Introduction
Studies within recent years have indicated that
tumor stem cells are significant in tumor formation and
progression. Tumor stem cells comprise some of the tumor tissue
found to possess characteristics associated with stem cells, i.e.,
the potential to self-renew and differentiate into various cell
types (1–3). Moreover, they are the source of the
growth, metastasis and relapse of tumors. At present, tumor stem
cells have been confirmed in gastric cancer cells, and they are
relevant to the formation, development and prognosis of gastric
cancer (4,5). Stem cell transcription factor Oct-3/4
is indispensable for the maintenance of cell totipotency, which is
involved in regulating growth of the embryo and tissues and
self-renewal of embryonic stem cells or primordial germ cells,
whereas in differentiated or mature tissues, its expression level
is reduced or even completely absent (6,7). It
has been reported that the expression of OCT3/4 in gastric cancer
tissues is associated with the invasion ability and prognosis of
gastric cancer (8). In order to
evaluate the effect of OCT3/4 on the invasion and metastasis
ability of gastric cancer, the present study first detected the
expression level of OCT3/4 in the gastric cancer tissues of
different tumor-node-metastasis (TNM) stages. Furthermore, the
correlation between the expression of OCT3/4 and the invasion
ability of gastric cancer cells, and the probable regulatory
mechanism were observed by RNA interference of OCT3/4 in gastric
cancer cell strain MKN28, so as to provide the molecular mechanism
for the occurrence and development of gastric cancer.
Materials and methods
Specimen source
The study involved 126 gastric cancer specimens
surgically obtained between 2003 and 2009 from 73 males and 53
females, with an average age of 61.3±11.7 years (range, 48–72
years). All surgical specimens were presented as paraffin sections,
and adjacent non-cancerous tissues were used as controls. The study
was approved by the ethics committee of Wuhan University (Wuhan,
China) and patients provided written informed consent.
For gastric cancer staging, the 7th International
Union Against Cancer TNM classification was adopted (9). T represented tumor invasion depth,
with Tis (primary tumor only confined to mucous layer) found in 14
cases, T1 (invasion to or below mucous layer) in 23 cases, T2
(invasion to muscular layer or plasma layer) in 35 cases, T3
(invasion through plasma layer) in 38 cases and T4 (invasion to
adjacent structures or intracavitary spreading to esophagus and
duodenum) in 16 cases. N represented the extent of lymph node
metastasis, with N0 (no pathological findings of involvement in the
dissected lymph nodes ≥15 in number) found in 35 cases, N1 (1–6
regional lymph node metastases) in 48 cases, N2 (7–15 regional
lymph node metastases) in 28 cases and N3 (≥16 regional lymph node
metastases) in 15 cases. M represented distant metastasis, with M0
(no distant metastasis) found in 98 cases and M1 (distant
metastasis to the pancreatic gland, mesentery or abdominal
paraaortic lymph nodes) in 28 cases.
Cell strains and antibodies
The human gastric cancer MKN28 cell strain was
maintained in liquid nitrogen at the Cancer Research Laboratory of
Wuhan University (Wuhan, China). Standard RPMI-1640 medium
containing 10% fetal bovine serum was purchased from Gibco-BRL (San
Francisco, CA, USA). Rabbit anti-human OCT3/4 (38 kDa) multiclone
antibodies were purchased from Santa Cruz Biotechnology, Inc.
(Santa Cruz, CA, USA), while rabbit anti-goat secondary antibodies
coated with horseradish peroxidase were purchased from Wuhan Boster
Biological Technology, Ltd. (Wuhan, China). Kits for the
immunohistochemical staining of labeled streptavidin biotin (LSAB;
Dako, Glostrup, Denmark), liposome Oligofectamine (Invitrogen Life
Technologies, Carlsbad, CA, USA) and OCT3/4 small interfering
(si)RNA double-stranded oligonucleotide, and Transwell chamber
models (Chemicon, Temecula, CA, USA) were used. Western blotting
kits were purchased from Wuhan Boster Biological Technology,
Ltd.
Construction of OCT3/4 an siRNA sequence
and transfection of the gastric cancer MKN28 strain
Three OCT3/4 siRNA oligonucleotides obtained from
Thermo Fisher Scientific (Waltham, MA, USA) were matched with a
human OCT3/4 cDNA sequence from GenBank following sequence
identification and contrasting with BLAST (http://www.ncbi.nlm.nih.gov/nucleotide/553727228?report=genbank&log$=nuclalign&blast_rank=16&RID=KJXJYKX501R).
The siRNA sense sequence was AAGGAUGUGGUCCGAGUGUGG. By contrast,
the siRNA negative control sense was formulated and synthesized as
AAGAACGGCAUCAAGGUGAAC. OCT3/4 siRNA was transfected into MKN28
cells (1×105 cells/ml) at concentrations of 6.25–100 nM
using Oligofectamine. The group design consisted of a blank control
group (Con-B group), an empty vector group (Con-A group),
transfection groups (groups S1-S3) and a negative control group (Sn
group). With the exception of the use of isocyatic
phosphate-buffered saline and an empty vector for the Con-B and
Con-A groups, respectively, the subsequent treatment of each group
was the same.
Immunohistochemical detection of OCT3/4
expression in gastric cancer tissues
LSAB kits were used for immunohistochemical
staining, according to the manufacturer’s instructions. Briefly,
OCT3/4 primary antibody (1:200 dilution) and biotin-labeled
secondary antibody (1:10,000 dilution) was added. The slices were
visualized for 5 minutes following staining, then restained with
hematoxylin and sealed. Positive cells were stained brown in the
nucleolus or cytolymph. Imag-pro-plus software was employed to
measure the percentage of the positively-stained cell area compared
with the reference strain area.
Western blot analysis
MKN28 cells in the exponential growth phase were
lysed by adding radioimmunoprecipitation assay buffer (Wuhan Boster
Biological Technology, Ltd.). Following centrifugation at 30,000 ×
g at 4°C for 5 min, the supernatant was obtained to determine the
protein level by the bicinchoninic acid method. Successively, 50 μg
protein was extracted and mixed with 2× loading buffer prior (Wuhan
Boster Biological Technology, Ltd.) to denaturation at 100°C for 5
min. Following separation by SDS-PAGE (Wuhan Boster Biological
Technology, Ltd.), the proteins were transferred to a
nitrocellulose filter (Wuhan Boster Biological Technology, Ltd.),
where they bound to specific antibodies and relative secondary
antibodies, were visualized by staining with enhanced
chemiluminescence (Wuhan Boster Biological Technology, Ltd.) and
were exposed, developed and fixed on X-ray films (Wuhan Boster
Biological Technology, Ltd.). Gray-scale analysis was performed
using BandScan software (ProZyme, Inc., Hayward, CA, USA).
Soft agar colony formation assay
The MKN28 cells at the exponential phase were
suspended in a given concentration of 1×103 cells/ml. A
mixture of 5% soft agar (Hyclone, Logan, MA, USA) plus medium at a
ratio of 1:9 was added to the culture dishes and kept at room
temperature for solidification. Additionally, 1.5 ml cell
suspension was mixed with an equivalent volume of 5% soft agar in a
5% CO2 incubator at 37°C for 2 weeks. Colony formation
conditions and rates were observed using the following formula:
Colony formation rate (%) = (colony number / incubated cell number)
× 100.
In vitro cellular invasion test
Transwell chamber models were employed to perform an
in vitro cell invasion test. Cell suspension with a given
concentration of 1×105 cells/ml was prepared, 50 μl of
which was added to the upper chamber. At 24 h post-incubation, the
cells on the upper chamber were wiped off and the number that had
migrated through the permeable membrane were counted by formalin
(10%) fixation and Giemsa dyeing.
Statistical analysis
The data are expressed as the mean ± SD. The
χ2 test and two-tailed t-test were conducted with SPSS
version 16.0 (SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Correlation between OCT3/4 expression and
the invasion and metastasis of gastric carcinoma
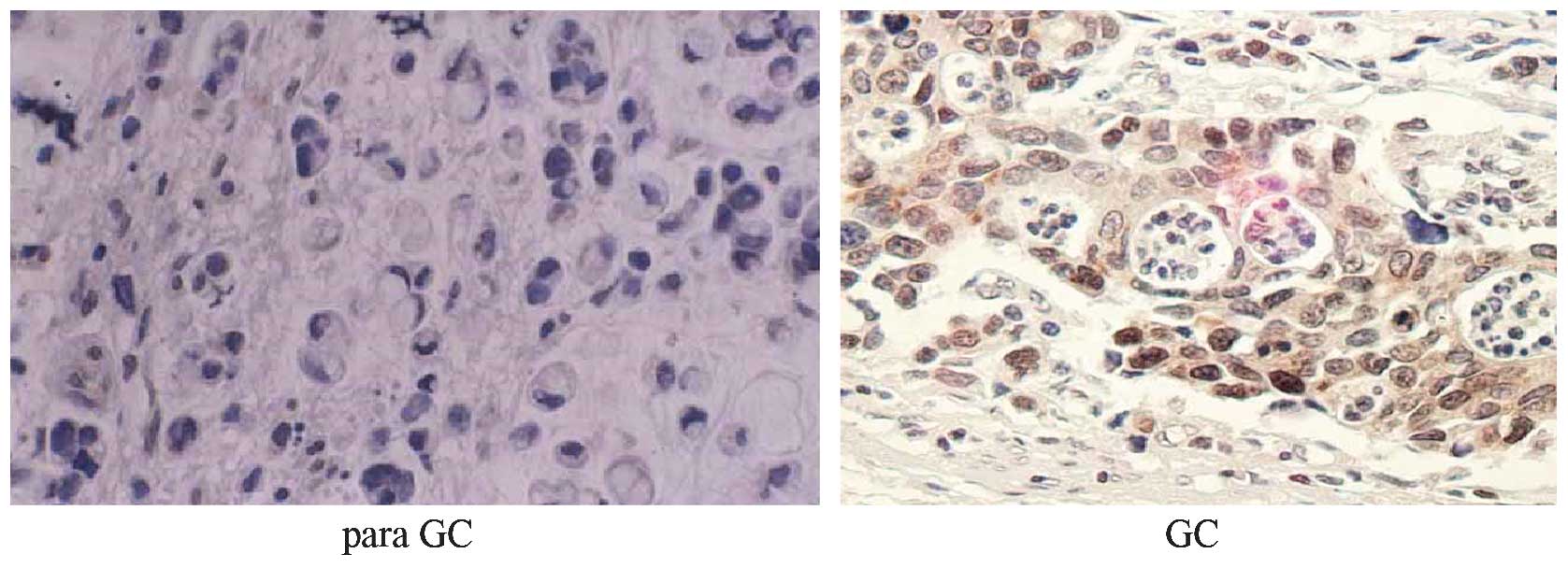
The immunohistochemical results showed an extremely
low level of OCT3/4 in the adjacent non-cancerous tissues
(1.12±0.18%) compared with that in the cancer tissues (22.56±8.72%)
of the 126 gastric cancer patients (P<0.01). Positive reactivity
of OCT3/4 was mainly restricted to the cell nuclei (Fig. 1). The expression of OCT3/4 was
associated with the invasion depth of the gastric cancer, which
presented a gradual rising trend from Tis-T4 stages. OCT3/4 had
significantly higher expression in the T2, T3 and T4 stages
compared with the Tis stage, with >10-fold higher expression in
the T4 stage compared with the Tis stage. The expression of OCT3/4
was observed to gradually increase as the extent of the lymph node
metastasis increased. A significant difference was also observed
between N2 and N0 (P<0.05). Likewise, ~5-fold higher expression
was found in N3 category compared with the N0 category. With regard
to distant metastasis, higher OCT3/4 expression was observed in M1
compared with M0 (P<0.01) (Table
I).
 | Table IExpression of OCT3/4 in gastric cancer
with different invasion degree. |
Table I
Expression of OCT3/4 in gastric cancer
with different invasion degree.
| n | OCT3/4, %a | F-value | P-value |
|---|
| Depth of
infiltration |
| Tis | 14 | 3.14±0.47 | | |
| T1 | 23 | 9.21±1.31 | T1/Tis, 6.069 | <0.01 |
| T2 | 35 | 16.23±2.03 | T2/T1, 7.013 | <0.01 |
| T3 | 36 | 26.01±3.46 | T3/T2, 9.7878 | <0.01 |
| T4 | 18 | 44.46±5.30 | T4/T3, 18.449 | <0.01 |
| Lymph node
metastasis |
| N0 | 35 | 5.46±2.39 | | |
| N1 | 48 | 6.63±1.67 | N1/N0, 1.169 | 0.039 |
| N2 | 28 | 13.69±2.80 | N2/N1, 7.057 | <0.01 |
| N3 | 15 | 26.86±4.10 | N3/N2, 13.167 | <0.01 |
| Distant
metastasis |
| M0 | 98 | 11.12±9.39 | M1/M0, 149.242 | <0.01 |
| M1 | 28 | 36.35±10.47 | | |
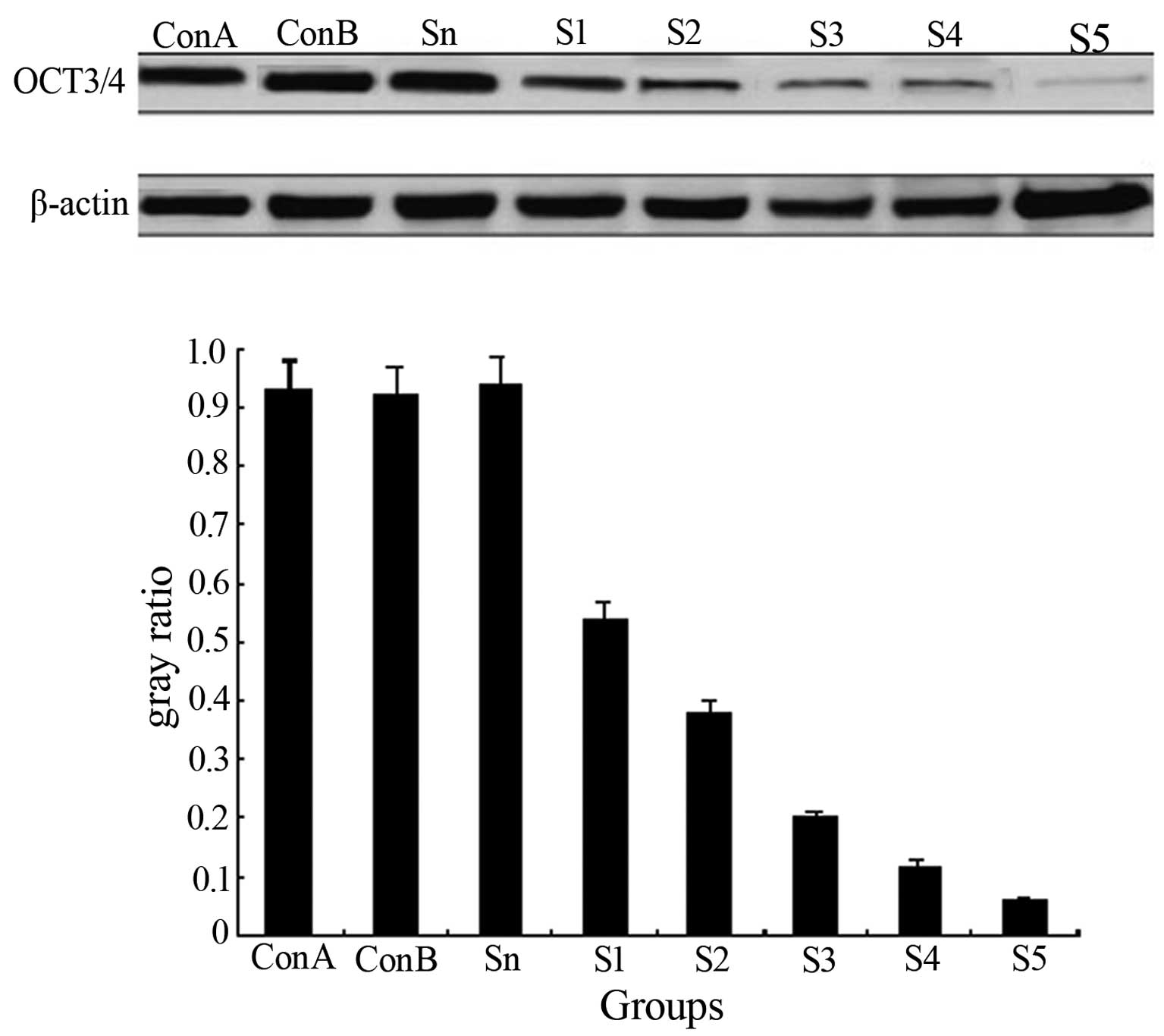
Effect of siRNA on OCT3/4 expression
Western blot analysis showed high OCT3/4 expression
in the Con-B, Sn and Con-A groups, but the difference among the
three groups was insignificant (P>0.05). OCT3/4 expression in
each transfection group was downregulated significantly
(P<0.01), particularly in the S3 group where the OCT3/4 level
had decreased by 90.6%, compared with the control group (Fig. 2). The results indicated that siRNA
transfection in the MKN28 cells was able to suppress OCT3/4
expression.
Effect of OCT3/4 siRNA on the
anchorage-independent growth of MKN28 cells
As S3 was indicated to be the most effective
interference sequence targeted to OCT3/4, OCT3/4 siRNA3 was
selected to continue the study. The soft agar colony formation
assay revealed that the MKN28 cells could form colonies
spontaneously in an in vitro culture system. Following
transfection of the MKN28 cells with different concentrations of
siRNA3 (0, 6.25, 12.5, 25, 50, and 100 nM), the colony formation
rate was observed to gradually decrease in a dose-dependent manner
as the transfection volume increased (Fig. 3).
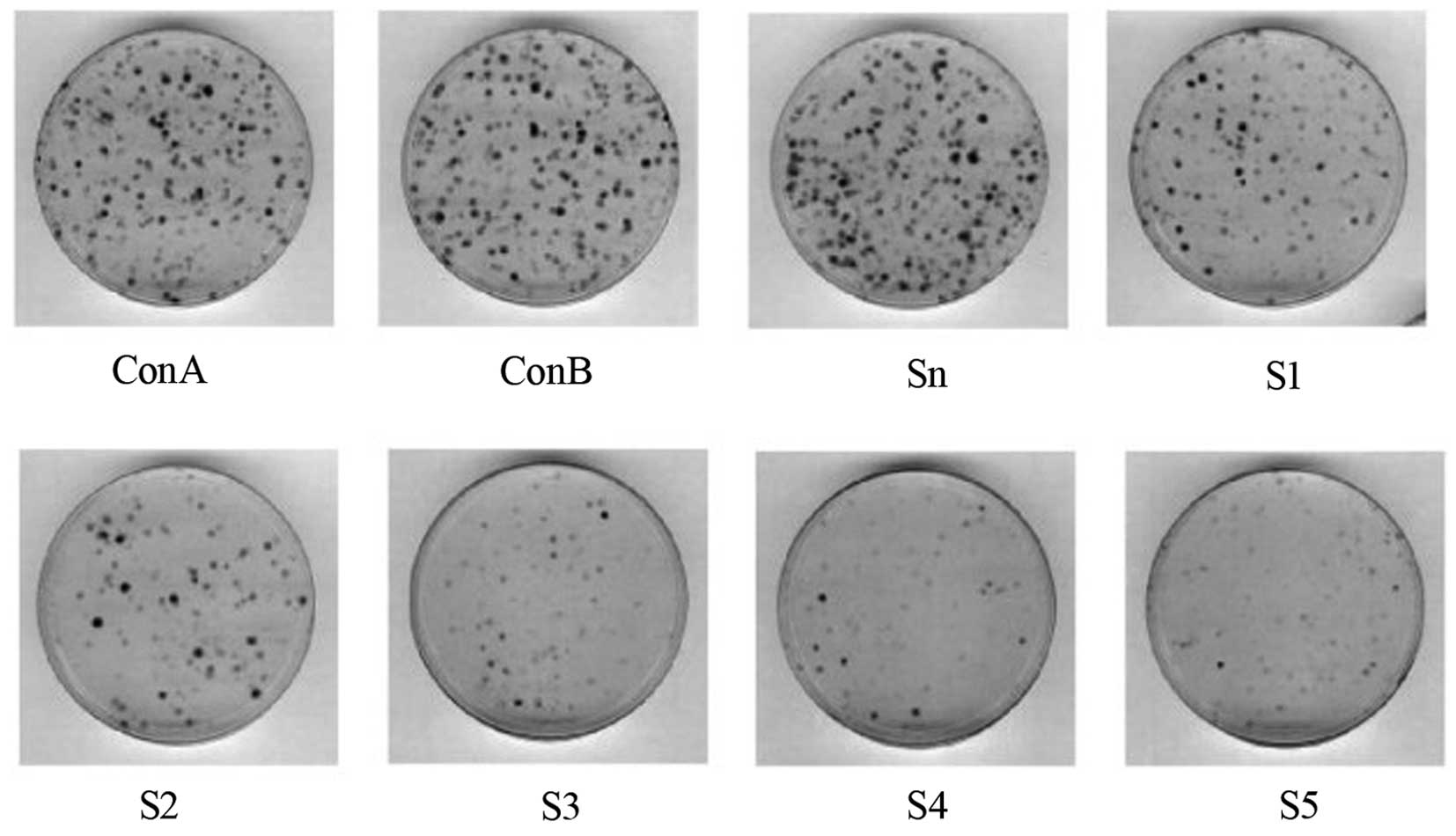 | Figure 3The colony formation rate was observed
to gradually decrease following transfection of the MKN28 cells
with various concentrations of siRNA3 (0, 6.25, 12.5, 25, 50 and
100 nM). Con A, empty vector; Con B, blank control; S1, 6.25 nM;
S2, 2.5 nM; S3, 25 nM; S4, 50 nM; and S5, 100 nM. |
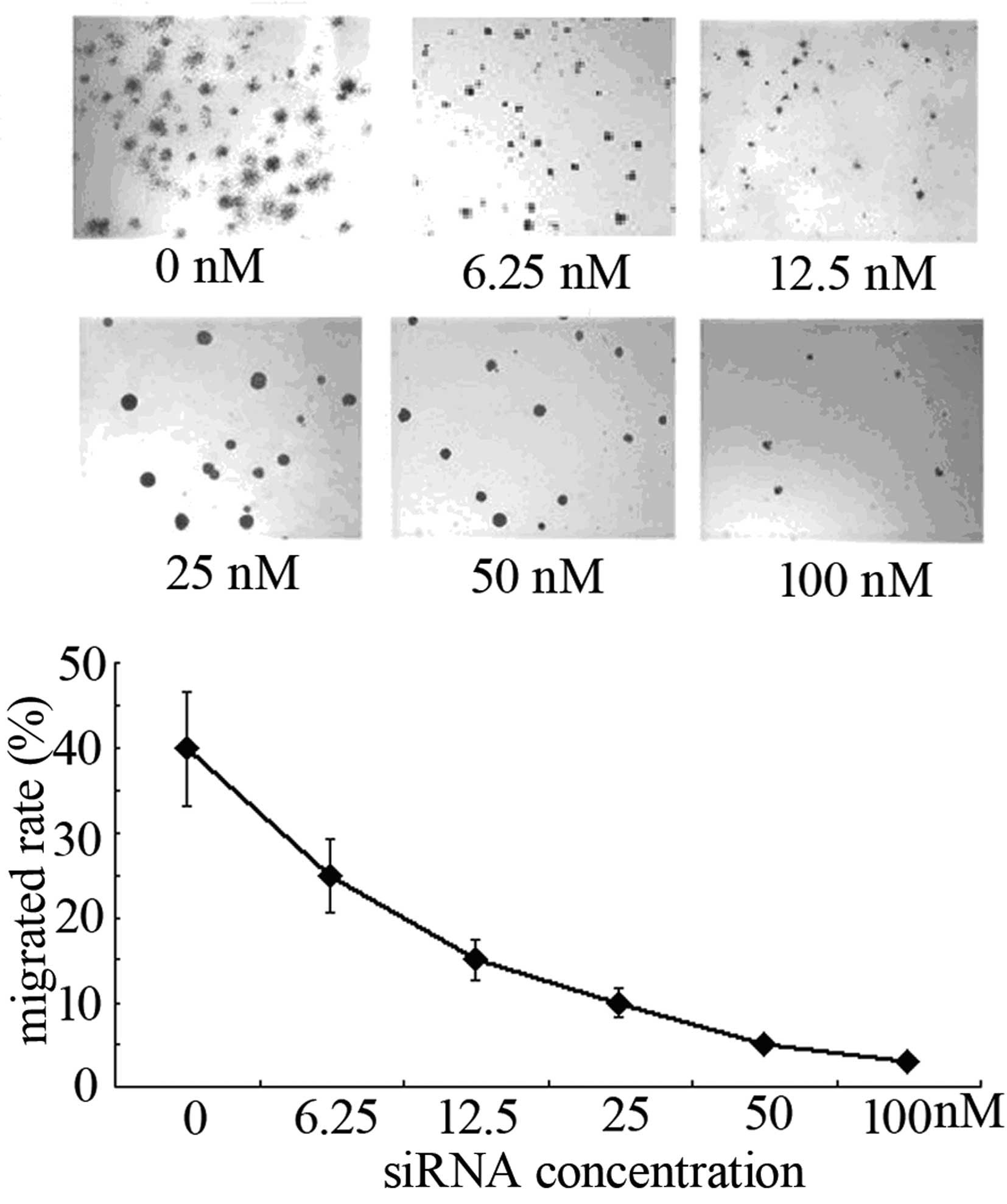
Effect of OCT3/4 interference on the
invasion capacity of MKN28 cells
At 48 h post-transfection of MKN28 cells with
different concentrations of siRNA3, Transwell chamber models were
employed to detect their invasion ability. The number of cells that
had migrated through the filter membrane was shown to have
decreased significantly following OCT3/4 interference and was
associated with the siRNA concentration (P<0.01; Fig. 4).
Discussion
With deeper study into tumor stem cells in recent
years, the possible existence of gastric cancer stem cells has come
to light. OCT3/4 is a major molecular marker for stem cells
(10). OCT3/4 is a POU
transcription factor that is encoded by the POU5F1 gene located on
human chromosome 6q21.3 and contains 324 amino acids at a molecular
weight of 38 kDa (11). By binding
to target gene promoter or octamer sequence ATGCAAAT in the
enhancer region, OCT3/4 is activated to start transcription. OCT3/4
is a transcription factor required to maintain the self-renewal of
embryonic stem cells and primordial germ cells (12); in well-differentiated tissues its
expression is reduced or even completely absent (7,13).
Certain studies have indicated that OCT3/4 is highly expressed in
multiple tumor tissues or tumor cells, such as germinal cell tumors
of the testis (14) and renal
medullary (15), esophageal
(16) and breast (17) carcinoma, and that this expression is
correlated with the diagnosis and prognosis of the tumor.
A recent study showed that the expression of OCT3/4
in gastric cancer tissues was correlated with the prognosis of the
gastric cancer patients (8). In the
present study, OCT3/4 was mainly located in the cell nuclei, with a
far higher level in the cancer tissues than in the adjacent
non-cancerous tissues. The expression of OCT3/4 followed a rising
trend with the worsening of tumor invasion, lymph node metastasis
and distant metastasis (Fig. 1;
Table I). Therefore, it can be
initially concluded that OCT3/4 plays a certain role in the
invasion and metastasis of gastric cancer and is significant for
the instruction of the TNM staging of gastric cancer. Chen et
al (18) used quantitative PCR
to detect the mRNA expression of OCT3/4 in 62 cases of gastric
cancer. The study reported higher OCT3/4 expression in the gastric
cancer tissues compared with the adjacent non-cancerous tissues,
atrophic gastritis or gastric ulcer samples. The study concluded
that the expression of OCT3/4 was correlated with the extent of
gastric cancer differentiation, but not with patient age, gender,
tumor size, TNM staging or lymph node metastasis. In contrast, the
present study highlighted the associations between the expression
level of OCT3/4 in gastric cancer tissues with the different
degrees of invasion, lymph node metastasis and distant metastasis.
Significant differences in OCT3/4 level were found in the gastric
cancer tissues with different extents of invasion, lymph node
metastasis and distant metastasis (P<0.01).
To further examine the role of OCT3/4 in the
invasion and metastasis of gastric cancer, the present study used
specific RNA interference to silence OCT3/4, and employed a soft
agar colony formation assay and Transwell chamber models to detect
cell anchorage-independence and invasion capability. Cell anchorage
dependence refers to the fact that normal cells must attach to a
solid surface for growth, while tumor cells have the properties of
anchorage-independent growth. Soft agar colony formation assays are
able to determine the anchorage-independence and the degree of
malignancy of tumor cells (19). A
stronger invasion ability in cancer cells gives rise to more colony
formation. The present study indicated that OCT3/4 siRNA could
suppress the colony formation of MKN28 cells on soft agar in a
concentration-dependent manner (Fig.
3). Accordingly, interfering with OCT3/4 can suppress the
invasion of MKN28 cells. The migration and invasion ability of
tumor cells is associated with the tumor microenvironment and the
extracellular matrix, thus a Transwell chamber model simulated in
accordance with the extracellular matrix is a reliable method for
studying cell invasion ability (20). The present study found that
subsequent to the interference of OCT3/4, the number of cells that
migrated through Transwell membrane was significantly decreased in
a siRNA concentration-dependent manner (Fig. 4).
The present study found that OCT3/4 in gastric
cancer may play a role similar to that of oncogenes. The expression
level of OCT3/4 may predict the malignant potential of gastric
cancer, and downregulation of its expression may suppress the cell
invasion ability. Asadi et al (21) found that a novel spliced variant of
OCT4 designated as OCT4B1 was highly expressed in a gastric cancer
cell strain, and that downregulation of its expression accelerated
cell apoptosis. The study also described OCT4B1 as a novel tumor
marker with potential value in the diagnosis and treatment of
gastric cancer.
In conclusion, the present study showed that the
expression of OCT3/4 increased with the worsening of gastric cancer
invasion and metastasis, and interfering with OCT3/4 therefore
attenuated the invasion ability of the gastric cancer cell MKN28
strain. These findings indicate that OCT3/4 plays a vital role in
regulating the invasion and metastasis of gastric cancer. Detection
of OCT3/4 is conducive to judging the malignant potential and
prognosis of gastric cancer. Furthermore, the downregulation of
OCT3/4 reduces the invasion and metastasis of gastric cancer,
therefore, OCT3/4 is expected to be a molecular target for the
treatment of this disease. The present study is specific to surface
phenomenon observations and sets the stage for a deeper
investigation into the role of OCT3/4 in the occurrence,
development and treatment of gastric cancer and the possible
mechanism behind this.
References
|
1
|
Collins AT, Berry PA, Hyde C, et al:
Prospective identification of tumorigenic prostate cancer stem
cells. Cancer Res. 65:10946–10951. 2005.
|
|
2
|
Liu S, Dontu G, Mantle ID, et al: Hedgehog
signaling and Bmi-1 regulate self-renewal of normal and malignant
human mammary stem cells. Cancer Res. 66:6063–6071. 2006.
|
|
3
|
van der Flier LG and Clevers H: Stem
cells, self-renewal, and differentiation in the intestinal
epithelium. Annu Rev Physiol. 71:241–260. 2009.
|
|
4
|
Chen T, Yang K, Yu J, et al:
Identification and expansion of cancer stem cells in tumor tissues
and peripheral blood derived from gastric adenocarcinoma patients.
Cell Res. 22:248–258. 2012.
|
|
5
|
Ryu HS, Park do J, Kim HH, et al:
Combination of epithelial-mesenchymal transition and cancer stem
cell-like phenotypes has independent prognostic value in gastric
cancer. Hum Pathol. 43:520–528. 2012.
|
|
6
|
Matoba R, Niwa H, Masui S, et al:
Dissecting Oct3/4-regulated gene networks in embryonic stem cells
by expression profiling. PLoS One. 1:e262006.
|
|
7
|
Takeda J, Seino S and Bell GI: Human Oct3
gene family: cDNA sequences, alternative splicing, gene
organization, chromosomal location, and expression at low levels in
adult tissues. Nucleic Acids Res. 20:4613–4620. 1992.
|
|
8
|
Matsuoka J, Yashiro M, Sakurai K, et al:
Role of the stemness factors sox2, oct3/4, and nanog in gastric
carcinoma. J Surg Res. 174:130–135. 2012.
|
|
9
|
Santiago JM, Sasako M and Osorio J:
TNM-7th edition 2009 (UICC/AJCC) and Japanese Classification 2010
in Gastric Cancer. Towards simplicity and standardisation in the
management of gastric cancer. Cir Esp. 89:275–281. 2011.(In
Spanish).
|
|
10
|
Song Z and Cheng L: Gastric stem cells and
gastric cancer stem cells. Zhong Hua Yi Xue Za Zhi. 89:1868–1870.
2009.(In Chinese).
|
|
11
|
Schreiber E, Tobler A, Malipiero U, et al:
cDNA cloning of human N-Oct3, a nervous-system specific POU domain
transcription factor binding to the octamer DNA motif. Nucleic
Acids Res. 21:253–258. 1993.
|
|
12
|
Looijenga LH, Stoop H, de Leeuw HP, et al:
POU5F1 (OCT3/4) identifies cells with pluripotent potential in
human germ cell tumors. Cancer Res. 63:2244–2250. 2003.
|
|
13
|
Kekuda R, Prasad PD, Wu X, et al: Cloning
and functional characterization of a potential-sensitive,
polyspecific organic cation transporter (OCT3) most abundantly
expressed in placenta. J Biol Chem. 273:15971–15979. 1998.
|
|
14
|
De Jong J, Stoop H, Dohle GR, et al:
Diagnostic value of OCT3/4 for pre-invasive and invasive testicular
germ cell tumours. J Pathol. 206:242–249. 2005.
|
|
15
|
Rao P, Tannir NM and Tamboli P: Expression
of OCT3/4 in renal medullary carcinoma represents a potential
diagnostic pitfall. Am J Surg Pathol. 36:583–588. 2012.
|
|
16
|
Wang Q, He W, Lu C, et al: Oct3/4 and Sox2
are significantly associated with an unfavorable clinical outcome
in human esophageal squamous cell carcinoma. Anticancer Res.
29:1233–1241. 2009.
|
|
17
|
Wang P, Branch DR, Bali M, et al: The POU
homeodomain protein OCT3 as a potential transcriptional activator
for fibroblast growth factor-4 (FGF-4) in human breast cancer
cells. Biochem J. 375:199–205. 2003.
|
|
18
|
Chen Z, Xu WR, Qian H, et al: Oct4, a
novel marker for human gastric cancer. J Surg Oncol. 99:414–419.
2009.
|
|
19
|
Kikuchi S, Katada N, Sakuramoto S, et al:
Factors associated with pN3 stage tumors according to the TNM
classification in advanced gastric cancer. Hepatogastroenterology.
50:1723–1726. 2003.
|
|
20
|
Omejc M, Juvan R, Jelenc F and Repse S:
Lymph node metastases in gastric cancer: correlation between new
and old UICC TNM classification. Int Surg. 86:14–19. 2001.
|
|
21
|
Asadi MH, Mowla SJ, Fathi F, et al:
OCT4B1, a novel spliced variant of OCT4, is highly expressed in
gastric cancer and acts as an antiapoptotic factor. Int J Cancer.
128:2645–2652. 2011.
|