Introduction
Primary cardiac tumors are rare, with an autopsy
incidence ranging from 0.001 to 0.03% (1). Cardiac myxoma is the most common
primary cardiac tumor worldwide, and myxomas may be sporadic or
part of genetic conditions, such as Carney complex or lentigines,
atrial myxoma and blue nevi syndrome (2,3).
Microscopically, myxomas exhibit a myxoid stroma
with plump spindle or stellate cells. Such elements have
endothelial characteristics and may be organized into
pseudovascular structures. In certain cases, a variably abundant
vascular component may also be present (4).
An increase in the number of mast cells in tumors
and a correlation between angiogenesis, mast cell number and growth
of the neoplasm has previously been reported (5–7).
Currently, no available studies have demonstrated
that the pathological characteristics of cardiac myxomas, such as
cell differentiation and vascularization, are correlated with the
angiogenic factors of mast cells. In the present study, via
immunohistochemical analysis, the role of mast cell tryptases in
cardiac myxomas was investigated using a series of 10 cardiac
myxomas (8). Furthermore, the
possible association between the tumorigenesis of myxomas and
current theories regarding endocardial development were
investigated.
Materials and methods
Materials
Archival formalin-fixed and paraffin-embedded
tissues were used to study sporadic left atrial myxomas and were
collected from 10 consecutive patients (four male and six female
patients; mean age, 56±4.7 years) who had undergone surgery at the
Department of Cardiac Surgery, School of Medicine, University Magna
Graecia (Catanzaro, Italy). The study was approved by the ethics
committee of the University Magna Graecia (Catanzaro, Italy).
Serial deparaffinated sections (4 μm-thick) were used for the
staining procedures, including hematoxylin and eosin, Alcian Blue
(pH 2.5; Bio-Optica Milano SpA, Milano, Italy) and
immunohistochemistry. All the procedures were performed at room
temperature. Patients provided written informed consent.
Immunohistochemistry
Mast cells in all cases were then
immunohistochemically stained for mouse monoclonal anti-human
tryptase (clone 10D11, 1:150 dilution; Leica, Mannheim, Germany),
mouse monoclonal anti-human cluster of differentiation (CD)31
(clone JC70A, 1:40 dilution; Dako, Carpinteria, CA, USA), mouse
monoclonal anti-human CD34 (clone QBEnd10, 1:250 dilution; Dako)
and rabbit polyclonal anti-human CD117 (1:100 dilution; Dako) with
an automated immunostainer (Bond™ Max; Leica Biosystems, Melbourne,
Australia) (9).
Blood vessel density was assessed by light
microscopy according to the method of Weidner et al
(10) and a score graded on a scale
of one to four was assigned: 1, 1–5 microvessels observed; 2, 6–10
microvessels observed; 3, 11–15 microvessels observed; 4, 16–20
microvessels observed.
Evaluation of positive cells
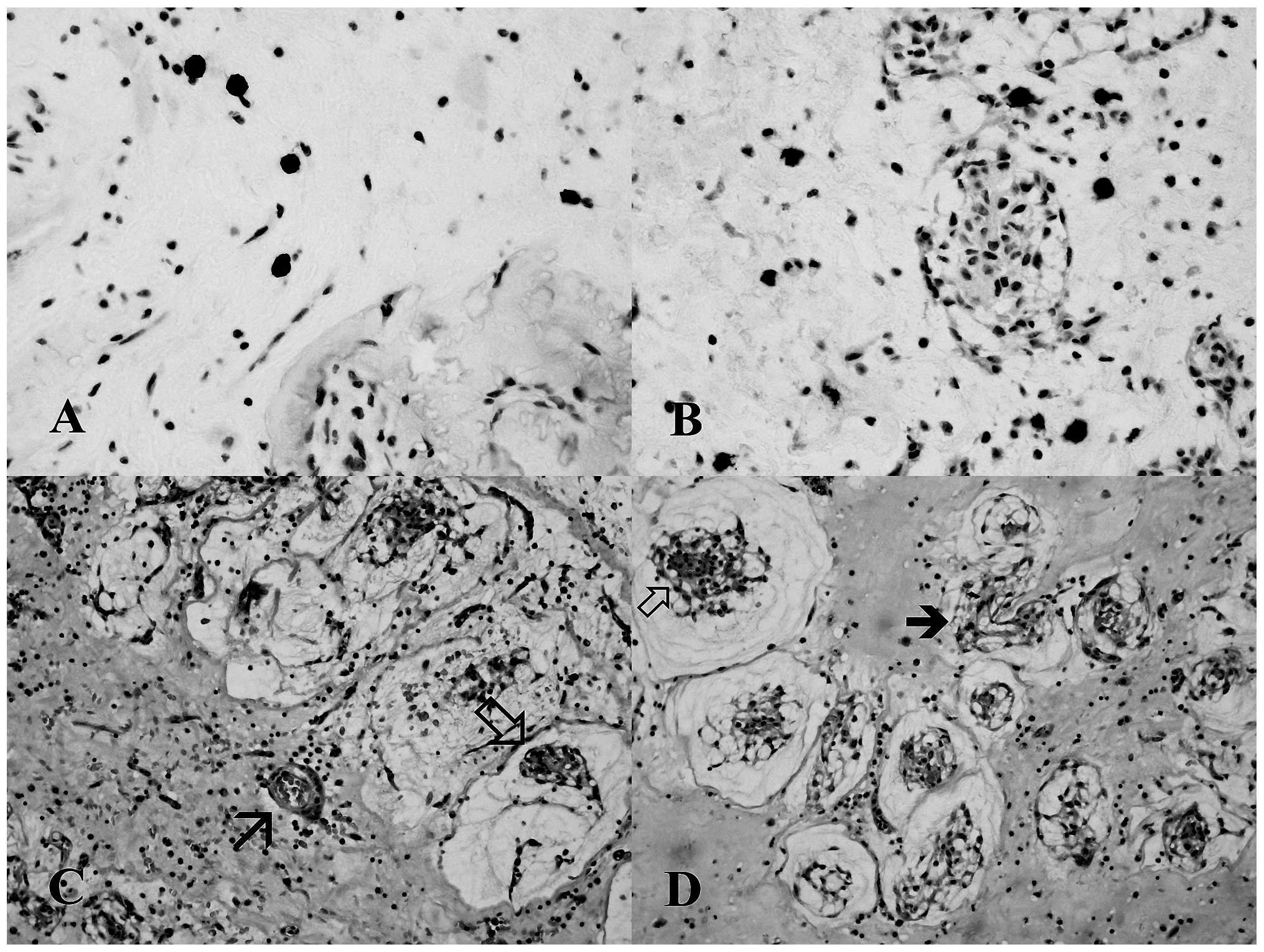
The number of mast cells that were tryptase-positive
(Fig. 1A) and CD117-positive cells
(Fig. 1B) was evaluated according
to the method of Benitez-Bribiesca et al (7).
Statistical analysis
Statistical analysis was performed in order to
calculate the correlation coefficient using least square regression
analysis between the blood vessel density score and the number of
tryptase-positive mast cells, as well as the associated
P-value.
Histopathological characteristics, such as the
presence of pseudovascular structures, abundant (≥10 pseudovascular
channels in five high power fields; magnification, ×400) or scanty
(<10 pseudovascular channels in five high power fields;
magnification, ×400, and the presence or absence of hemorrhages
were recorded. Pseudovascular structures were recognized as the
lumen lacked red blood cells and was lined by larger, often
multinucleated, cells.
Student’s t-test was used to compare the number of
tryptase-positive mast cells in the two groups of tumors with
abundant or scanty pseudovascular structures. In addition, the
correlation between the tumor size and the number of
tryptase-positive cells was examined by a correlation index.
P<0.05 was considered to indicate a statistically significant
difference. Analyses were performed using the online ‘Statistics to
Use’ software (http://www.physics.csbsju.edu/stats/).
Results
Main findings
The immunohistochemical findings and tumor size are
summarized in Tables I and II, respectively. Statistical analysis
demonstrated a positive correlation between angiogenesis and the
number of tryptase-positive mast cells (r=0.797; P=0.006).
 | Table IMorphological patterns and
immunohistochemical analysis of myxomas. |
Table I
Morphological patterns and
immunohistochemical analysis of myxomas.
| Case | Morphological
patterns | No. of
tryptase-/CD117-positive cellsa | Blood vessel density
score |
|---|
| 1 | APS, H | 18.4/23.0 | 4 |
| 2 | APS, H | 12.6/14.5 | 4 |
| 3 | APS | 15.4/17.4 | 4 |
| 4 | APS | 7.6/11.4 | 3 |
| 5 | SPS | 6.2/6.6 | 3 |
| 6 | SPS | 6.0/7.4 | 2 |
| 7 | SPS | 8.2/10.4 | 2 |
| 8 | SPS | 8.8/12.4 | 3 |
| 9 | SPS | 5.4/7.0 | 1 |
| 10 | SPS | 6.4/9.2 | 1 |
 | Table IITumor size (cm). |
Table II
Tumor size (cm).
| Case | Size |
|---|
| 1 | 5.8 |
| 2 | 3.7 |
| 3 | 3.8 |
| 4 | 4.9 |
| 5 | 3.8 |
| 6 | 1.7 |
| 7 | 2.3 |
| 8 | 2.6 |
| 9 | 3.4 |
| 10 | 3.5 |
Results of the statistical analysis
The results of Student’s t-test allowed us to
reject the null hypothesis in our series (P=0.009) concerning the
two groups of tumors with abundant and scanty pseudovascular
structures. Moreover, the number of CD117-positive cells,
attributed only to mast cells, as basophils, endothelial and
neoplastic elements are known to be only feebly positive or
negatively stained (11,12), were increased in all the cases
compared with the number of tryptase-positive elements, suggesting
degranulation of mastocytes (Table
I). Tumor size was not correlated with the number of
tryptase-positive cells (r=0.584; P=0.076).
Morphological observations
Qualitative analysis of our series suggests that
developing pseudovascular structures may be segregated or
intermixed with vessels. Notably, such structures clearly originate
from these complex architectures (Fig.
1C and D).
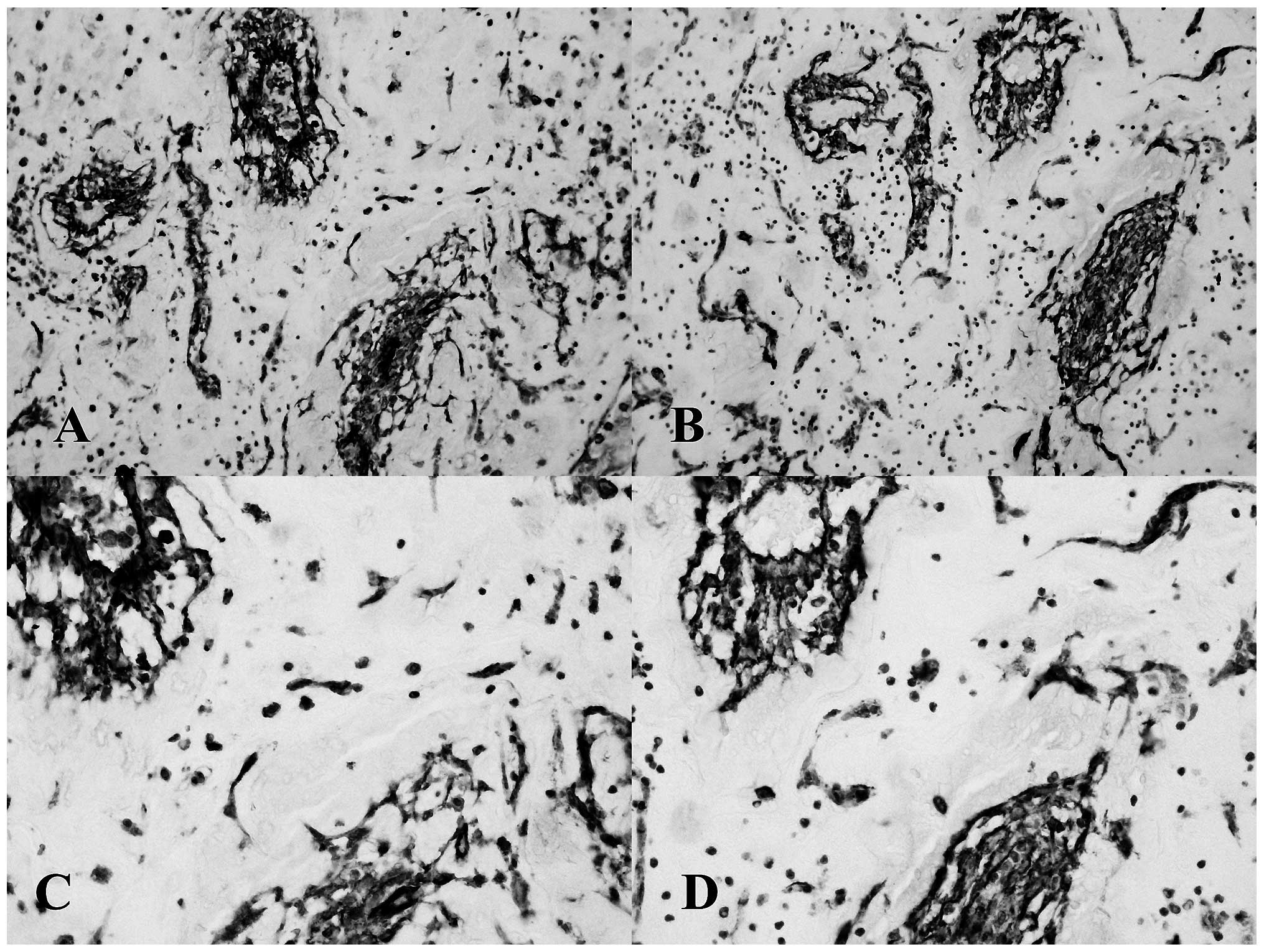
Isolated tumor tissues were CD31- and CD34-positive
with an irregular staining distribution on the cell membrane;
furthermore, tumor tissues exhibiting vascular and pseudovascular
structures were always stained positive for CD31 and CD34 (Fig. 2A and D).
Discussion
Cardiac myxoma is a rare neoplasia with an obscure
origin; its endothelial characteristics permit us to hypothesize
that the factors inducing angiogenesis are also important for the
growth and differentiation of this tumor type.
Cardiac tissue typically consists of mast cells in
the myocardium and endocardium. Notably, such elements, as well as
in cardiac myxomas, are predominantly located in the atrium
(13).
Cell receptors and molecules of the extracellular
matrix have previously been identified as possible factors of
growth and angiogenesis in cardiac myxomas (14). However, previous literature has
focused on the phenotype of cells, suggesting that cardiac myxoma
cells may derive from adult developmental remnants in the presence
of myocytic antigens (15). In such
a setting, it is important to correlate our data with other
available studies. CD117 expression may be considered as a key
factor in order to distinguish putative cardiac progenitor cells
(negative) from mast cells (positive) in child and adult human
hearts (16).
Numerous observations support a model in which the
endocardium is a spatially restricted population of the
endothelium, arising as a result of de novo vasculogenesis
from precursor cells present in the cardiac crescent (17). It is likely that myxoma cells are
independent endocardial precursors of endothelial cells expressing
CD31 (18).
The pattern of CD34 and CD31 expression and the
reciprocal location of vascular and pseudovascular proliferation
found in our series, suggests that in cardiac myxomas, endothelial
precursors of endocardial type, through an intermediate stage of
tumor cells, may also differentiate into vascular endothelia. CD34
is a cell surface glycoprotein expressed on hematopoietic stem and
progenitor cells and on the luminal cell membrane of endothelial
cells of small blood and lymphatic vessels (19–21).
Moreover, a small subset of CD34-positive precursor cells remain
present in later passages of primary endothelial cell cultures and
also in immortalized endothelial cell lines (22). Such cells have been identified as
endothelial elements able to regulate angiogenesis (23). In the present study, the pool of
isolated elements of myxomas was CD34-positive. Such elements may
be tumoral stem cells with angiogenic properties. Moreover, in our
myxoma series, angiogenesis was often intermixed with
pseudovascular structures. Thus, the angiogenic factors of mast
cells may play a pivotal role in regulating the growth and
differentiation of such a primitive endothelial population.
The ambiguous correlation between mast cells and
tumors has been previously investigated (24,25)
and among the functions of mast cells promoting tumor development,
their contribution to neoangiogenesis appears to be highly
important. Angiogenesis was measured and microvessels were counted
in human endometrial carcinoma (26). The number of microvessels correlated
with the number of tryptase-positive cells and these parameters
increased with tumor progression. A similar outcome was observed in
uterine cervix carcinoma (7),
pulmonary adenocarcinoma (27) and
gastrointestinal cancers (28). The
density of mast cells is also parallel to microvessel density in
the progression of gastric carcinoma (29). This correlation was observed for
chymase- and tryptase-positive cells.
In conclusion, the present study demonstrated a
significant correlation between angiogenesis and the number of mast
cells present in cardiac myxomas. As tumors rich in pseudovascular
structures contain a significantly higher number of mastocytes,
such cellular elements may play a role in the development and
differentiation of tumoral cells with an endothelial origin.
Finally, as tryptase is important for tumor
progression, the inhibition of this proteinase is a promising
technique in patients not surgically treatable. Compounds targeting
tryptase, although designed as anti-allergenics, may also exert
antitumor effects (25,30).
References
|
1
|
Riberi A, Gariboldi V, Grisoli D, et al:
Cardiac tumors. Rev Pneumol Clin. 66:95–103. 2010.
|
|
2
|
Carney JA, Gordon H, Carpenter PC, et al:
The complex of myxomas, spotty pigmentation, and endocrine
overactivity. Medicine (Baltimore). 64:270–283. 1985.
|
|
3
|
Rhodes AR, Silverman RA, Harrist TJ, et
al: Mucocutaneous lentigines, cardiomucocutaneous myxomas, and
multiple blue nevi: the ‘LAMB’ syndrome. J Am Acad Dermatol.
10:72–82. 1984.
|
|
4
|
Burke AP, Tazeelar H, Gomez-Roman JJ, et
al: Benign tumours of pluripotent mesenchyme. World Health
Organization Tumours of the Lung, Pleura, Thymus and Heart. Travis
WD, Brambilla E, Muller-Hermelink HK and Harris CC: IARC Press;
Lyon: pp. 260–265. 2004
|
|
5
|
Ribatti D and Crivellato E: The
controversial role of mast cells in tumor growth. Int Rev Cell Mol
Biol. 275:89–131. 2009.
|
|
6
|
Ribatti D, Crivellato E, Roccaro AM, et
al: Mast cell contribution to angiogenesis related to tumour
progression. Clin Exp Allergy. 34:1660–1664. 2004.
|
|
7
|
Benitez-Bribiesca L, Wong A, Utrera D, et
al: The role of mast cell tryptase in neoangiogenesis of
premalignant and malignant lesions of the uterine cervix. J
Histochem Cytochem. 49:1061–1062. 2001.
|
|
8
|
McNeil HP, Adachi R and Stevens RL: Mast
cell-restricted tryptases: structure and function in inflammation
and pathogen defense. J Biol Chem. 282:20785–20789. 2007.
|
|
9
|
Ammendola M, Zuccala V, Patruno R, et al:
Tryptase-positive mast cells and angiogenesis in keloids: a new
possible post-surgical target for prevention. Updates Surg.
65:53–57. 2013.
|
|
10
|
Weidner N, Semple JP, Welch WR, et al:
Tumor angiogenesis and metastasis - correlation in invasive breast
carcinoma. N Engl J Med. 324:1–8. 1991.
|
|
11
|
Acebo E, Val-Bernal JF and Gomez-Roman JJ:
Thrombomodulin, calretinin and c-kit (CD117) expression in cardiac
myxoma. Histol Histopathol. 16:1031–1036. 2001.
|
|
12
|
Arock M, Schneider E, Boissan M, et al:
Differentiation of human basophils: an overview of recent advances
and pending questions. J Leukoc Biol. 71:557–564. 2002.
|
|
13
|
Sperr WR, Bankl HC, Mundigler G, et al:
The human cardiac mast cell: localization, isolation, phenotype,
and functional characterization. Blood. 84:3876–3884. 1994.
|
|
14
|
Donato G, Conforti F, Zuccala V, et al:
Expression of tenascin-c and CD44 receptors in cardiac myxomas.
Cardiovasc Pathol. 18:173–177. 2009.
|
|
15
|
Orlandi A, Ciucci A, Ferlosio A, et al:
Cardiac myxoma cells exhibit embryonic endocardial stem cell
features. J Pathol. 209:231–239. 2006.
|
|
16
|
Zhou Y, Pan P, Yao L, et al:
CD117-positive cells of the heart: progenitor cells or mast cells?
J Histochem Cytochem. 58:309–316. 2010.
|
|
17
|
Harris IS and Black BL: Development of the
endocardium. Pediatr Cardiol. 31:391–399. 2010.
|
|
18
|
Milgrom-Hoffman M, Harrelson Z, Ferrara N,
et al: The heart endocardium is derived from vascular endothelial
progenitors. Development. 138:4777–4787. 2011.
|
|
19
|
Andrews RG, Singer JW and Bernstein ID:
Monoclonal antibody 12-8 recognizes a 115-kd molecule present on
both unipotent and multipotent hematopoietic colony-forming cells
and their precursors. Blood. 67:842–845. 1986.
|
|
20
|
Krause DS, Fackler MJ, Civin CI, et al:
CD34: structure, biology, and clinical utility. Blood. 87:1–13.
1996.
|
|
21
|
Nielsen JS and McNagny KM: CD34 is a key
regulator of hematopoietic stem cell trafficking to bone marrow and
mast cell progenitor trafficking in the periphery.
Microcirculation. 16:487–496. 2009.
|
|
22
|
van Beijnum JR, van der Linden E and
Griffioen AW: Angiogenic profiling and comparison of immortalized
endothelial cells for functional genomics. Exp Cell Res.
314:264–272. 2008.
|
|
23
|
Siemerink MJ, Klaassen I, Vogels IM, et
al: CD34 marks angiogenic tip cells in human vascular endothelial
cell cultures. Angiogenesis. 15:151–163. 2012.
|
|
24
|
Theoharides TC and Conti P: Mast cells:
the Jekyll and Hyde of tumor growth. Trends Immunol. 25:235–241.
2004.
|
|
25
|
Dyduch G, Kaczmarczyk K and Okon K: Mast
cells and cancer: enemies or allies? Pol J Pathol. 63:1–7.
2012.
|
|
26
|
Ribatti D, Finato N, Crivellato E, et al:
Neovascularization and mast cells with tryptase activity increase
simultaneously with pathologic progression in human endometrial
cancer. Am J Obstet Gynecol. 193:1961–1965. 2005.
|
|
27
|
Takanami I, Takeuchi K and Naruke M: Mast
cell density is associated with angiogenesis and poor prognosis in
pulmonary adenocarcinoma. Cancer. 88:2686–2692. 2000.
|
|
28
|
Ammendola M, Sacco R, Donato G, et al:
Mast cell positivity to tryptase correlates with metastatic lymph
nodes in gastrointestinal cancer patients treated surgically.
Oncology. 85:111–116. 2013.
|
|
29
|
Ribatti D, Guidolin D, Marzullo A, et al:
Mast cells and angiogenesis in gastric carcinoma. Int J Exp Pathol.
91:350–356. 2010.
|
|
30
|
Groot Kormelink T, Abudukelimu A and
Redegeld FA: Mast cells as target in cancer therapy. Curr Pharm
Des. 5:1868–1878. 2009.
|