The PCR fragments were sequenced
at the Magic Biotech Company (Shanghai, China).
Statistical analysis
Data analysis was performed using SPSS version 17.0
software (SPSS, Inc., Chicago, IL, USA). Bilateral χ2
tests were used to analyze demographic variables, environmental
risk factors and APC genotype distributions between the case and
control groups. Hardy-Weinberg equilibrium for genotypic
frequencies was determined using the goodness-of-fit χ2
test. Univariate and multivariate unconditional logistic regression
analyses were used to obtain odds ratios (OR) and corresponding 95%
confidence intervals (CI). Stratified analysis was used to assess
the effect of various factors on the incidence of CRC. P<0.05
was considered to indicate a statistically significant
difference.
Results
Subject characteristics
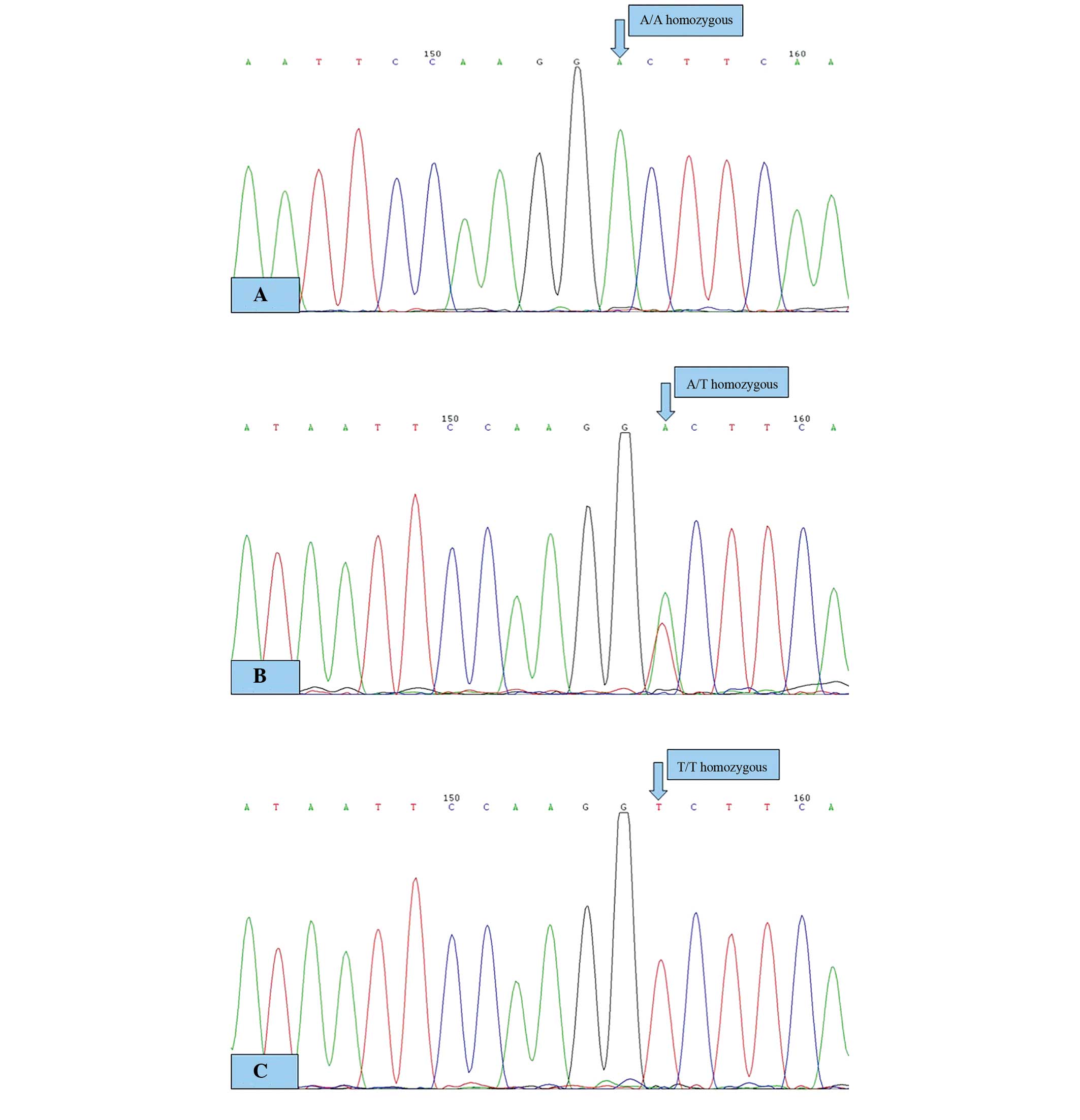
The direct sequencing histogram of the PCR products
is shown in Fig. 2. A single peak
represents homozygous products and double peaks represent
heterozygous products.
The characteristics of the cases and controls are
shown in Table I. The mean age of
the case group was 59.6±12.9 years compared with 59.2±13.0 years in
the control group (P=0.463). Similarly, there was no significant
difference in gender distribution between the cases and controls
(P=0.591).
 | Table IDemographic characteristics of the
cases (n=196) and controls (n=279). |
Table I
Demographic characteristics of the
cases (n=196) and controls (n=279).
| Characteristics | Case, n (%) | Controls, n (%) | χ2 | P-value |
|---|
| Gender |
| Male | 126 (64.29) | 185 (66.31) | 0.290 | 0.591 |
| Female | 70 (35.71) | 94 (33.69) | | |
| Age, years |
| <65 | 122 (62.24) | 188 (67.38) | 1.341 | 0.247 |
| ≥65 | 74 (37.76) | 91 (32.62) | | |
APC genotype and allele frequencies of
the two groups
The APC gene AA genotype was present in 226 subjects
in the normal control group, the AT genotype was found in 50
subjects and the TT genotype in three subjects. The T allele
frequency was 0.1004 and the A allele frequency was 0.8996. The
genotype distributions of APC D1822V were in agreement with the
Hardy-Weinberg equilibrium (P=0.95).
In the case group, the AA genotype was present in
172 cases, the AT genotype in 23 cases and the AT genotype in one
case. The T allele frequency was 0.0681 and the A allele frequency
was 0.9362. The results are shown in Table II.
 | Table IIAPC gene D1822V SNP and colorectal
cancer risk. |
Table II
APC gene D1822V SNP and colorectal
cancer risk.
| Genotype | Cases, n | Controls, n | OR (95% CI) | P-value |
|---|
| A/A | 172 | 226 | 1.00 | |
| A/T | 23 | 50 | 0.604
(0.355–1.029) | 0.062 |
| T/T | 1 | 3 | 0.438
(0.045–4.247) | 0.822 |
| A | 367 | 502 | 1.00 | |
| T | 25 | 56 | 0.611
(0.374–0.997) | 0.047 |
Association between the APC gene D1822V
polymorphism and the susceptibility to CRC
There were no significant differences between the
case and control groups with respect to the ratio of the AT (OR,
0.604; 95% CI, 0.355–1.029) and TT (OR, 0.438; 95% CI, 0.045–4.247)
genotypes compared with the AA genotype (Table II). The proportion of T alleles was
significantly higher in the case group compared with the control
group (P=0.047). The OR (95% CI) was 0.611 (0.374–0.997). These
findings indicate that the T allele may be a protective factor for
CRC.
Associations between the APC gene D1822V
polymorphism and the clinical features of CRC
There was no significant association between the APC
D1822V SNP and the risk of CRC among subjects of a different gender
or smoking and alcohol status. In addition, there was no link
between the CRC risk and pre-existing hypertension or diabetes
(Table III). However, the
frequency of AT genotype was higher in the subjects that were
>45 years old at the time of diagnosis compared with those
<45 years old (P<0.05). The OR (95% CI) was 0.271
(0.093–0.790). This result indicates that the AT genotype may
correlate with age to predict the risk of developing CRC. Thus, the
AT genotype may play a protective role in the subgroup of patients
>45 years old (Table III).
 | Table IIIStratified analyses for the variant
D1822V genotype in the cases and controls. |
Table III
Stratified analyses for the variant
D1822V genotype in the cases and controls.
| Variable | AT | AA | OR (95% CI) | P-value |
|---|
| Gender | 23 | 172 | | |
| Female | 8 | 62 | 1.00 | |
| Male | 15 | 110 | 1.057
(0.424–2.633) | 0.906 |
| Age, years |
| <45 | 6 | 15 | 1a | |
| ≥45 | 17 | 157 | 0.271
(0.093–0.790) | 0.012 |
| Smoking status |
| Non-smokers | 13 | 115 | 1.00 | |
| Smokers | 10 | 56 | 1.580
(0.653–3.824) | 0.308 |
| Alcohol
consumption |
| No | 18 | 139 | 1.00 | |
| Yes | 5 | 32 | 1.207
(0.417–3.492) | 0.729 |
| Hypertension |
| Yes | 18 | 132 | 1.00 | |
| No | 5 | 39 | 0.940
(0.328–2.695) | 0.909 |
| Diabetes |
| No | 20 | 154 | 1.00 | |
| Yes | 3 | 17 | 1.359
(0.366–5.050) | 0.646 |
| Depth of tumor
invasionb,c |
| Tis | 0 | 6 | | |
| T1 | 1 | 3 | | |
| T2 | 4 | 20 | −0.402 | 0.688 |
| T3 | 6 | 65 | | |
| T4 | 12 | 74 | | |
| Lymph node
metastasisa |
| Positive | 8 | 80 | 1.00 | |
| Negative | 15 | 91 | 0.607
(0.244–1.506) | 0.278 |
| Location |
| Colon | 12 | 77 | 1.00 | |
| Rectum | 11 | 95 | 0.743
(0.311–1.776) | 0.503 |
The effect of the D1822V SNP in APC was further
evaluated to investigate possible associations with the
clinicopathological characteristics of CRC. However, the variant
homozygote TT genotype showed no significant association with the
depth of tumor invasion, lymph node metastasis or the tumor
location (Table III).
Discussion
The development of CRC has been shown to result from
an interaction of multiple factors. APC gene mutation is a cause of
the inherited autosomal dominant disease, FAP. The types of
mutation involved in this disease process include missense
mutations, small insertions or deletions. Each of these has the
potential to advance the stop codon, resulting in the generation of
a truncated APC protein, with altered function (8). Mismatched repair gene mutations are
associated with another rare disease known as hereditary
non-polyposis CRC (11). However,
these two hereditary diseases account for only 1–2% of all cases of
CRC (11).
Associations between genotype and phenotype indicate
that different types of APC mutations will have varying clinical
manifestations (12). Although the
presence of the D1822V SNP in the APC gene has been reported, the
most frequently occurring mutation of this type, the prevalence of
the AT genotype, has been reported to be as high as 30% in control
populations (13).
Codon 1,822 is located between the 4th and 57th
amino acid repeat region in the APC protein. The wild-type in this
area has the effect of downregulating β-catenin (Fig. 1). The specific biological function
of this missense mutation remains uncertain. However, it is
believed to be a result in a change from aspartate to valine, which
may have important clinical significance.
Recently, a review and meta-analysis indicated that
APC is a candidate gene for colorectal neoplasia susceptibility,
and provided evidence that APC mutation may result in aberrant
cellular proliferation, leading to the early stages of CRC
(7). Based on this assumption, the
possible associations between the APC gene D1822V SNP and the
susceptibility to CRC was investigated.
There are currently >20 ways to detect SNPs.
These include TaqMan, mass spectrometry, chip methods and direct
sequencing (14). Of these options,
direct gene sequencing is the most reliable, and this was the
method used in the present study. For cases of uncertain
sequencing, fragments from the genome were re-amplified and
subsequently the product was re-sequenced. This procedure provided
a high level of accuracy.
The majority of studies on SNPs use peripheral
blood, which is more convenient and more economical, particularly
for patients with unresectable lesions. A number of studies have
confirmed that oncogene mutations, consistent with the primary
tumors, are present in DNA extracted from peripheral blood
(15–17). Based on this experience, the present
study used direct sequencing to genotype peripheral blood specimens
collected from 196 CRC cases and 279 healthy controls. The
frequency of the T allele in the control group was similar to that
reported in the in HapMap Project of the Chinese Han population in
Beijing (18). The frequency of the
AT and TT genotypes was significantly lower than the frequency of
the AA genotype in the case and control groups. However, the
frequency of the A allele was significantly higher compared with
the T allele in the groups (P=0.047), indicating that the T allele
may be a protective factor for CRC. These results are in accordance
with previous findings showing that AT heterozygotes for this site
were associated with a low odds ratio for CRC, indicating that the
presence of the T allele at this site may protect against the
development of CRC (8). However, no
TT homozygotes were identified in this previous population.
In another study, APC D1822V was shown to exert a
protective effect in patients with colon (OR, 0.76; 95% CI,
0.60–0.97) and rectal cancer (OR, 0.73; 95% CI, 0.56–0.95),
respectively (18). A study using
the denaturing high-performance liquid chromatography technique
also showed that individuals with homozygous mutant alleles of the
variant were at a reduced risk of developing CRC (19). However, a study in Tunisian subjects
found no statistically significant association between the D1822V
variant and CRC risk (20).
APC D1822V is traditionally considered to be a risk
factor for colorectal tumorigenesis. In 1999 (21), a VV homozygous mutation was found in
patients with FAP, and also in a proportion of healthy subjects.
However, APC D1822V was not found in the control population,
indicating that the D1822V mutation may be a morbid change that is
associated with an increased risk of developing CRC.
An increasing body of research has focused on
investigating the association between the APC D1822V variant and
CRC risk. In a previous study, the D1822V variant of the APC gene
was found not to be associated with a family history of CRC, but it
has the potential to increase the risk of adenoma transformation
(22). In the same study,
individuals with the AA genotype who had a history of symptomatic
adenomas had a 2-fold increase in the risk of developing CRC (OR,
1.91; 95% CI, 0.56–6.52; P=0.02). This risk further increased in T
allele carriers.
Another large study (23) found an association between the TT
genotype and a reduced risk of colon cancer among individuals
diagnosed at >65 years of age, and among those maintained on a
low-fat diet. This finding is partly consistent with a study
demonstrating a protective role of the TT genotype in CRC, in the
absence of any consistent association between the protective effect
of D1822V specific to patients with a low-fat diet or advanced age
(13). A further study in 196 cases
and 200 controls was undertaken to explore the interaction between
the D1822V polymorphism, APC gene and dietary intake in subjects
with CRC (24). A study that
evaluated nutritional status and lifestyle found that a high
cholesterol intake was associated with an increased risk of CRC
(OR, 1.66; 95% CI, 1.00–2.76) only in non-carriers (DD) of the
D1822V APC allele. A large case-control study in Scotland also
reported a reduced risk of CRC in individuals consuming a diet that
was low in fat (total, trans and saturated and monounsaturated
fatty acids) (25). However, this
study did not observe any associations between these variants and
the CRC risk in the population as a whole.
Each of these previously reported studies supports
the findings of the present study, showing that the T allele for
the APC gene D1822V variant appears to confer a protective effect
against CRC. Other studies have shown that the APC Asp1822Val and
Gly2502Ser polymorphisms are not associated with an increased risk
of CRC or adenoma (26). However,
the same studies found a significant correlation between the
Asp1822Val genotype and the use of a postmenopausal hormone
(P=0.03). All these results support the idea that the incidence of
CRC is due to genetic, environmental and lifestyle factors.
The present study lacks information on diet, and was
therefore unable to analyze the interaction between diet, lifestyle
and genetic factors. The study population was also relatively
small, and larger studies will therefore be required to validate
its findings. The present study does, however, provide evidence for
the first time that the T allele of the APC gene D1822V
polymorphism may be a protective factor for CRC in Chinese
subjects, particularly in those >45 years of age.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (No. 81072152).
References
|
1
|
Zhang SW, Lei ZL, Li GL, Zou LN, Zhao P
and Chen WC: A report of cancer incidence and mortality from 34
cancer registries in China, 2006. Chin Cancer. 19:356–365.
2010.
|
|
2
|
Thliveris A, Albertsen H, Tuohy T, et al:
Long-range physical map and deletion characterization of the
1100-kb NotI restriction fragment harboring the APC gene. Genomics.
34:268–270. 1996.
|
|
3
|
Näthke IS: The adenomatous polyposis coli
protein: the Achilles heel of the gut epithelium. Annu Rev Cell Dev
Biol. 20:337–366. 2004.
|
|
4
|
Hirohashi S and Kanai Y: Cell adhesion
system and human cancer morphogenesis. Cancer Sci. 94:575–581.
2003.
|
|
5
|
Wendeler MW, Praus M, Jung R, et al:
Ksp-cadherin is a functional cell-cell adhesion molecule related to
LI-cadherin. Exp Cell Res. 294:345–355. 2004.
|
|
6
|
Thomson RB, Igarashi P, Biemesderfer D, et
al: Isolation and cDNA cloning of Ksp-cadherin, a novel
kidney-specific member of the cadherin multigene family. J Biol
Chem. 270:17594–17601. 1995.
|
|
7
|
Liang J, Lin C, Hu F, et al: APC
polymorphisms and the risk of colorectal neoplasia: a HuGE review
and meta-analysis. Am J Epidemiol. 177:1169–1179. 2013.
|
|
8
|
Chen SP, Tsai ST, Jao SW, et al: Single
nucleotide polymorphisms of the APC gene and colorectal cancer
risk: a case-control study in Taiwan. BMC Cancer. 6:832006.
|
|
9
|
Jung R, Wendeler MW, Danevad M,
Himmelbauer H and Gessner R: Phylogenetic origin of LI-cadherin
revealed by protein and gene structure analysis. Cell Mol Life Sci.
61:1157–1166. 2004.
|
|
10
|
Fearnhead NS, Britton MP and Bodmer WF:
The ABC of APC. Hum Mol Genet. 10:721–733. 2001.
|
|
11
|
de la Chapelle A: Genetic predisposition
to colorectal cancer. Nat Rev Cancer. 4:769–780. 2004.
|
|
12
|
Takayama T, Miyanishi K, Hayashi T, Sato Y
and Niitsu Y: Colorectal cancer: genetics of development and
metastasis. J Gastroenterol. 41:185–192. 2006.
|
|
13
|
Cleary SP, Kim H, Croitoru ME, et al:
Missense polymorphisms in the adenomatous polyposis coli gene and
colorectal cancer risk. Dis Colon Rectum. 51:1467–1474. 2008.
|
|
14
|
Zhao GR, Yang F, Yuan YJ, et al: Progress
in detection methods of single nucleotide polymorphisms. Yi Chuan.
27:123–129. 2005.(In Chinese).
|
|
15
|
Yoon KA, Park S, Lee SH, Kim JH and Lee
JS: Comparison of circulating plasma DNA levels between lung cancer
patients and healthy controls. J Mol Diagn. 11:182–185. 2009.
|
|
16
|
Maheswaran S, Sequist LV, Nagrath S, et
al: Detection of mutations in EGFR in circulating lung-cancer
cells. N Engl J Med. 359:366–377. 2008.
|
|
17
|
Diehl F, Schmidt K, Choti MA, et al:
Circulating mutant DNA to assess tumor dynamics. Nat Med.
14:985–990. 2008.
|
|
18
|
Picelli S, Zajac P, Zhou XL, et al: Common
variants in human CRC genes as low-risk alleles. Eur J Cancer.
46:1041–1048. 2010.
|
|
19
|
Zhou XL, Eriksson U, Werelius B, et al:
Definition of candidate low risk APC alleles in a Swedish
population. Int J Cancer. 110:550–557. 2004.
|
|
20
|
Bougatef K, Marrakchi R, Ouerhani S, et
al: No evidence of the APC D1822V missense variant’s pathogenicity
in Tunisian patients with sporadic colorectal cancer. Pathol Biol
(Paris). 57:e67–e71. 2009.
|
|
21
|
Wallis YL, Morton DG, McKeown CM and
Macdonald F: Molecular analysis of the APC gene in 205 families:
extended genotype-phenotype correlations in FAP and evidence for
the role of APC amino acid changes in colorectal cancer
predisposition. J Med Genet. 36:14–20. 1999.
|
|
22
|
Menéndez M, González S, Blanco I, et al:
Colorectal cancer risk and the APC D1822V variant. Int J Cancer.
112:161–163. 2004.
|
|
23
|
Slattery ML, Samowitz W, Ballard L, et al:
A molecular variant of the APC gene at codon 1822: its association
with diet, lifestyle, and risk of colon cancer. Cancer Res.
61:1000–1004. 2001.
|
|
24
|
Guerreiro CS, Cravo ML, Brito M, et al:
The D1822V APC polymorphism interacts with fat, calcium, and fiber
intakes in modulating the risk of colorectal cancer in Portuguese
persons. Am J Clin Nutr. 85:1592–1597. 2007.
|
|
25
|
Theodoratou E, Campbell H, Tenesa A, et
al: Modification of the associations between lifestyle, dietary
factors and colorectal cancer risk by APC variants. Carcinogenesis.
29:1774–1780. 2008.
|
|
26
|
Tranah GJ, Giovannucci E, Ma J, Fuchs C
and Hunter DJ: APC Asp1822Val and Gly2502Ser polymorphisms and risk
of colorectal cancer and adenoma. Cancer Epidemiol Biomarkers Prev.
14:863–870. 2005.
|