Introduction
Esophageal squamous cell carcinoma (ESCC) is a
common gastrointestinal cancer with a poor prognosis mainly due to
metastasis. The death-associated protein kinase (DAPK) family, a
family of pro-apoptotic proteins, has previously been discovered as
one of the genes isolated by the ‘technical knock-out’ (TKO)
approach in a functional screen, based on the random knockdown of
gene expression (1). DAPK has been
shown to be involved in a number of apoptotic signal transduction
pathways, initiating tumorigenesis though unbalanced cell
proliferation and death, though the accumulation of mutated genes
and via a prolonged cell growth period (2–4). Ezrin
has been demonstrated to be significant in metastasis (5). DAPK and ezrin interact with E-cadherin
in metastasis. In the present study, the expression of DRP-1 (a
member of the DAPK family), ezrin and E-cadherin was examined in
tumor tissues excised from ESCC patients from Handan, Hebei, a high
ESCC incidence area, using in situ hybridization and
immunohistochemistry methods, and analyzed their roles in the
carcinogenesis and development of ESCC in order to discover and
develop novel biomarkers for the condition.
Materials and methods
Specimens and Patients
Surgical tissues were collected from 76 patients who
underwent surgery in the Department of Thoracic Surgery of the
Affiliated Hospital of Hebei University of Engineering (Handan,
Hebei, China) between July 2008 and July 2010, and used to prepare
tissue arrays. The patients included 49 males and 27 females (with
a male to female ratio of 1.8:1), aged 39 to 73 years, with a
median age of 61±5.1 years. None of the patients had received
radiotherapy or chemotherapy prior to the surgery. In total, 26, 33
and 17 were confirmed to have well-, moderately- and
poorly-differentiated ESCC by post-operative pathological
examination, respectively. A total of 10 patients were stage T1, 21
were T2, 29 were T3 and 16 were T4. Overall, 41 patients presented
with lymphatic metastasis, while 35 did not. The samples were
provided by the pathological department of the Affiliated Hospital
of Hebei University of Engineering (Handan, China). This study was
approved by the ethics committee of the Affiliated Hospital of
Hebei University of Engineering.
Methods
Tissue Array
Tissue paraffin blocks were sectioned and stained
with hematoxylin and eosin (HE). To prepare the tissue arrays (10×8
mm), eight holes (2 mm in diameter) were created using Beecher
Tissue Arrayer (Beecher Instruments, Inc., Sun Prairie, WI, USA) on
a control block, and filled up with tissues obtained from the donor
blocks according to positions precisely mapped on HE film. The
identification number of the tissue was recorded for each hole. For
each sample, tumor and paracancerous normal (3–5 cm away from the
tumor tissue) tissues were used. The arrays were sectioned to a
thickness of 3–4 μm, melted, stained with HE and examined by
pathologists prior to subsequent analysis.
Immunohistochemistry and in situ
hybridization
The tissue sections were analyzed
immunohistochemically using an immunohistochemistry kit (Beijing
Zhongshan Jinqiao Biological Technology, Ltd., Beijing, China),
following the manufacturer’s instructions. Rabbit antihuman
polyclonal DAPK anybody (Boster, Wuhan, China) was diluted 150
times prior to use and counterstained with diaminobenzidine (Zymed
Co., Invitrogen Life Technologies, Carlsbad, CA, USA), according to
manufacturer’s instructions. For the control, phosphate-buffered
saline was used in place of the primary antibody. All solutions
used in the in situ hybridization, such as
streptavidin-biotin alkaline phosphatase system (SA-Bio-AP),
5-bromo-4-chloro-3-indolyl-phosphate (BCIP)/nitroblue tetrazolium
(NBT), were purchased from Wuhan Boster Biological Technology, Ltd.
(Wuhan, Hubei, China). 5′-biotin-labeled, phosphorothioated probe
(5′-CAGCTCGCCACCTGCAACGA) was synthesized by Beijing Bioko
Biotechnology (Beijing, China).
The specimens were dewaxed in freshly prepared
xylene and hydrated in a series of ethanol solution, and then
dipped in freshly prepared H2O2 solution
(0.5%) for 30 min to deactivate any endogenous peroxidase. The
slices were then immersed in freshly prepared 3% citrate buffer (pH
6.0) containing trypsin (0.01 g/l) and incubated at 37°C for 10 min
to digest DNA binding proteins. Each slide was administered 20 μl
pre-heated (42°C) pre-hybridization solution without the probe and
incubated for 4 h. The slide was then administered the
hybridization solution with the probe (1 ng/l), and hybridized in a
moist chamber for 12 h at 42°C. Subsequent to being washed with
0.1× standard sodium citrate at 42°C, SA-Bio-AP was added and the
slides were incubated at 37°C for 10 min. The slides were then
washed and administered BCIP/NBT for coloration in the dark for 2
to 4 h. The negative control was treated as the samples, but
without the probe.
Evaluation criteria
DRP-1 staining was considered positive if
light-yellow to brown-colored granules were seen inside the
cytoplasm. The expression level was graded based on the percentage
of positive cells and the staining intensity, as previously
described (6) and as follows:
Samples with <10% positive cells scored 1, 10–50% scored 2 and
>50% scored 3. Based on staining intensity, negative staining
scored 0, light yellow (or blue) scored 1, medium light yellow (or
blue) scored 2 and brown-yellow (or purple-blue) scored 3.
Categorically, score 0 was graded as (−), 2 as (+) and ≥3 as (++),
where (++) represented normal expression, (−) no expression and (+)
reduced expression.
For ezrin staining, the cells with brown granules
distributed diffusely in the cytoplasm were rated as positive.
Based on the staining intensity and the number of positive cells,
the ezrin expression was classified into (−) no expression, (+)
with 50% of cells positive or with light staining, and (++) with
≥50% of cells being positive and highly stained (7), where (++) represents
overexpression.
For E-cadherin expression, the cells with small
yellow or brown granules on the membrane were considered to be
positive, while those showing granules in the cytoplasm, but not on
membrane, were rated negative. The expression level of E-cadherin
was graded according to Gonzalez’s criteria, where (−) represents
cells with no staining, (+) with <75% of cells stained and (++)
with ≥75% cells being positive (8).
Expression at (−) to (+) was considered to be negative or reduced,
or abnormal expression.
Statistical analysis
The data were analyzed with the aid of SPSS 13.0
software (SPSS, Inc., Chicago, IL, USA). The differences were
tested by χ2 test, and Spearman’s rank correlation was
performed. P<0.05 was considered to indicate a significant
difference.
Results
Expression of DRP-1, ezrin and E-cadherin
in ESCC and paracancerous cells
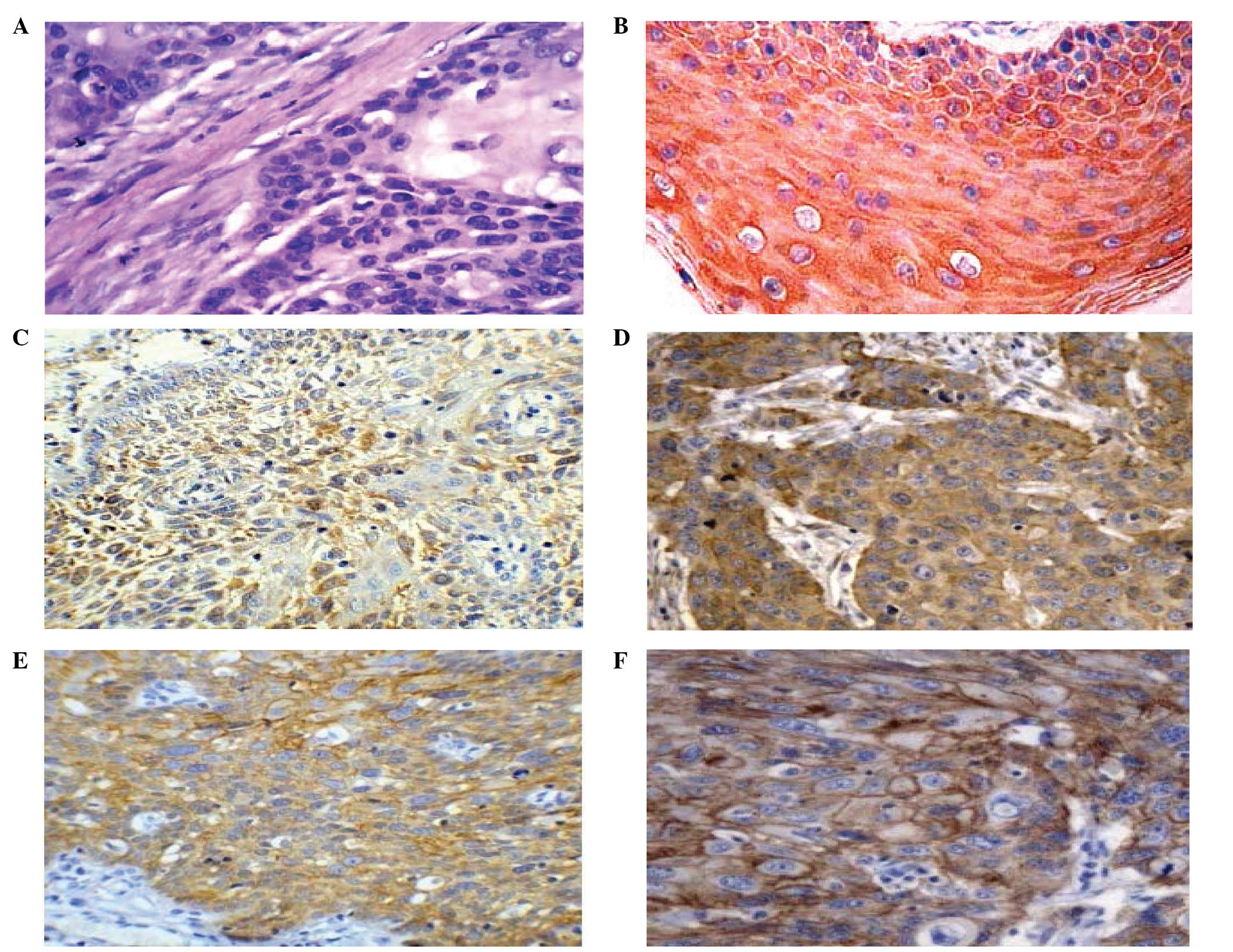
Following immunological staining, DRP-1 protein was
localized in the cytoplasm, visualized as light yellow to brown
granules in ESCC, but not in the control samples (Fig. 1A and B). Meanwhile, ezrin was
observed to be expressed in the ESCC and paracancerous normal
squamous epithelial cells, macrophages and lymphocytes in the
interstitial tissues. In the epithelial tissue, ezrin was highly
expressed in prickle cells and coenocytes, and weakly expressed or
absent in basal cells. In the cancer tissues, ezrin was mainly
expressed in the cytoplasm adjacent to the cell membrane, with few
observed on the cell membrane (Fig. 1C
and D). E-cadherin was mainly expressed on the cell membrane as
brown granules in the cancer and paracancerous normal squamous
epithelial tissues. In the epithelial tissues, it was intensely
expressed in the basal and prickle cells (Fig. 1E and F). The expression levels of
the three genes in the ESCC and paracancerous normal epithelial
tissues were statistically different (Table I).
 | Table IExpression of DRP-1, ezrin and
E-cadherin in ESCC and paracancerous tissues. |
Table I
Expression of DRP-1, ezrin and
E-cadherin in ESCC and paracancerous tissues.
| DRP-1, n (%) | | Ezrin, n (%) | | E-cadherin, n
(%) | |
|---|
|
| |
| |
| |
|---|
| Tissue | (+) | (−) | P-value | (+) | (−) | P-value | (+) | (−) | P-value |
|---|
| Paracancerous normal
tissue | 65 (85.5) | 11 (14.4) | | 35 (46.1) | 41 (53.9) | | 74 (97.36) | 2 (2.63) | |
| Cancer tissue | 27 (35.5) | 49 (64.4) | <0.001 | 69 (90.7) | 7 (9.2) | <0.001 | 21 (27.63) | 55 (72.36) | <0.001 |
| χ2 | 39.762 | | 51.05 | | 78.849 | |
Expression of DRP-1, ezrin and
E-cadherin, and the clinical biology of ESCC
The present analysis indicated that the expression
of DRP-1 was not associated with the age or gender of the patients,
nor with the degree of tumor differentiation, but that it was
associated with the invasiveness of the tumor and lymph node
metastasis. Ezrin expression was associated with the tumor
invasiveness and lymph node metastasis, but not with patient age
and gender, or tumor size and degree of differentiation. Meanwhile,
no association was found between the expression of E-cadherin and
patient age and gender, or tumor size and invasiveness, however, an
association with tumor differentiation and lymph node metastasis
was observed (Table II).
 | Table IIAssociation between expression of
DRP-1, ezrin, E-cadherin and clinical parameters of ESCC. |
Table II
Association between expression of
DRP-1, ezrin, E-cadherin and clinical parameters of ESCC.
| | DRP-1 level, n | | | Ezrin level, n | | | E-cadherin level,
n | | |
|---|
| |
| | |
| | |
| | |
|---|
| Clinical
parameters | n | − | + | ++ | Positive, % | P-value | − | + | ++ | Positive, % | P-value | − | + | ++ | Positive, % | P-value |
|---|
| Gender |
| Male | 49 | 21 | 10 | 18 | 36.7 | | 9 | 19 | 21 | 42.9 | | 25 | 10 | 14 | 28.6 | |
| Female | 27 | 11 | 7 | 9 | 33.3 | 0.838 | 4 | 10 | 13 | 48.1 | 0.621 | 17 | 3 | 7 | 25.9 | 0.702 |
| zc | | | −0.205 | | | | | −0.494 | | | | | −0.383 | | | |
| Age, years |
| <60 | 44 | 17 | 11 | 16 | 36.4 | | 10 | 15 | 19 | 43.2 | | 27 | 4 | 13 | 29.5 | |
| ≥60 | 32 | 16 | 5 | 11 | 34.4 | 0.844 | 8 | 11 | 13 | 40.6 | 0.796 | 15 | 9 | 8 | 25.0 | 0.467 |
| zc | | | −0.196 | | | | | −0.259 | | | | | −0.728 | | | |
| Tumor size, cm |
| <5 | 33 | 14 | 9 | 10 | 30.3 | | 15 | 6 | 12 | 36.4 | | 16 | 8 | 9 | 27.3 | |
| ≥5 | 43 | 18 | 8 | 17 | 39.5 | 0.635 | 19 | 5 | 19 | 44.2 | 0.681 | 26 | 5 | 12 | 27.9 | 0.736 |
| zc | | | −0.475 | | | | | −0.412 | | | | | −0.338 | | | |
| Differentiation |
| Well | 26 | 11 | 6 | 9 | 34.6 | | 8 | 8 | 10 | 38.5 | | 8 | 6 | 12 | 46.2 | |
| Moderate | 33 | 14 | 7 | 12 | 36.4 | | 9 | 11 | 13 | 39.4 | | 21 | 5 | 7 | 21.2 | |
| Low | 17 | 7 | 4 | 6 | 35.3 | 0.997 | 4 | 5 | 8 | 47.1 | 0.841 | 13 | 2 | 2 | 11.8 | 0.006 |
| χ2
value | | | 0.006 | | | | | 0.346 | | | | | 10.148 | | | |
| Invasion |
| Not to
adventitia | 31 | 22 | 4 | 5 | 16.1 | | 14 | 10 | 7 | 22.6 | | 16 | 5 | 10 | 32.3 | |
| To tissue outside
adventitia | 45 | 10 | 13 | 22 | 48.9 | 0.000 | 10 | 13 | 22 | 48.9 | 0.012 | 26 | 8 | 11 | 24.4 | 0.450 |
| zc | | | −3.981 | | | | | −2.522 | | | | | −0.775 | | | |
| Lymph node
metastasis |
| Yes | 41 | 13 | 8 | 20 | 48.8 | | 10 | 10 | 21 | 51.2 | | 28 | 7 | 6 | 14.6 | |
| No | 35 | 19 | 9 | 7 | 20.0 | 0.016 | 14 | 12 | 9 | 25.7 | 0.032 | 14 | 6 | 15 | 42.8 | 0.006 |
| zc | | | −2.409 | | | | | −2.141 | | | | | −2.758 | | | |
Associations among the expression of
DRP-1, ezrin and E-cadherin in ESCC
In the 76 ESCC samples, 27 were positive and 49 were
negative for DRP-1. Of the positive and negative samples, 15 were
positive and 6 were negative for E-cadherin, respectively.
Therefore, there was a higher percentage of samples with E-cadherin
expression in the DRP-1-positive samples than in the DRP-1-negative
samples (P<0.05), indicating that the expression of the two
genes was positively correlated. There were 22 and 47
ezrin-positive samples in the DRP-1-positive and -negative samples,
respectively, indicating that there were significantly less ezrin
samples in the DRP-1-positive samples than in the negative samples
(P<0.05), indicating that the expression of the two genes was
negatively correlated (Table
III). In our previous study, a total of 16 and 43 positive
samples were observed in the samples expressing normal or abnormal
levels of E-cadherin, respectively, indicating that there was a
decreased number of ezrin-positive samples in the tissues with
normal E-cadherin expression than in those with abnormal
expression, indicating that the two genes were negatively
correlated (17).
 | Table IIIAssociation between expression of
DRP-1, ezrin and E-cadherin in ESCC. |
Table III
Association between expression of
DRP-1, ezrin and E-cadherin in ESCC.
| | Ezrin | | E-cadherin | |
|---|
| |
| |
| |
|---|
| Sample | n | (+) | (−) | P-value | (+) | (−) | P-value |
|---|
| DRP-1-positive | 27 | 22 | 5 | | 15 | 12 | |
| DRP-1-negative | 49 | 47 | 2 | <0.05 | 6 | 43 | <0.01 |
| χ2
value | | 4.338 | | 16.330 | |
Discussion
Metastasis and recurrence are the basic
characteristics of malignant tumors, with 90% of tumor patients
succumbing to distant metastasis (9). Studies have shown that DRP-1 is a
calcium calmodulin-regulated serine/threonine kinase involved in
apoptotic and autophagic cell death, and the suppression of tumors
and metastasis (10,11). Decreased or absent DRP-1 expression
has been found in a number of tumor cells and tissues, which may be
associated with the methylation changes at CpG sites, and play
significant roles in tumorigenesis and development (12,13).
Ezrin has been demonstrated to be involved in the interactions
between the cells and between the cells and the stroma through
regulating adhesion molecules and signal transduction. Ezrin
therefore is significant in tumor progression, invasion and
metastasis. A high level of ezrin expression has been shown to be
positively associated with malignancy and metastasis, and has been
observed to be an indicator of poor prognosis (14,15).
The calcium dependent glycoprotein, E-cadherin, is widely
distributed in epithelial cells. The main role of E-cadherin is to
mediate cellular adhesion between homogenous cells, with functional
roles in the cytoskeleton to maintain structural integrity and
epithelial polarity. E-cadherin mediates the absence or decrease of
cell adhesion, which is an important step in the metastasis of the
majority of tumors. Cell adhesion is weakened by this decrease or
absence of E-cadherin expression, resulting in tumor cells that are
easy to separate and that can grow invasively, leading to
metastasis (16). Therefore, the
three genes, DRP-1, ezrin and E-cadherin, are of significance in
tumorigenesis and disease progression.
Results from the present study showed that although
DRP-1 was expressed in the ESCC tissues and paracancerous cells,
the expression level was significantly lower in the ESCC cells,
indicating that tumors with lower DRP-1 expression are highly
invasive. Furthermore, the results showed that the abnormal
expression of the DRP-1 gene was associated with the depth of the
invasion of the cancer and lymph node metastasis, indicating that
abnormal expression may be used as an indicator for a poor
prognosis in order to improve our understanding of the biology of
the cancer. The DRP-1 gene may also provide a novel option for the
early diagnosis and treatment of ESCC. Ezrin was highly expressed
to a significant degree in the ESCC tissues compared with the
paracancerous cells, indicating that it may play roles in the
progression and metastasis of ESCC. Furthermore, its expression was
found to be associated with the invasiveness of the cancer and
lymph node metastasis, indicating that the expression of ezrin is
an indication of tumor invasion and lymph node metastasis in
patients, thereby resulting in a poor prognosis. Therefore, the
detection of ezrin expression can be used to determine an early
prognosis (17). In ovarian
carcinoma, weak or absent ezrin expression in serous ovarian
carcinoma has been associated with an unfavorable prognosis in
patients (18). In the present
study, E-cadherin expression was associated with the
differentiation and lymph node metastasis of ESCC, but had no
association with the age and gender of the patient, or the tumor
size and invasiveness. The positive rate of E-cadherin expression
was lower in the cancer tissues of the patients with lymph node
metastasis than in those without (P<0.05), and was lower in the
poorly-differentiated cancer tissues than in the
well-differentiated tissues (P<0.05), indicating that a
reduction in the expression of E-cadherin may be attributed to the
differentiation and lymph node metastasis of ESCC. E-cadherin was
similarly expressed in the cancer tissues with and without
adventitia invasion, indicating that E-cadherin is not responsible
for ESCC invasiveness. Further analysis indicated that there were
negative and positive associations between DRP-1 and ezrin
(P<0.01), and DRP-1 and E-cadherin (P<0.01), respectively,
and the expression of ezrin and E-cadherin was found to be
negatively associated (P<0.01). The results indicated that the
three genes are interconnected, interactive and correlate with each
other in their roles in tumorigenesis, progression, invasion and
metastasis.
Tumor invasion and metastasis are multistage,
multistep and multifactor processes, affected by the
characteristics of the tumor cells, the overall immune status of
the host and the characteristics of the local tissues being
metastasized. The present results showed that DRP-1, ezrin and
E-cadherin are involved in the tumorigenesis and metastasis of
ESCC. DRP-1 and E-cadherin were shown to be negatively associated
with ezrin, indicating that they may be antagonistic to each other.
Simultaneous analysis of the expression of the three genes would
aid in the determination of the differentiation degree, the
invasiveness and the potential for metastasis, as well as serving
as novel indicators for prognosis. Future studies are required to
further elucidate the associations among the three genes.
Acknowledgements
The study was financially supported by Science
Supports from the Hebei Science and Technology Department (grant
no. 112061176D).
References
|
1
|
Deiss LP, Feinstein E, Berissi H, Cohen O
and Kimchi A: Identification of a novel serine/threonine kinase and
a novel 15-kD protein as potential mediators of the gamma
interferon-induced cell death. Genes Dev. 9:15–30. 1995.
|
|
2
|
Brabender J, Arbab D, Huan X, Vallböhmer
D, Grimminger P, Ling F, Neiss S, Bollschweiler E, Schneider PM,
Hölscher AH and Metzger R: Death-associated protein kinase (DAPK)
promoter Methylation and response to neoadjuvant radiochemotherapy
in esophageal cancer. Ann Surg Oncol. 16:1378–1383. 2009.
|
|
3
|
Tong A, Lynn G, Ngo V, Wong D, Moseley SL,
Ewbank JJ, Goncharov A, Wu YC, Pujol N and Chisholm AD: Negative
regulation of Caenorhabditis elegans epidermal damage
responses by death-associated protein kinase. Proc Natl Acad Sci
USA. 106:1457–1461. 2009.
|
|
4
|
Li H, Ray G, Yoo BH, Erdogan M and Rosen
KV: Down-regulation of death-associated protein kinase-2 is
required for beta-catenin-induced anoikis resistance of malignant
epithelial cells. Biol Chem. 284:2012–2022. 2009.
|
|
5
|
McClatchey AI: Merlin and ERM proteins:
unappreciated roles in cancer development? Nat Rev Cancer.
3:877–883. 2003.
|
|
6
|
Zhai J, Yang X, Zhang Y, Qi Q, Hu J and
Wang Q: Reduced expression levels of the death-associated protein
kinase and E-cadherin are correlated with the development of
esophageal squamous cell carcinoma. Experimental And Therapeutic
Med. 5:972–976. 2013.
|
|
7
|
Mathew J, Hines JE, Obafunwa JO, Burr AW,
Toole K and Burt AD: CD44 is expressed in hepatocellular carcinomas
showing vascular invasion. J Pathol. 179:74–79. 1996.
|
|
8
|
Gonzalez MA, Pinder SE, Wencyk PM, Bell
JA, Elston CW, Nicholson RI, Robertson JF, Blamey RW and Ellis IO:
An immunohistochemical examination of the expression of E-cadherin,
alpha- and beta/gamma-catenins, and alpha2- and betal-integrins in
invasive breast cancer. J Pathol. 187:523–529. 1999.
|
|
9
|
Cavallaro U and Christofori G:
Multitasking in tumor progression: signaling function of cell
adhension molecules. Ann NY Acad Sci. 1014:58–66. 2004.
|
|
10
|
Bajbouj K, Poehlmann A, Kuester D, Drewes
T, Haase K, Hartig R, Teller A, Kliche S, Walluscheck D, Ivanovska
J, Chakilam S, Ulitzsch A, Bommhardt U, Leverkus M, Roessner A and
Schneider-Stock R: Identification of phosphorylated p38 as a novel
DAPK-interacting partner during TNFalpha-induced apoptosis in
colorectal tumor cells. Am J Pathol. 175:557–570. 2009.
|
|
11
|
Zalckvar E, Berissi H, Eisenstein M and
Kimchi A: Phosphorylation of Beclin 1 by DAP-kinase promotes
autophagy by weakening its interactions with Bcl-2 and Bcl-XL.
Autophagy. 5:720–722. 2009.
|
|
12
|
Hoffmann AC, Vallböhmer D, Prenzel K,
Metzger R, Heitmann M, Neiss S, Ling F, Hölscher AH, Schneider PM
and Brabender J: Methylated DAPK and APC promoter DNA detection in
peripheral blood is significantly associated with apparent residual
tumor and outcome. J Cancer Res Clin Oncol. 135:1231–1237.
2009.
|
|
13
|
Fendri A, Masmoudi A, Khabir A,
Sellami-Boudawara T, Daoud J, Frikha M, Ghorbel A, Gargouri A and
Mokdad-Gargouri R: Inactivation of RASSF1A, RARbeta2 and DAP-kinase
by promoter methylation correlates with lymph node metastasis in
nasopharyngeal carcinoma. Cancer Biol Ther. 8:444–451. 2009.
|
|
14
|
Akisawa N, Nishimori I, Iwamura T, Onishi
S and Hollingsworth MA: High levels of ezrin expressed by human
pancreatic adenocarcinoma cell lines with high metastatic potentia.
Biochem Biophys Res Commun. 258:395–400. 1999.
|
|
15
|
Makitie T, Carpén O, Vaheri A and Kivelä
T: Ezrin as a prognostic indicator and its relationship to tumor
characteristics in uveal malignant melanoma. Invest Ophthalmol Vis
Sci. 42:2442–2449. 2001.
|
|
16
|
Santos-García A, Abad-Hernández MM,
Fonseca-Sánchez E, Julián-González R, Galindo-Villardón P,
Cruz-Hernández JJ and Bullón-Sopelana A: E-cadherin, laminin and
collagen IV expression in the evolution from dysplasia to oral
squamous cell carcinoma. Med Oral Patol Oral Cir Bucal.
11:E100–E105. 2006.
|
|
17
|
Zhai JW, Yang XG, Yang FS, Hu JG and Hua
WX: Expression and clinical significance of Ezrin and E-cadherin in
esophageal squamous cell carcinoma. Chin J Cancer. 29:317–320.
2010.
|
|
18
|
Moilanen J, Lassus H, Leminen A, Vaheri A,
Bützow R and Carpén O: Ezrin immunoreactivity in relation to
survival in serous ovarian carcinoma patients. Gynecol oncol.
90:273–281. 2003.
|