Introduction
In the global population, metastasis is the most
frequent and life-threatening complication associated with cancer
(1). Metastasis leads to >90% of
mortalities in cancer patients (1),
and ~95% of patients who succumb to metastatic disease have
metastasis in the lung, as indicated by autopsy (2). For osteosarcoma (OS), >80% of
patients develop a recurrent disease within 2 years. When treated
by surgery alone, more than half of patients will develop
metastasis within 6 months (3).
Studies have shown that MSCs promote the growth and pulmonary
metastasis of breast cancer and OS (4,5).
Several studies have also shown that the injection of MSCs into a
vein promoted the metastasis established in subcutaneous or primary
sites (6,7). In addition, a study showed that B16
melanoma cells transplanted into allogeneic mice did not form
tumors unless co-injected with MSCs (8). Subcutaneous inoculation of COS1NR
cells followed by intravenous injection of MSCs at weeks 3 and 5
significantly increased the number of lung nodules (9). It has also been demonstrated that MSCs
enhance the survival of follicular lymphoma B cells derived from
human tumors. Additionally, treating MSCs with tumor necrosis
factor-α increased the protective effect of MSCs; however, the
mechanism by which MSCs are involved in the regulation of tumor
cells remains elusive (as reviewed in 8). MSCs appear to play a significant role
in the adaptation of these traits by carcinoma cells, initiating
carcinoma cell phenotypes (10).
These studies also indicated that the tumor cells may interact with
MSCs, and MSCs subsequently promote the established
micrometastasis.
Having a strong carcinogenic capability, UMR-106 is
an aggressive, poorly immunogenic OS cell line with an osteoblastic
phenotype (11). In 2001, UMR-106
cells were first used in orthotopic implantation animal models by
inoculation into the tibia of athymic mice (12). The present study aimed to
investigate whether homologous MSCs could interact with UMR-106
cells and promote UMR-106 cell growth and pulmonary metastasis
within a normal immune system. Various established animal models
with or without co-injection of UMR-106 cells and homologous MSCs
were also compared. The aim of the present study was to explore the
function of MSCs in the pulmonary metastasis of UMR-106 cells and
the possible underling mechanisms of MSCs in promoting the
emergence of UMR-106 cells, as well as the growth of pulmonary
metastasis in rats with a normal immune system.
Materials and methods
OS cell line
The UMR-106 cell line, syngenic to Sprague-Dawley
(SD) rats, was purchased from the American Type Culture Collection
(Manassas, VA, USA). The UMR-106 cells were cultured in Dulbecco’s
modified Eagle’s medium (DMEM) in 10% fetal bovine serum (FBS)
(Sigma Aldrich, St. Louis, MO, USA) supplemented with L-glutamine
(2 mM; Sigma Aldrich), penicillin (100 U/ml; Sigma Aldrich) and
streptomycin (100 μg/ml; Sigma Aldrich), and confirmed to be
mycoplasma-free by routine testing.
Cell culture of MSCs
Male SD rats, 2 weeks old, were purchased from the
Laboratory Animal Research Center of the Fourth Military Medical
University (Xi’an, China). The rats were maintained in
micro-isolator cages under specific pathogen-free conditions. The
temperature was maintained at 24°C and the animals were exposed to
a 24-h circadian rhythm with free access to water and food. The
study was previously approved by the Fourth Military Medical
University Ethics Committee for Animal Research. The bone marrow
was aseptically collected and subsequently cultured using
whole-marrow differential adherence methods (12). MSCs were obtained by multiple
digestions and passages. MSCs were identified by the cellular
surface marker expression [i.e., cluster of differentiation 29
(CD29), CD34, CD45 and CD90] using flow cytometry (13). Third generation MSCs were used in
subsequent experiments and were pre-labeled with 4 μg/ml
chloromethyl-dialkylcarbocyanine (CM-Dil; Invitrogen Life
Technologies, Carlsbad, CA, USA) for 5 min at 37°C in pre-warmed
phosphate-buffered saline (PBS), followed by incubation for 15 min
at 4°C before the implantation experiment.
Implantation technique
Four-week-old male SD rats were divided into six
groups at random, with or without injected UMR-106 into the tibia
(IT) or the caudal vein (IV) and with or without injection of MSCs
into the caudal vein (IV). All animals were subsequently
anesthetized intraperitoneally with 10% chloral hydrate at a dosage
of 0.1 ml/30 g body weight and the operative field was prepared
with iodine and draped. Orthotropic implantation models of the rats
were performed by IT injection directly into the rats with a
syringe (25-gauge needle) for inoculation with UMR-106 cells.
Respectively, UMR-106 cells (1×107 cells in 100 μl) were
injected intraosseously into the proximal part of the tibia shaft.
The tumor size was determined by measuring the largest and smallest
diameter. The metastatic tumor volume was calculated according to
the following formula: Tumor volume (mm3) = [largest
diameter (mm) × smallest diameter (mm)2]/2.
Histopathological and immunohistochemical
examination
Following fixation in buffered isotonic formaldehyde
(100 ml of 37% formaldehyde solution, 900 ml distilled water, 4 g
monobasic sodium phosphate and 6.5 g dibasic sodium phosphate),
implantation tumor and lung sections were embedded in paraffin for
24 h. Samples were then immersed in 70% alcohol and stained with
hematoxylin-eosin. The samples were examined by a pathologist in a
blinded manner (12).
Co-cultured MSCs and UMR-106 cells in a suspension
of 1×104 cells (MSCs:UMR-106 cell ratio, 1:1) in 2 mL
DMEM and 10% FBS were added into each dish. Following incubation
for 12 h, the medium was replaced with DMEM and 1% FBS.
Subsequently, after incubation for 24 h, the cells were washed with
PBS, fixed with 4% paraformaldehyde for 30 min, and then prepared
for histopathological and immunohistochemical examination. Each
experiment was performed in triplicate. Cell climbing slices were
treated with 3% hydrogen peroxide in methanol for 10 min to
inactivate endogenous peroxidases and were then treated with a VEGF
rabbit anti-mouse, anti-rat and anti-human polyclonal primary
antibody (Abcam, Cambridge, MA, USA) overnight at 4°C. Subsequent
to rinsing with PBS, the cell climbing slices were treated for 20
min with pre-diluted biotin-conjugated broad-spectrum
immunoglobulin G polyclonal goat anti-rabbit, and anti-mouse
secondary antibody (SBS Genetech Co., Ltd., Beijing, China), and
then visualized using streptavidin-conjugated horseradish
peroxidase provided with the Real Envision Detection kit (SBS
Genetech Co., Ltd.) following instructions specified by the
manufacturer.
ELISA assay
To determine the secretion of VEGF in the
supernatants from UMR-106, MSCs or co-cultured UMR-106 cells +
MSCs, cells were plated in medium containing 1% FBS. After the
cells were cultured for 48 h, the supernatants were collected
according to the manufacturer’s instructions. The media were
analyzed by a commercially available sandwich VEGF ELISA kit
(Invitrogen Life Technologies). Assays were performed in
quadruplicate. Results were normalized for the number of producing
cells and reported as picograms of VEGF in 1×106 cells
per 48 h.
Statistical analysis
SPSS 11.0 (SPSS, Inc., Chicago, IL, USA) was used
for data variation analysis. Data were obtained from at least three
independent experiments and presented as the mean ± standard
deviation. Comparisons between two groups were performed with
Student’s t-test, and the statistical significance of mean
differences among multiple groups was obtained by analysis of
variance followed by Dunnett’s post-hoc test. P<0.05 was
considered to indicate a statistically significant difference.
Results
OS pulmonary metastasis is promoted by
co-injection with MSCs
The development of OS metastasis was tested in
response to MSCs to study the metastasis interaction between OS and
MSCs and the underlying mechanism. Injection of UMR-106 cells with
or without injection of MSCs through the caudal vein resulted in
the tumors of the leg expanding vigorously with a time-lapse in the
first 3 weeks. The tumor size was measured and calculated weekly
post-inoculation, as in our previous study (12). There were no metastatic sarcomas in
the liver, spleen and kidney for all groups. The pulmonary tumor
metastatic rate is shown in Table
I. Data were obtained from the measurement of the pulmonary
metastasis rate of the UMR-106 cells. It was shown that there were
no metastatic tumors in the control group, which were injected with
normal saline solution, and that there were also no metastatic
tumors in the MSC group. While in the other four groups, the number
of rats having macroscopic and microscopic visible tumors
increased. In the UMR-106 (IT) + MSCs (IV) group, 6 out of 10 rats
had metastatic tumors. However, the number of rats with metastatic
tumors (10/10) in the UMR-106 (IV) and UMR-106 (IV) + MSCs (IV)
groups was identical.
 | Table ITumor metastatic rate. |
Table I
Tumor metastatic rate.
| Group | Time to
autopsya, weeks | Rats with macroscopic
tumors, n | Rats with microscopic
tumors, n |
|---|
| Control | 5 | 0/10 | 0/10 |
| UMR-106 (IT) | 5 | 5/10 | 5/10 |
| MSCs (IV) | 5 | 0/10 | 0/10 |
| UMR-106 (IT) and MSCs
(IV) | 5 | 6/10 | 6/10 |
| UMR-106 (IV) | 5 | 10/10 | 10/10 |
| UMR-106 (IV) and MSCs
(IV) | 5 | 10/10 | 10/10 |
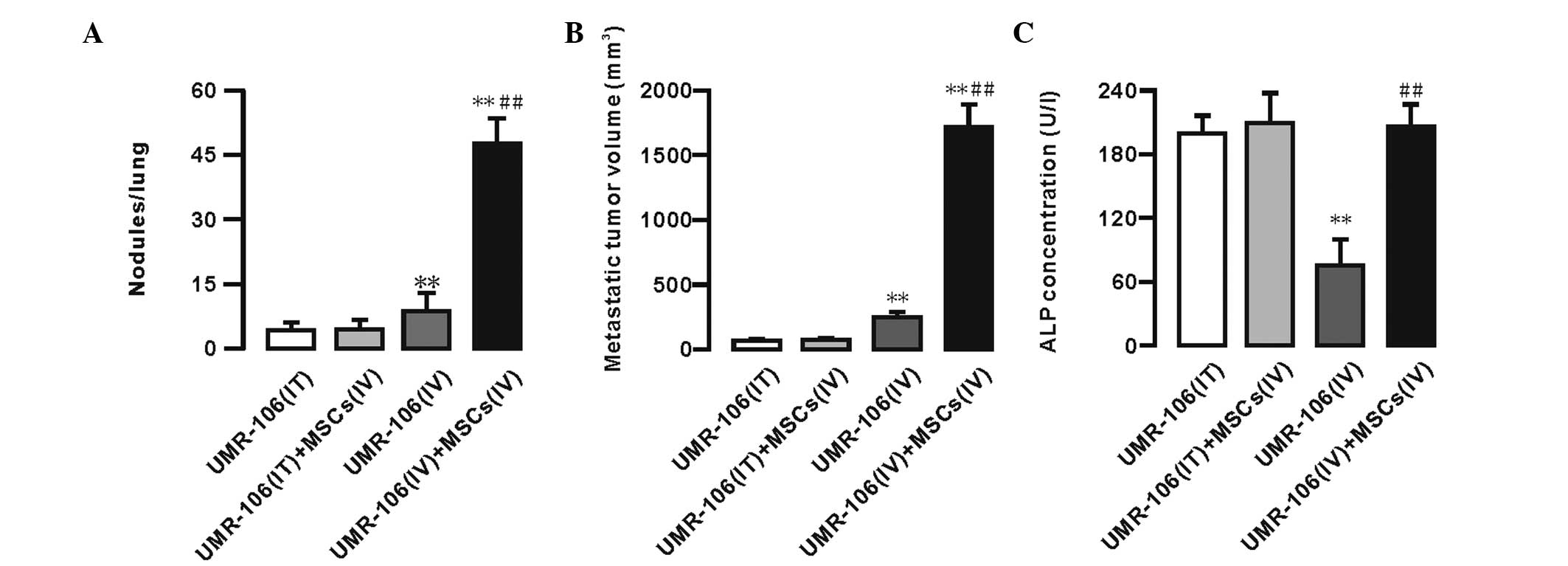
Furthermore, 5 weeks after the injection, the
metastatic tumor nodules and volume per lung were measured. No
significant differences in the number of metastatic tumor nodules
and the metastatic tumor volume were identified between the UMR-106
(IT) and UMR-106 (IT) + MSCs (IV) groups. However, an increased
number of metastatic tumor nodules and an enhanced metastatic tumor
volume was observed in the UMR-106 (IV) + MSCs (IV) group (Fig. 1A and B). The data showed that the
number of metastatic tumor nodules in the UMR-106 (IV) + MSCs (IV)
group was significantly increased compared with that in the UMR-106
(IV) group (47.84±5.51 vs. 8.63±3.70; n=10; P<0.01). The
metastatic tumor volume in the UMR-106 (IV) + MSCs (IV) group was
significantly increased compared with that in the UMR-106 (IV)
group (1737.4±199.61 vs. 251.84±56.04; n=10; P<0.01). The
primary tibia tumor volume of the UMR-106 (IT) + MSCs (IV) group
was greater than that of the UMR-106 (IT) group before the third
week, but there was no difference between the UMR-106 (IT) and
UMR-106 (IT) + MSCs (IV) groups in the fifth week (data not shown).
Furthermore, the levels of alkaline phosphatase (ALP) in the blood
serum were measured at week 5 to determine the progression of OS
metastasis. The data showed that the serum ALP levels were not
significantly different between the UMR-106 (IT) and UMR-106 (IT) +
MSCs (IV) groups (198.39±16.92 vs. 208.04±30.71 U/l; n=10;
P>0.05). In the UMR-106 (IV) group, the ALP levels were
significantly decreased compared with those in the UMR-106 cells
(IT) group, whereas the ALP levels were significantly enhanced in
the UMR-106 (IV) + MSCs (IV) group compared with those in the
UMR-106 (IV) group (205.29±23.59 vs. 75.12±24.12; n=10; P<0.01).
However, there were no significant differences between the UMR-106
(IV) + MSCs (IV) group compared with the UMR-106 (IT) and UMR-106
(IT) + MSCs (IV) groups (Fig.
1C).
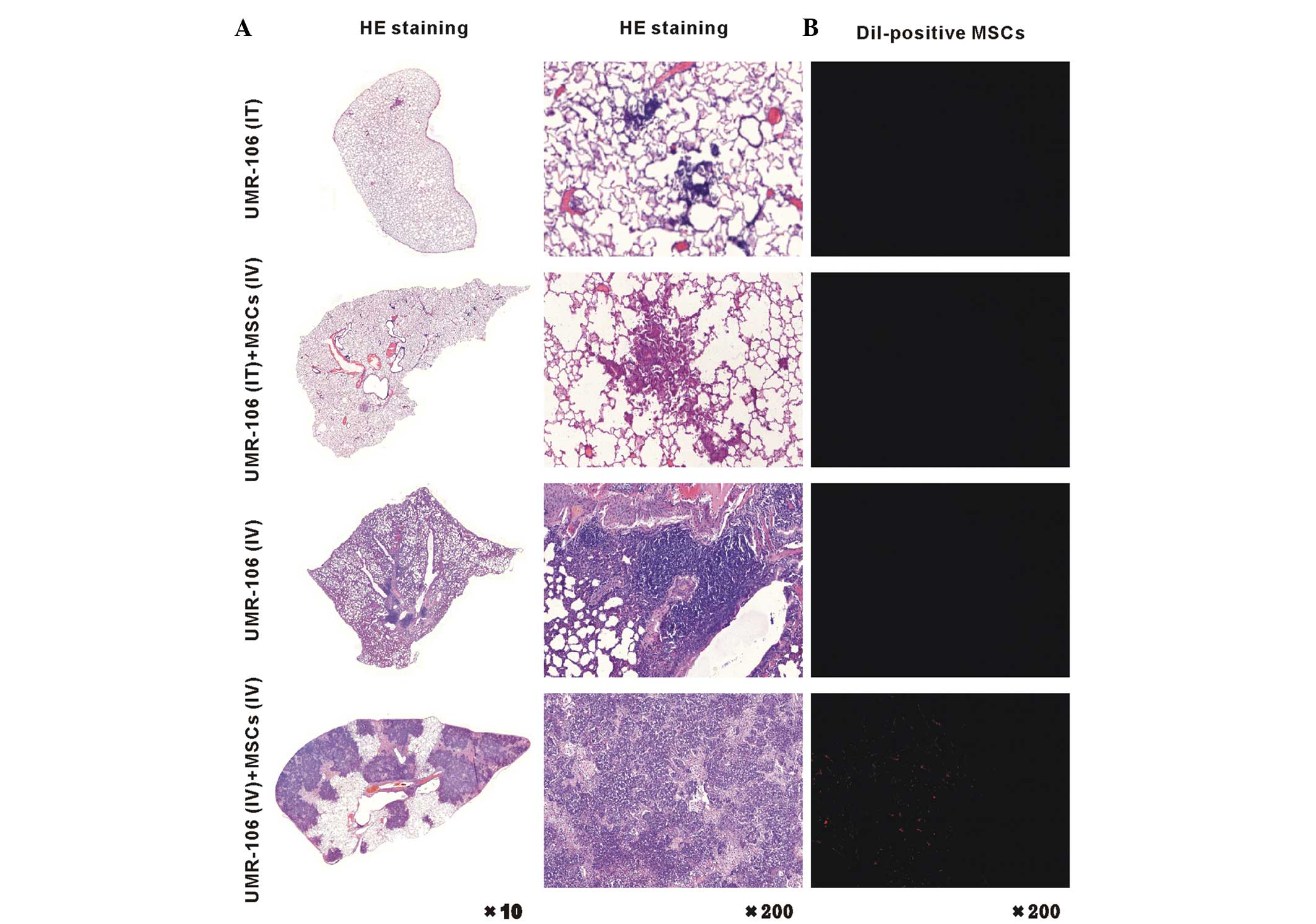
MSCs increase in the OS tumor pulmonary
metastatic site
Pathology results of the distribution of OS
pulmonary metastatic site are shown in Fig. 2. The pulmonary metastatic OS was
significantly increased in the UMR-106 (IV) + MSCs (IV) rats,
compared with that in the UMR-106 (IT) + MSCs (IV) and UMR-106 (IV)
rats (Fig. 2, left panel). Five
weeks after injection of the CM-Dil-labeled MSCs, which can stain
the MSC cell membrane red, an enhanced large portion of MSCs was
found in the lung of the UMR-106 (IV) + MSCs (IV) group (Fig. 2, right panel). We propose that the
UMR-106 cells were driven to undergo pulmonary metastasis by
components that were secreted by MSCs, or that chemoattraction
caused the UMR-106 cells and MSCs to intricately interact,
resulting in the development of pulmonary metastasis. Overall,
these observations indicate that the development and progression of
OS pulmonary metastasis were promoted in response to MSCs.
VEGF expression and secretion is enhanced
in the MSCs and UMR-106 cells co-culture system
Growth of the UMR-106 cells and MSCs in co-culture
system is shown in Fig. 3A. These
UMR-106 cells showed a colony-like growth and the MSCs distributed
between the colonies in a dense area. In the fluorescence
microscopy images, the blue fluorescence were the UMR-106 cells
labeled with Hoechest, and the red fluorescence were the MSCs
labeled with Dil (x100). The expression of VEGF was also analyzed
in the UMR-106 cells and MSCs by immunohistochemistry. Positive
immunohistochemical staining for VEGF is shown in Fig. 3B. The VEGF protein was detected in
the cytoplasm and membrane of MSCs and/or UMR-106 cells. The basal
VEGF expression of MSCs and UMR-106 cells was low. However, in the
UMR-106 + MSCs co-culture model system, when the two cells were
co-cultured for 48 h, an increase in VEGF expression was observed.
The levels of VEGF were also measured in the supernatants of the
MSCs and UMR-106 cells co-culture system by ELISA (Fig. 3C) in the following groups: UMR-106
cells (1.0×106 cells) alone, MSCs (1.0×106
cells) alone and co-culture of UMR-106 cells + MSCs
(0.5×106 cells each). The data showed that an extremely
low level of VEGF was secreted in cultured MSC supernatants, while
in the UMR-106 cells group, the VEGF levels were significantly
higher compared with the MSCs group (66.23±17.85 vs. 14.04±5.97
pg/1×106 cells/48 h; n=4; P<0.01). However, in the
supernatants of the MSCs co-cultured with UMR-106 cells group, the
concentration of VEGF was significantly increased (184.45±22.44
pg/1×106 cells/48 h; n=4; P<0.01) compared with the
MSCs or UMR-106 cells group (Fig.
3C).
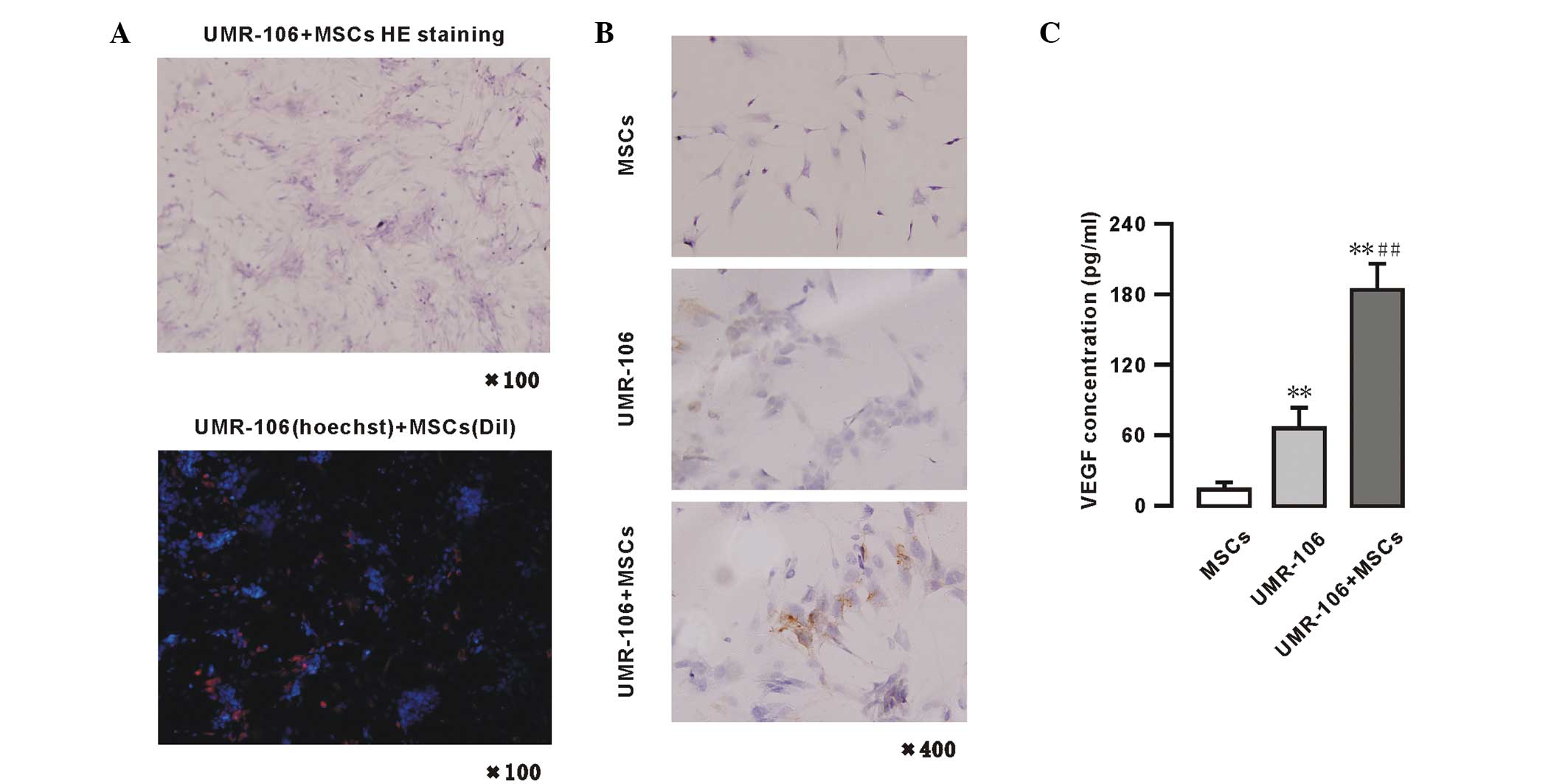 | Figure 3VEGF protein expressed in MSCs and
UMR-106 cells in a co-culture system. (A) Pathological (HE
staining; magnification, ×100) and fluorescence microscopy (blue,
UMR-106 cells labeled with Hoechest; and red, MSCs labeled with
Dil; magnification, ×100) analyses of a co-culture of UMR-106 cells
and MSCs. (B) Immunohistochemistry for VEGF of UMR-106 cells and
MSC colonies (magnification, ×400); (C) VEGF secretion in MSCs and
UMR-106 cells, as determined by ELISA. Results are expressed as the
mean ± standard deviation. *P<0.05 and
**P<0.01, vs. the MSC group. #P<0.05
and ##P<0.01, vs. the UMR-106 group. VEGF, vascular
endothelial growth factor; MSCs, mesenchymal stem cells; HE,
hematoxylin and eosin; Dil, dialkylcarbocyanine. |
Discussion
The present study showed that homologous MSCs
promoted the pulmonary metastasis significantly subsequent to
UMR-106 entering into circulation in the SD rat model, and MSCs
were present in the pulmonary metastatic nodules. In addition, the
UMR-106 cells and MSCs expressed little VEGF separately, but
UMR-106 cells and MSCs expressed high levels of VEGF in a mixed
culture. These results demonstrate that the interaction with MSCs
causes the survival of UMR-106 cells and establishes metastasis in
pulmonary parenchyma.
The cross-talk between tumor cells and the
surrounding peri-tumoral stroma has been studied recently (14). The contribution of MSCs is believed
to regulate carcinoma cell growth and motility (15). The homologous Dil-labeled MSCs were
found in the metastatic colonies and MSCs increased the metastatic
nodules in the lung (Fig. 2).
However, other studies have also shown that prior to dissemination
of the metastatic tumor cells the environment of the lung was
altered in mice bearing subcutaneous metastatic melanomas or lung
carcinomas (16–18). In these studies, by directing the
recruitment of bone marrow-derived cells to the lungs, the tumors
effected alterations in the distant lung parenchyma, in which
disseminated tumor cells subsequently settled.
Metastasis is a cascade of molecular and cellular
events, which involve tumor cell intravasation, transport and
immune evasion in the circulatory system; arrest at a secondary
site; extravasation; and finally colonization and growth (19). Once the cancer cells have entered
the blood circulation, the number of cancer cells that eventually
generate metastatic foci is even less (20,21)
The possible mechanisms underlying the tumor and host MSCs
interactions are associated with the steps of the metastasis. These
include MSCs chemoattracted to UMR-106 cells that then become
trapped UMR-106 cells in the circulation. The two types of cells
interact with each other and express VEGF and achieve metastasis in
pulmonary parenchyma. Notably, bone marrow-derived inflammatory
cells have been found in elevated concentrations in the blood of
patients with cancer (22). VEGF is
one of these factors, which is secreted by tumor-associated
inflammatory cells and fibroblasts, and acts pleiotropically to
affect tumor cell proliferation, invasion and angiogenesis
(5). The data of the present study
showed that MSCs and UMR-106 cells expressed a low level of VEGF
separately, but their mixed colonies expressed a high level of
VEGF. This indicates that they interacted with each other in the
mixed culture system and also upregulated the expression of VEGF.
OS with lung metastasis has been reported to exhibit a high
expression of VEGF (18,23,24,25).
Our previous study showed that VEGF could determine the endothelial
cell activation, proliferation and migration (26). VEGF is also a known OS angiogenesis
inducer (24). OS with lung
metastasis has been reported to exhibit a high expression of VEGF.
VEGF promotes mitosis of vascular endothelial cells, dilates blood
vessels, increases vascular permeability and induces the expression
of a number of genes involved in the degradation of the vascular
basement membrane (27–29). Tumors that exhibited a positive VEGF
expression presented a worse prognosis (26).
Primary tumors cells recruit and induce the MSC
differentiation residing locally in their origin sites. In
addition, these tumors may release signals to induce the
mesenchymal progenitor cells that circulate to extravasate and take
up residence in the tumor stroma, and these tumor cells may also be
induced to differentiate into various mesenchymal lineages.
Previous findings indicate that a third of tumors release endocrine
signals to impinge on the bone marrow, where these signals induce
various types of stromal precursor cells to form and mobilize into
the circulation, even prior to the mobilization of tumor cells into
the circulation (30,31). Various types of tumors have an
organ-specific preference for metastasis; while the metastatic
behavior of OS varies, >80% of all OS metastasis arise in the
lungs and other organs usually remain unaffected (32). The results of the present study
showed that MSCs promote the pulmonary metastasis of OS, and the
two cell types (MSCs and UMR-106) could interact with each other
and increase the level of VEGF. These partly explain the mechanisms
of metastasis of OS. However, why the metastasis has arisen in the
lungs and how to modulate the expression of VEGF is unclear.
The present study demonstrated that MSCs promoted
pulmonary metastasis following dissemination of UMR-106 and the
level of VEGF increased in the UMR-106 and MSCs co-culture system.
However, the steps of metastasis, whereby MSCs aid UMR-106 cells to
achieve immune evasion within the circulatory system and how they
interact with each other to upregulate the expression of VEGF,
requires further investigation. These will help to develop
strategies to block the OS invasion-metastasis cascade and to know
the process occurring during the tumor cell dissemination from the
primary site.
Acknowledgements
The present study was supported by grants from the
National Nature Science Foundation of China (no. 81072194). The
authors would like to thank the English editor, Dong Shuhua, from
Guangdong University of Foreign Studies.
Abbreviations:
|
CM-Dil
|
chloromethyl-dialkylcarbocyanine
|
|
MSCs
|
mesenchymal stem cells
|
|
OS
|
osteosarcoma
|
|
VEGF
|
vascular endothelial growth factor
|
References
|
1
|
Sleeman J and Steeg PS: Cancer metastasis
as a therapeutic target. Eur J Cancer. 46:1177–1180. 2010.
|
|
2
|
Meyers PA and Gorlick R: Osteosarcoma.
Pediatr Clin North Am. 44:973–989. 1997.
|
|
3
|
Aung L, Gorlick R, Healey JH, et al:
Metachronous skeletal osteosarcoma in patients treated with
adjuvant and neoadjuvant chemotherapy for nonmetastatic
osteosarcoma. J Clin Oncol. 21:342–348. 2003.
|
|
4
|
Chen X, Yang TT, Wang W, et al:
Establishment and characterization of human osteosarcoma cell lines
with different pulmonary metastatic potentials. Cytotechnology.
61:37–44. 2009.
|
|
5
|
Karnoub AE, Dash AB, Vo AP, et al:
Mesenchymal stem cells within tumour stroma promote breast cancer
metastasis. Nature. 449:557–563. 2007.
|
|
6
|
Aboody KS, Brown A, Rainov NG, et al:
Neural stem cells display extensive tropism for pathology in adult
brain: evidence from intracranial gliomas. Proc Natl Acad Sci USA.
97:12846–12851. 2000.
|
|
7
|
De Palma M, Venneri MA, Roca C and Naldini
L: Targeting exogenous genes to tumor angiogenesis by
transplantation of genetically modified hematopoietic stem cells.
Nat Med. 9:789–795. 2003.
|
|
8
|
Klopp AH, Gupta A, Spaeth E, Andreeff M
and Marini FR III: Concise review: Dissecting a discrepancy in the
literature: do mesenchymal stem cells support or suppress tumor
growth? Stem Cells. 29:11–19. 2011.
|
|
9
|
Tsukamoto S, Honoki K, Fujii H, et al:
Mesenchymal stem cells promote tumor engraftment and metastatic
colonization in rat osteosarcoma model. Int J Oncol. 40:163–169.
2012.
|
|
10
|
Schall TJ, Jongstra J, Dyer BJ, et al: A
human T cell-specific molecule is a member of a new gene family. J
Immunol. 141:1018–1025. 1988.
|
|
11
|
Forrest SM, Ng KW, Findlay DM, et al:
Characterization of an osteoblast-like clonal cell line which
responds to both parathyroid hormone and calcitonin. Calcif Tissue
Int. 37:51–56. 1985.
|
|
12
|
Yu Z, Sun H, Fan Q, Long H, Yang T and Ma
B: Establishment of reproducible osteosarcoma rat model using
orthotopic implantation technique. Oncol Rep. 21:1175–1180.
2009.
|
|
13
|
Tu XH, Song JX, Xue XJ, et al: Role of
bone marrow-derived mesenchymal stem cells in a rat model of severe
acute pancreatitis. World J Gastroenterol. 18:2270–2279. 2012.
|
|
14
|
Wang H and Chen L: Tumor microenviroment
and hepatocellular carcinoma metastasis. J Gastroenterol Hepatol.
28(Suppl 1): S43–S48. 2013.
|
|
15
|
Xu WT, Bian ZY, Fan QM, Li G and Tang TT:
Human mesenchymal stem cells (hMSCs) target osteosarcoma and
promote its growth and pulmonary metastasis. Cancer Lett.
281:32–41. 2009.
|
|
16
|
Hiratsuka S, Nakamura K, Iwai S, et al:
MMP9 induction by vascular endothelial growth factor receptor-1 is
involved in lung-specific metastasis. Cancer Cell. 2:289–300.
2002.
|
|
17
|
Hiratsuka S, Watanabe A, Aburatani H and
Maru Y: Tumour-mediated upregulation of chemoattractants and
recruitment of myeloid cells predetermines lung metastasis. Nat
Cell Biol. 8:1369–1375. 2006.
|
|
18
|
Kaplan RN, Riba RD, Zacharoulis S, et al:
VEGFR1-positive haematopoietic bone marrow progenitors initiate the
pre-metastatic niche. Nature. 438:820–827. 2005.
|
|
19
|
Chambers AF, Groom AC and MacDonald IC:
Dissemination and growth of cancer cells in metastatic sites. Nat
Rev Cancer. 2:563–572. 2002.
|
|
20
|
Fidler IJ and Nicolson GL: Fate of
recirculating B16 melanoma metastatic variant cells in parabiotic
syngeneic recipients. J Natl Cancer Inst. 58:1867–1872. 1977.
|
|
21
|
Kaplan RN, Psaila B and Lyden D: Bone
marrow cells in the ‘pre-metastatic niche’: within bone and beyond.
Cancer Metastasis Rev. 25:521–529. 2006.
|
|
22
|
Zumsteg A and Christofori G: Corrupt
policemen: inflammatory cells promote tumor angiogenesis. Curr Opin
Oncol. 21:60–70. 2009.
|
|
23
|
Worth LL, Lafleur EA, Jia SF and
Kleinerman ES: Fas expression inversely correlates with metastatic
potential in osteosarcoma cells. Oncol Rep. 9:823–827. 2002.
|
|
24
|
Plate K: From angiogenesis to
lymphangiogenesis. Nat Med. 7:151–152. 2001.
|
|
25
|
Oda Y, Yamamoto H, Tamiya S, Matsuda S,
Tanaka K, Yokoyama R, Iwamoto Y and Tsuneyoshi M: CXCR4 and VEGF
expression in the primary site and the metastatic site of human
osteosarcoma: analysis within a group of patients, all of whom
developed lung metastasis. Mod Pathol. 19:738–745. 2006.
|
|
26
|
Zhang P, Dong L, Yan K, et al:
CXCR4-mediated osteosarcoma growth and pulmonary metastasis is
promoted by mesenchymal stem cells through VEGF. Oncol Rep.
30:1753–1761. 2013.
|
|
27
|
Furudoi A, Tanaka S, Haruma K, et al:
Clinical significance of vascular endothelial growth factor C
expression and angiogenesis at the deepest invasive site of
advanced colorectal carcinoma. Oncology. 62:157–166. 2002.
|
|
28
|
Kitadai Y, Amioka T, Haruma K, et al:
Clinicopathological significance of vascular endothelial growth
factor (VEGF)-C in human esophageal squamous cell carcinomas. Int J
Cancer. 93:662–666. 2001.
|
|
29
|
Tian X, Song S, Wu J, Meng L, Dong Z and
Shou C: Vascular endothelial growth factor: acting as an autocrine
growth factor for human gastric adenocarcinoma cell MGC803. Biochem
Biophys Res Commun. 286:505–512. 2001.
|
|
30
|
McAllister SS, Gifford AM, Greiner AL, et
al: Systemic endocrine instigation of indolent tumor growth
requires osteopontin. Cell. 133:994–1005. 2008.
|
|
31
|
Shojaei F, Wu X, Qu X, et al:
G-CSF-initiated myeloid cell mobilization and angiogenesis mediate
tumor refractoriness to anti-VEGF therapy in mouse models. Proc
Natl Acad Sci USA. 106:6742–6747. 2009.
|
|
32
|
PosthumaDeBoer J, Witlox MA, Kaspers GJ
and van Royen BJ: Molecular alterations as target for therapy in
metastatic osteosarcoma: a review of literature. Clin Exp
Metastasis. 28:493–503. 2011.
|