Introduction
Recurrent or advanced gastric cancer (AGC) is one of
the leading causes of cancer-related mortalities worldwide
(1–3), with a high incidence rate in Asia
(4). A large number of patients
have unresectable, locally advanced or metastatic gastric cancer at
the initial diagnosis, indicating a poor outcome (5). AGC patients have a median survival
time of between three and five months, and a five-year survival
rate of <10%, if left untreated (6–8).
Previous studies have shown that chemotherapy may
improve a patient's survival time and quality of life (6–9).
Several types of single drug regimens exist that have certain
effects on AGC, including 5-fluorouracil (5-FU), mitomycin,
cisplatin and etoposide, with effective rates of 10–20%. In recent
years, novel chemotherapeutic agents have included the taxanes
(docetaxel and paclitaxel) and oral fluoropyrimidines (capecitabine
and S-1), as well as oxaliplatin and irinotecan (10–14).
Several studies have analyzed the use of taxanes (paclitaxel and
docetaxel) in AGC as single agents or in combination (10,12,13).
In V325, a large randomized phase III study, the combination of
docetaxel, cisplatin and 5-FU (DCF) was shown to significantly
improve the time to progression (TTP), the survival time and the
response rate (RR) in untreated AGC patients compared with
cisplatin and 5-FU (CF). However, DCF treatment resulted in a
certain level of increased toxicity (14). Further studies have demonstrated
that paclitaxel plus 5-FU (PF) and docetaxel plus 5-FU (DF) appear
to have similar efficacy against advanced or recurrent gastric
cancer, with different, but acceptable, safety profiles (15,16).
Capecitabine (N4-pentoxycarbonyl-50-deoxy-5-fluorocytidine; Xeloda;
Roche Holding AG, Basel, Switzerland) is a 5-FU prodrug developed
to reduce the toxicity and enhance the intratumoral concentrations
of 5-FU. Capecitabine has been used in preclinical xenograft
models, and has been shown to be highly active against several
types of tumors, including breast, colorectal, gastric and cervical
tumors (17,18), and also against 5-FU-sensitive and
-resistant tumors (19).
Capecitabine has also been shown to be active in previously
untreated AGC patients, as a single agent (20) or in combination with other drugs,
including cisplatin, oxaliplatin, epirubicin and docetaxel
(20–23). The combination of paclitaxel and
capecitabine (PX) in AGC, however, has rarely been reported.
The present retrospective study was conducted to
investigate the efficacy and tolerability of the combination of PX
in patients with AGC as first-line therapy.
Materials and methods
Patients
Patients with advanced or recurrent gastric cancer
who were treated with PX as first-line chemotherapy between January
2001 and December 2012 at the Zhejiang Cancer Hospital (Hangzhou,
China) were retrospectively investigated. Patients eligible for
this study had histologically-confirmed advanced or recurrent
gastric cancer. Furthermore, the eligibility criteria included at
least one measurable lesion of ≥1 cm in the longest diameter or
lymphonodus of ≥1.5 cm in the shortest diameter. Patients were
treated with PX as first-line therapy. The study was approved by
the ethics committee and institutional review board of Zhejiang
Cancer Hospital and conducted in compliance with the Helsinki
Declaration.
Chemotherapy
Paclitaxel (75 mg/m2) was administered
intravenously for 3 h on days 1 and 8 of the 21-day cycle (or 150
mg/m2 on day one of the 21-day cycle), combined with
capecitabine (850 mg/m2) peroral twice daily on days
1–14. Dose adjustments were made according to the specific
situation.
Adverse effects
Toxicity was measured using the National Cancer
Institute-Common Toxicity Criteria version 2.0 (10) toxicity scales. Grade 3 to 4 toxicity
was recorded according to the medical records.
Assessment and statistics
Response was evaluated every two cycles of treatment
using the Response Evaluation Criteria in Solid Tumors (24). In cases of partial response (PR) or
complete response (CR), a confirmative computed tomography scan was
performed four weeks later. CR was defined as the complete
disappearance of all evaluable lesions, persisting for four weeks
or more. PR was defined as a ≥30% reduction in the sum of the
products of the largest perpendicular diameters in all measurable
lesions for more than four weeks, without the development of new
lesions. Progressive disease (PD) was defined as an increase in a
previous lesion by >20%, or the development of any new lesion.
Stable disease (SD) was defined as any change in a previous lesion
that did not conform with the PR or PD categories. The primary
endpoint was progression-free survival (PFS) and the secondary
endpoints were overall survival (OS), RR and toxicity. Survival
time was analyzed using the Kaplan-Meier software of SPSS version
15.0 (SPSS, Inc., Chicago, IL, USA).
Results
Patient characteristics
Due to the exclusion of cases with incomplete data
as a result of incomplete medical records and follow-up, 36
patients were investigated between January 2001 and December 2012
at the Zhejiang Cancer Hospital, and their baseline characteristics
are shown in Table I. All patients
received PX as first-line therapy and among them, 25 were male and
11 were female with a median age of 53.5 years (range, 28–75
years). A median of 4 treatment cycles were administered (range
2–8). In addition, eight patients underwent radical surgery.
 | Table IBaseline characteristics of the study
population (n=36). |
Table I
Baseline characteristics of the study
population (n=36).
| Variables | Value | % |
|---|
| Gender, n |
| Male | 25 | 69.4 |
| Female | 11 | 30.6 |
| Age, years |
| Median | 53.5 | |
| Range | 28–75 | |
| Primary site, n |
| Esophagogastric
junction | 6 | 16.7 |
| Body of stomach | 21 | 58.3 |
| Gastric antrum | 2 | 5.6 |
| Diffuse gastric
lesions | 7 | 19.4 |
| Histology, n |
|
Well-differentiated | 0 | 0.0 |
|
Moderately-differentiated | 11 | 30.6 |
|
Poorly-differentiated | 25 | 69.4 |
| Surgical history,
n |
| Yes | 8 | 22.2 |
| No | 28 | 77.8 |
Efficacy
Out of the 36 patients evaluated, one achieved a CR,
seven achieved a PR, 24 exhibited SD and four exhibited PD. The
objective RR was 22.2% (8/36) and the disease control rate was
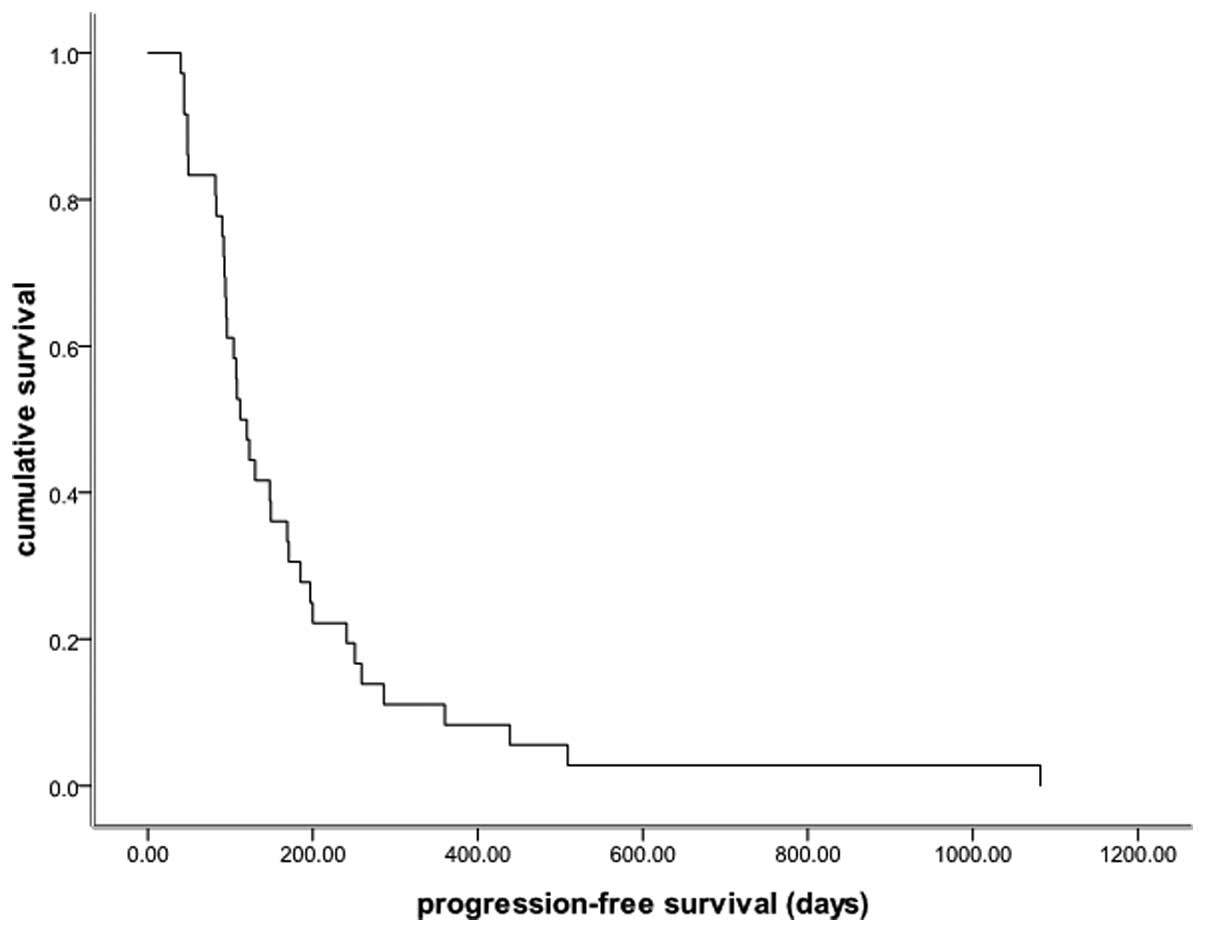
88.9% (32/36). The median PFS time was 3.7 months (95% CI, 2.9–4.5
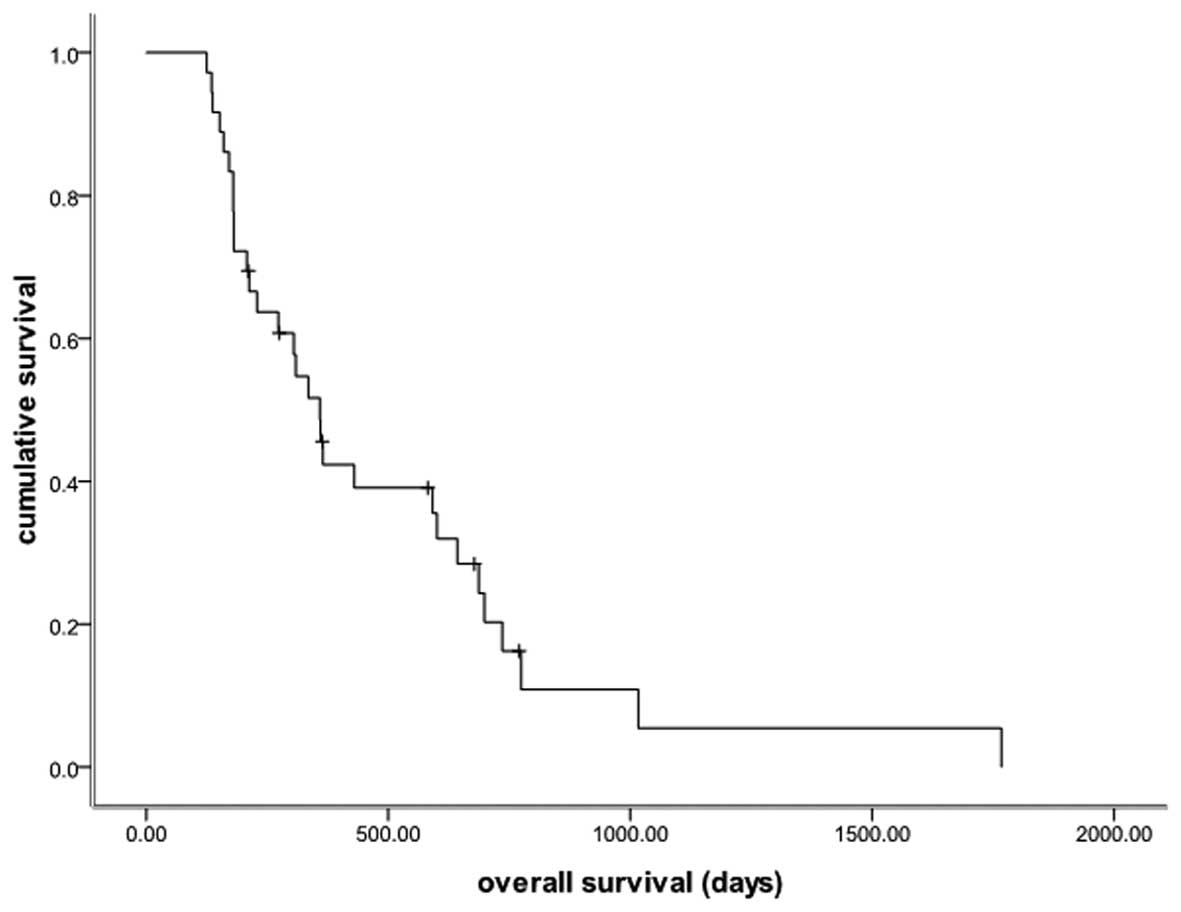
months; Fig. 1) and the median OS
time was 12.0 months (95% CI, 9.8–14.1 months; Fig. 2). The subgroup analysis showed no
difference in PFS time, regardless of gender, age, radical surgery
and tumor site (Table II).
 | Table IICox regression analysis concerning the
PFS of AGC patients. |
Table II
Cox regression analysis concerning the
PFS of AGC patients.
| Variables | HR | 95% CI | P-value |
|---|
| Gender | 0.469 | 0.186–1.182 | 0.108 |
| Age | 0.842 | 0.378–1.874 | 0.674 |
| Primary site | 0.786 | 0.756–4.233 | 0.226 |
| Surgical history | 1.788 | 0.533–1.161 | 0.186 |
Toxicity
All 36 patients were assessed for treatment safety
and the main adverse event was found to be hematological toxicity.
Grade 3 or 4 adverse events included neutropenia (2.8%), hand-foot
syndrome (2.8%) and vomiting (2.8%). No neutropenic fever or
treatment-related mortalities were observed. In addition, no other
adverse events were recorded in the medical records and no dosage
reduction occurred.
Discussion
The present study retrospectively investigated AGC
patients who were treated with PX as first-line chemotherapy
between January 2001 and December 2012 at the Zhejiang Cancer
Hospital. As few studies have been published concerning PX as
first-line chemotherapy in AGC patients, this investigation was
significant, however, it was not a prospective study or large-scale
randomized trial. In the AGC patients treated with PX as first-line
chemotherapy, the objective response and disease control rates were
22.2 and 88.9%, respectively, and the overall median survival time
was 12.0 months. These results are comparable to those reported in
the study by Kang et al (25), with a tumor RR of 48.9%, a median
TTP of 5.6 months and a median OS time of 11.3 months. The
combination of PX was well tolerated in the present study, with
only mild adverse effects [grade 3/4 neutropenia (2.8%), grade 3/4
hand-foot syndrome (2.8%) and grade 3/4 vomiting (2.8%)]. Only one
patient was reported with a grade 3 to 4 gastrointestinal reaction,
which included nausea and vomiting. Although the gender, age,
radical surgery and tumor sites differed between the patients, the
subgroup analysis showed no differences between the PFS and OS
times.
To the best of our knowledge, AGC patients have a
poor prognosis. Thus, treatment for such patients is a critical
issue facing medical workers worldwide. Systemic chemotherapy is
widely accepted as a palliative treatment for patients with AGC,
and has been confirmed to improve quality of life and prolong
survival time. In a number of Asian countries, chemotherapy
doublets are frequently used, while in Western countries, triplet
regimens are more widely adopted. However, the median survival
time, even with contemporary regimens, is typically less than one
year (14,26,27).
No standard chemotherapy regimen has been established worldwide
(28). The V325 trial, a randomized
phase III trial, has demonstrated that adding docetaxel to CF
significantly improves RR (37 vs. 25%), TTP (5.6 vs. 3.7 months)
and OS time (9.2 vs. 8.6 months), but does result in a certain
increase in toxicity, including grade 3 or 4 neutropenia (82 vs.
57%), which limits its application in clinical management (14). Further tests have revealed that PF
and DF appear similar in efficacy against advanced or recurrent
gastric cancer, with different, but acceptable, safety profiles
(15,16). PX has begun to emerge in the
treatment of AGC, and thymidine phosphorylase is an important
enzyme in the progress of capecitabine conversion to 5-FU. In a
human colon cancer xenograft model, thymidine phosphorylase was
upregulated, and synergy with PX was observed (29). This reveals that the combination of
PX may have a coordinated effect. A phase II study reported by Kang
et al (25) also showed that
in 45 AGC Korean patients treated with PX as first-line combination
chemotherapy, the tumor RR was 48.9%, the median TTP was 5.6 months
and the median OS time was 11.3 months. In addition, grade 3 or 4
adverse events included neutropenia (46.5% of patients), hand-foot
syndrome (9.3%), arthralgia (9.3%) and asthenia (4.7%). However,
few large-scale randomized trials have been conducted on Chinese
patients.
As this study was not a prospective study or
large-scale trial, it has evident deficiencies. Due to incomplete
medical records and follow-up data, only 36 patients were enrolled,
which affected the overall value of the study. However, this
retrospective study may also be considered meaningful, as few
studies have yet been published concerning PX treatment with a good
outcome in AGC patients.
In conclusion, the combination of PX may present as
a valuable first-line therapy for advanced or recurrent gastric
cancer. We hypothesize that PX may be convenient even in
maintenance chemotherapy. Future large-scale studies are urgently
required. In addition, for increased survival times and an improved
performance status, studies at the molecular biological level,
including resistance mechanisms and targeted therapy (for example,
antiangiogenic biologicals or trastuzumab in patients positive for
HER-2), are likely to be significant issues in the future.
Acknowledgements
The authors would like to express their gratitude to
all the patients who participated in the study, and thank Ms. Wang
Zeng from the Department of Pharmacy of the Zhejiang Cancer
Hospital (Hangzhou, China) for providing assistance with language
processing.
References
|
1
|
Wagner AD and Wedding U: Advances in the
pharmacological treatment of gastro-oesophageal cancer. Drugs
Aging. 26:627–646. 2009.
|
|
2
|
Bittoni A, Maccaroni E, Scartozzi M,
Berardi R and Cascinu S: Chemotherapy for locally advanced and
metastatic gastric cancer: state of the art and future
perspectives. Eur Rev Med Pharmacol Sci. 14:309–314. 2010.
|
|
3
|
Catalano V, Labianca R, Beretta GD, Gatta
G, De Braud F, et al: Gastric cancer. Crit Rev Oncol Hematol.
71:127–164. 2009.
|
|
4
|
Moore MA, Eser S, Igisinov N, Igisinov S,
Mohagheghi MA, Mousavi-Jarrahi A, Ozentürk G, Soipova M, Tuncer M
and Sobue T: Cancer epidemiology and control in North-Western and
Central Asia - past, present and future. Asian Pac J Cancer Prev.
11:17–32. 2010.
|
|
5
|
Tsai JY and Safran H: Status of treatment
for advanced gastric carcinoma. Curr Oncol Rep. 5:210–218.
2003.
|
|
6
|
Murad AM, Santiago FF, Petroianu A, Rocha
PR, Rodrigues MA and Rausch M: Modified therapy with
5-fluorouracil, doxorubicin, and methotrexate in advanced gastric
cancer. Cancer. 72:37–41. 1993.
|
|
7
|
Pyrhönen S, Kuitunen T, Nyandoto P, et al:
Randomised comparison of fluorouracil, epidoxorubicin and
methotrexate (FEMTX) plus supportive care with supportive care
alone in patients with nonresectable gastric cancer. Br J Cancer.
71:587–591. 1995.
|
|
8
|
Glimelius B, Ekstrom K, Hoffman K, Graf W,
Sjoden PO, Haglund U, Svensson C, Enander LK, Linne T, Sellstrom H
and Heuman R: Randomized comparison between chemotherapy plus best
supportive care with best supportive care in advanced gastric
cancer. Ann Oncol. 8:163–168. 1997.
|
|
9
|
Schipper DL and Wagener DJ: Chemotherapy
of gastric cancer. Anticancer Drugs. 7:137–149. 1996.
|
|
10
|
Cascinu S, Graziano F, Cardarelli N, et
al: Phase II study of paclitaxel in pretreated advanced gastric
cancer. Anticancer Drugs. 9:307–310. 1998.
|
|
11
|
Chao Y, Li CP, Chao TY, et al: An open,
multi-centre, phase II clinical trial to evaluate the efficacy and
safety of paclitaxel, UFT, and leucovorin in patients with advanced
gastric cancer. Br J Cancer. 95:159–163. 2006.
|
|
12
|
Einzig AI, Neuberg D, Remick SC, et al:
Phase II trial of docetaxel (Taxotere) in patients with
adenocarcinoma of the upper gastrointestinal tract previously
untreated with cytotoxic chemotherapy: the Eastern Cooperative
Oncology Group (ECOG) results of protocol E1293. Med Oncol.
13:87–93. 1996.
|
|
13
|
Sakamoto J, Morita S, Yumiba T, et al: A
phase II clinical trial to evaluate the effect of paclitaxel in
patients with ascites caused by advanced or recurrent gastric
carcinoma: a new concept of clinical benefit response for
nonmeasurable type of gastric cancer. Jpn J Clin Oncol. 33:238–240.
2003.
|
|
14
|
Van Cutsem E, Moiseyenko VM, Tjulandin S,
et al: Phase III study of docetaxel and cisplatin plus fluorouracil
compared with cisplatin and fluorouracil as first-line therapy for
advanced gastric cancer: a report of the V325 Study Group. J Clin
Oncol. 24:4991–4997. 2006.
|
|
15
|
Park SH, Lee WK, Chung M, et al:
Paclitaxel versus docetaxel for advanced gastric cancer: a
randomized phase II trial in combination with infusional
5-fluorouracil. Anticancer Drugs. 17:225–229. 2006.
|
|
16
|
Chon HJ, Rha SY, Im CK, Kim C, Hong MH,
Kim HR, An JR, Noh SH, Chung HC and Jeung HC: Docetaxel versus
paclitaxel combined with 5-FU and leucovorin in advanced gastric
cancer: combined analysis of two phase II trials. Cancer Res Treat.
41:196–204. 2009.
|
|
17
|
Ishikawa T, Sekiguchi F, Fukase Y, Sawada
N and Ishitsuka H: Positive correlation between the efficacy of
capecitabine and doxifluridine and the ratio of thymidine
phosphorylase to dihydropyrimidine dehydrogenase activities in
tumors in human cancer xenografts. Cancer Res. 58:685–690.
1998.
|
|
18
|
Ishikawa T, Utoh M, Sawada N, Nishida M,
Fukase Y, Sekiguchi F and Ishitsuka H: Tumor selective delivery of
5-fluorouracil by capecitabine, a new oral fluoropyrimidine
carbamate, in human cancer xenografts. Biochem Pharmacol.
55:1091–1097. 1998.
|
|
19
|
Cao S, Lu K, Ishitsuka H and Rustum YM:
Antitumor efficacy of capecitabine against fluorouracil-sensitive
and -resistant tumors. Proc Am Soc Clin Oncol. 16:266a1997.
|
|
20
|
Hong YS, Song SY, Lee SI, Chung HC, Choi
SH, Noh SH, Park JN, Han JY, Kang JH, Lee KS and Cho JY: A phase II
trial of capecitabine in previously untreated patients with
advanced and/or metastatic gastric cancer. Ann Oncol. 15:1344–1347.
2004.
|
|
21
|
Kang YK, Kim TW, Chang HM, Ryu MH, Yook
JH, Oh ST, Kim BS and Lee JS: A phase I/II trial of docetaxel,
capecitabine, and cisplatin as a first line chemotherapy for
advanced gastric cancer. Proc Am Soc Clin Oncol. 22:40662004.
|
|
22
|
Park YH, Ryoo BY, Choi SJ and Kim HT: A
phase II study of capecitabine and docetaxel combination
chemotherapy in patients with advanced gastric cancer. Br J Cancer.
90:1329–1333. 2004.
|
|
23
|
Cho EK, Lee WK, Im SA, Lee SN, Park SH,
Bang SM, Park DK, Park YH, Shin DB and Lee JH: A phase II study of
epirubicin, cisplatin and capecitabine combination chemotherapy in
patients with metastatic or advanced gastric cancer. Oncology.
68:333–340. 2005.
|
|
24
|
Eisenhauer EA, Therasse P, Bogaerts J, et
al: New response evaluation criteria in solid tumours: revised
RECIST guideline (version 1.1). Eur J Cancer. 45:228–247. 2009.
|
|
25
|
Kang HJ, Chang HM, Kim TW, et al: A phase
II study of paclitaxel and capecitabine as a first-line combination
chemotherapy for advanced gastric cancer. Br J Cancer. 98:316–322.
2008.
|
|
26
|
Cunningham D, Starling N, Rao S, et al;
Upper Gastrointestinal Clinical Studies Group of the National
Cancer Research Institute of the United Kingdom. Capecitabine and
oxaliplatin for advanced esophagogastric cancer. N Engl J Med.
358:36–46. 2008.
|
|
27
|
Webb A, Cunningham D, Scarffe JH, et al:
Randomized trial comparing epirubicin, cisplatin, and fluorouracil
versus fluorouracil, doxorubicin, and methotrexate in advanced
esophagogastric cancer. J Clin Oncol. 15:261–267. 1997.
|
|
28
|
Foukakis T, Lundell L, Gubanski M, et al:
Advances in the treatment of patients with gastric adenocarcinoma.
Acta Oncol. 46:277–285. 2007.
|
|
29
|
Sawada N, Ishikawa T, Fukase Y, et al:
Induction of thymidine phosphorylase activity and enhancement of
capecitabine efficacy by taxol/taxotere in human cancer xenografts.
Clin Cancer Res. 4:1013–1019. 1998.
|