Introduction
Chronic infection and inflammation are important
epigenetic factors contributing to tumorigenensis and tumor
progession (1). Toll-like receptors
(TLRs) are important pattern recognition receptors expressed by
immune cells. Toll-like receptor 4 (TLR4) expression has been
investigated in tumor cells or cell lines, including gastric
carcinoma, extranodal marginal zone B-cell lymphomas, pituitary
epithelial tumor cell lines, hepatocellular carcinoma cells, colon
cancer cells and human prostate epithelial PC3 cells. Although TLR4
is expressed in numerous non-immune cells and tumor cells, the
functional association of TLR4 with tumor progression requires
further elucidation. The TLR4 single-nucleotide polymorphisms
(SNPs), Asp299 and Thr399, have been reported to be involved in
inflammation and cancer (2,3).
It was hypothesized that the presence of TLR4
variants may lead to the development of ovarian cancer. In the
present study, the relevance of TLR4 SNPs, Asp299Gly (rs4986790)
and Thr399Ile (rs4986791) are investigated in 105 ovarian cancer
patients retrospectively with an extended follow-up and complete
representative adjuvant therapy (chemotherapy with or without
surgical treatment).
Patients and methods
Patients and tissue samples
Tissue specimens of 105 ovarian cancer patients
(mean age, 54±11.7 years) were collected by the Department of
Pathology, Shandong Cancer Hospital and Institute (Jinan, China).
All patients were diagnosed and treated at the Department of
Gynecologic Oncology, Shandong Cancer Hospital and Institute
between 2004 and 2012. Treatment strategies were determined based
on consensus recommendations from gynecological oncologists, which
were based on guidelines for ovarian cancer treatment at the time
(4). All patients provided written
informed consent for the use of their tissues and participation in
the study. The tissue samples were obtained during diagnostic or
therapeutic surgery. Overall, 75 (71%) patients received paclitaxel
+ carboplatin chemotherapy prior or subsequent to surgery. A total
of 19 (18%) patients received other chemotherapy prior or following
surgery, and 11 (11%) patients received only surgery. Regular
follow-up procedures were performed and the median follow-up in
patients who had survived until the time of analysis was 26±17.6
months (range, 1–96 months).
Sequence analysis of TLR4
As described in a previous study (5), DNA samples were extracted from 10-μm
sections of formalin-fixed, paraffin-embedded tumor tissue. The
germline mutations, TLR4 Asp299Gly (rs4986790) and Thr399Ile
(rs4986791) were analyzed in all patients using pyrosequencing. For
TLR4 Asp299Gly (rs4986790) and Thr399Ile (rs4986791),
pyrosequencing was performed with the forward primer,
5′-TCTGGCTGGTTTAGAAGTCCA-3′; and the reverse primer,
5′-AATTGCCAGCCATTTTCAAG-3′; resulting in a 698-bp fragment.
Following denaturation at 95°C, 35 cycles of DNA amplification were
performed using Taq DNA Polymerase 2× Master Mix Red
(Ampliqon-Biomol, Hamburg, Germany) at 95°C for 30 sec, 60°C for 30
sec and 72°C for 60 sec, with a final extension for 5 min at 72°C.
Once the amplification was confirmed, the polymerase chain reaction
product was digested for 1 h at 37°C with the restriction enzyme
(Invitrogen Life Technologies, Carlsbad, CA, USA), Ncol
(6). The SNP assays were purchased
from Applied Biosystems, Inc., (ABI; Carlsbad, CA, USA) and
performed on the ABI StepOnePlusTM system. Data were
analyzed using ABI StepOneTM Software.
Results
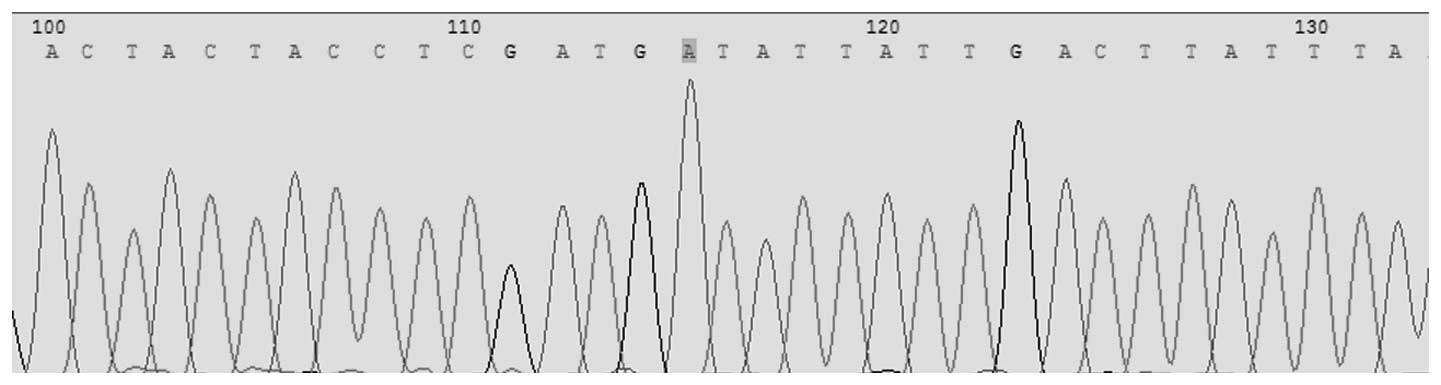
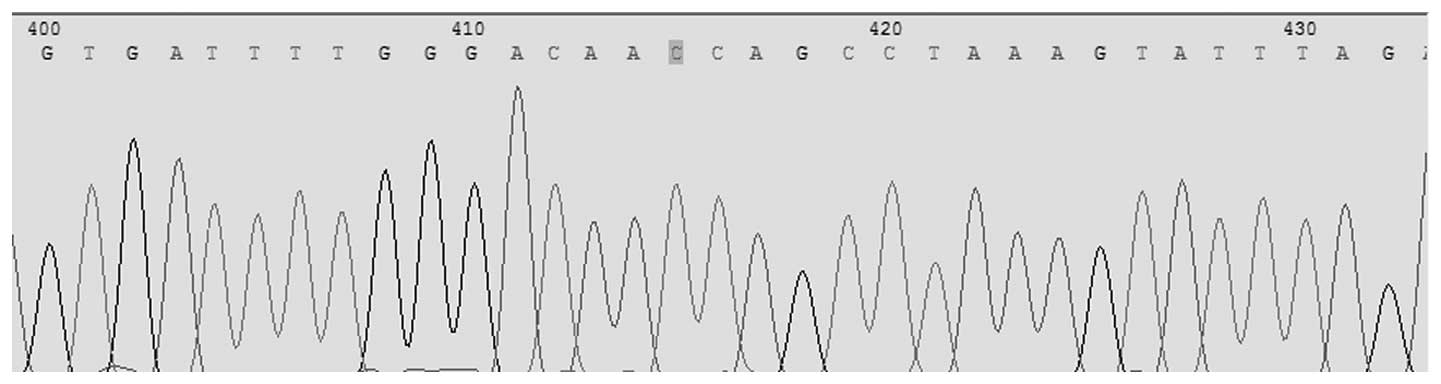
Pyrosequencing was conducted for the simultaneous
detection of Asp299Gly (rs4986790) and Thr399Ile (rs4986791) in the
TLR4 gene. The allele frequencies for TLR4 Asp299Gly identified in
this population were 1.00 (A) and 0.00 (G); for TLR4 Thr399Ile the
allele frequencies were, 1.00 (C) and 0.00 (T). For TLR4 Asp299Gly
the observed genotype frequency was 1.00 (AA), 0.00 (AG) and 0.0
(GG). In TLR4 Thr399Ile the observed genotype frequencies were 1.00
(CC), 0.00 (CT) and 0.00 (TT; Figs.
1 and 2).
Discussion
Chronic inflammation is significant in the
progression of various human cancers. Previous studies have
revealed that inflammation-induced TLRs are involved in
tumorigenesis (7–11). Additionally, certain studies have
shown that the activation of TLR4 signaling may correlate with
tumor progression (12). Thus, TLRs
may be candidates as independent prognostic cancer markers
(13).
The response to TLR ligands may be impaired by SNPs
that are present in TLR genes, resulting in a modified
susceptibility to the outcome of infectious or inflammatory
diseases. Furthermore, the TLR genes have been shown to be
polymorphic (6).
Currently, two SNPs within the human TLR4 gene have
been focused on. The first is an A-G substitution at the 896-bp
region, which results in an aspartic acid to glycine replacement at
the 299 position of the amino acid sequence (Asp299Gly), and the
other is a C-T substitution at the 1,196-bp region, which results
in a threonine to isoleucine exchange at position 399 in the amino
acid sequence (Thr399Ile) (2).
In breast cancer, data shows that patients who
express the TLR4 loss of function allele experience a relapse more
rapidly, following radiotherapy and chemotherapy, when compared
with those patients who express the normal allele (14). For marginal zone B cells, TLR4 is
the major receptor for lipopolysaccharide, and the rare TLR4
Asp299Gly allele attenuates the receptor signaling and decreases
the inflammatory response. Data has shown that heterozygous
genotypes are expressed significantly less frequently in patients
with gastric mucosa-associated lymphoid tissue (MALT) lymphoma
compared with that of Helicobacter pylori-infected control
subjects. The TLR4 Asp299Gly genotype acts as a protective factor
during the development of gastric MALT lymphoma in Caucasians
(15).
Notably, there is an opposite conclusion for the
TLR4 Asp299Gly/Thr399Ile polymorphism that is associated with
gastric cancer. A study by Kato et al (16) reported no association between the
TLR4 Asp299Gly polymorphism and gastric pre-cancerous lesions.
However, de la Trejo et al (17) reported that the Asp299Gly
polymorphism in TLR4 was significantly associated with duodenal
ulcers and that there was a trend for an association with gastric
cancer, with Asp299Gly polymorphism values similar in patients with
or without the H. pylori infection. The Thr399Ile
polymorphism in TLR4 was also identified as a genetic risk factor
for gastritis and pre-cancerous lesions in a northern Indian
population (18). Santini et
al (19) demonstrated that the
TLR4 Thr399Ile polymorphism is linked with an increased
susceptibility to gastric cancer. However, other data indicates
that TLR4 Asp299Gly and Thr399Ile are extremely rare in the
Japanese population and, therefore, they may not be significant
factors in establishing the outcome of H. pylori-infected
Japanese patients (20). Guo et
al (21) demonstrated that the
polymorphism of cluster of differentiation, but not the TLR4
Asp299Gly mutation, was associated with a presence of colorectal
cancer in Chinese patients. Zhang et al (22) indicated that using additional
genetic models for rs4986790 and rs4986791 complicates analysis.
Their meta-analysis indicated that the two SNPs (rs4986790 and
rs4986791) in TLR4 were associated with an increased cancer risk,
however, one SNP in TLR4 (rs1927911) was associated with a
decreased cancer risk. Thus, the frequency of different
polymorphisms has been shown to vary significantly across the
different ethnic populations worldwide.
In conclusion, the TLR4 Asp299Gly and Thr399Ile
alleles were not detected in the ovarian cancer patients in the
present study. The results indicate that the TLR4 299Gly and 399Ile
alleles have a markedly reduced frequency in northern Chinese
ovarian cancer patients compared with those presented in the study
by Zhang et al (21).
Although detailed mechanisms and regulation of the TLR4 functions
in tumor pathogenesis remain to be elucidated, TLR4 may be a
promising target for the development of anticancer agents in the
future.
References
|
1
|
Balkwill F and Coussens LM: Cancer: an
inflammatory link. Nature. 431:405–406. 2004.
|
|
2
|
Arbour NC, Lorenz E, Schutte BC, et al:
TLR4 mutations are associated with endotoxin hyporesponsiveness in
humans. Nat Genet. 25:187–191. 2000.
|
|
3
|
Lorenz E, Patel DD, Hartung T and Schwartz
DA: Toll-like receptor 4 (TLR4)-deficient murine macrophage cell
line as an in vitro assay system to show TLR4-independent
signaling of Bacteroides fragilis lipopolysaccharide. Infect
Immun. 70:4892–4896. 2002.
|
|
4
|
Poveda Velasco A, Casado Herráez A,
Cervantes Ruipérez A, et al: Treatment guidelines in ovarian
cancer. Clin Transl Oncol. 9:308–316. 2007.
|
|
5
|
Lehnerdt GF, Franz P, Zaqoul A, et al:
Overall and relapse-free survival in oropharyngeal and
hypopharyngeal squamous cell carcinoma are associated with
genotypes of T393C polymorphism of the GNAS1 gene. Clin Cancer Res.
14:1753–1758. 2008.
|
|
6
|
Montes AH, Asensi V, Alvarez V, et al: The
Toll-like receptor 4 (Asp299Gly) polymorphism is a risk factor for
Gram-negative and haematogenous osteomyelitis. Clin Exp Immunol.
143:404–413. 2006.
|
|
7
|
Fukata M, Chen A, Vamadevan AS, et al:
Toll-like receptor-4 promotes the development of colitis-associated
colorectal tumors. Gastroenterology. 133:1869–1881. 2007.
|
|
8
|
Lee SJ and Lim KT: UDN glycoprotein
regulates activities of manganese-superoxide dismutase, activator
protein-1, and nuclear factor-kappaB stimulated by reactive oxygen
radicals in lipopolysaccharide-stimulated HCT-116 cells. Cancer
Lett. 254:274–287. 2007.
|
|
9
|
Wang JH, Manning BJ, Wu QD, Blankson S,
Bouchier-Hayes D and Redmond HP: Endotoxin/lipopolysaccharide
activates NF-kappa B and enhances tumor cell adhesion and invasion
through a beta 1 integrin-dependent mechanism. J Immunol.
170:795–804. 2003.
|
|
10
|
Cianchi F, Cortesini C, Fantappiè O, et
al: Cyclooxygenase-2 activation mediates the proangiogenic effect
of nitric oxide in colorectal cancer. Clin Cancer Res.
10:2694–2704. 2004.
|
|
11
|
Molteni M, Marabella D, Orlandi C and
Rossetti C: Melanoma cell lines are responsive in vitro to
lipopolysaccharide and express TLR-4. Cancer Lett. 235:75–83.
2006.
|
|
12
|
Huang B, Zhao J, Li H, et al: Toll-like
receptors on tumor cells facilitate evasion of immune surveillance.
Cancer Res. 65:5009–5014. 2005.
|
|
13
|
Castro FA, Försti A, Buch S, et al: TLR-3
polymorphism is an independent prognostic marker for stage II
colorectal cancer. Eur J Cancer. 47:1203–1210. 2011.
|
|
14
|
Apetoh L, Ghiringhelli F, Tesniere A, et
al: Toll-like receptor 4-dependent contribution of the immune
system to anticancer chemotherapy and radiotherapy. Nat Med.
13:1050–1059. 2007.
|
|
15
|
Hellmig S, Fischbach W, Goebeler-Kolve ME,
Fölsch UR, Hampe J and Schreiber S: Association study of a
functional Toll-like receptor 4 polymorphism with susceptibility to
gastric mucosa-associated lymphoid tissue lymphoma. Leuk Lymphoma.
46:869–872. 2005.
|
|
16
|
Kato I, Canzian F, Plummer M, et al:
Polymorphisms in genes related to bacterial
lipopolysaccharide/peptidoglycan signaling and gastric precancerous
lesions in a population at high risk for gastric cancer. Dig Dis
Sci. 52:254–261. 2007.
|
|
17
|
Trejo-de la OA, Torres J, Pérez-Rodríguez
M, et al: TLR4 single-nucleotide polymorphisms alter mucosal
cytokine and chemokine patterns in Mexican patients with
Helicobacter pylori-associated gastroduodenal diseases. Clin
Immunol. 129:333–340. 2008.
|
|
18
|
Achyut BR, Ghoshal UC, Moorchung N and
Mittal B: Association of Toll-like receptor-4 (Asp299Gly and
Thr399Ileu) gene polymorphisms with gastritis and precancerous
lesions. Hum Immunol. 68:901–907. 2007.
|
|
19
|
Santini D, Angeletti S, Ruzzo A, et al:
Toll-like receptor 4 Asp299Gly and Thr399Ile polymorphisms in
gastric cancer of intestinal and diffuse histotypes. Clin Exp
Immunol. 154:360–364. 2008.
|
|
20
|
Tahara T, Arisawa T, Shibata T, Hirata I
and Nakano H: Absence of common polymorphisms of toll like receptor
4 (TLR4): Asp299Gly, Thr399Ile in patients with gastroduodenal
diseases in Japan. J Clin Biochem Nutr. 40:62–65. 2007.
|
|
21
|
Guo Q, Zhu J and Xia B: Polymorphism of
CD14 gene but not the mutation of TLR4 gene is associated with
colorectal cancer in Chinese patients. J Gastroenterol Hepatol.
21:92–77. 2006.
|
|
22
|
Zhang K, Zhou B, Wang Y, Rao L and Zhang
L: The TLR4 gene polymorphisms and susceptibility to cancer: a
systematic review and meta-analysis. Eur J Cancer. 49:946–954.
2013.
|